Abstract
l-Thyroxine is converted to 3,5,3′-l-triiodothyronine (T3) as well as to 3,3′,5′-l-triiodothyronine (reverse T3). One product of further deiodination is 3,3′-diiodothyronine (3,3′T2). The serum levels of reverse T3 and 3,3′T2 change considerably in various physiological and disease states. We previously found that reverse T3 and 3,3′T2 bind to the solubilized hepatic nuclear “receptors” for thyroid hormones. This led us to study binding and actions of these metabolites in cultured rat pituitary cells in which glucose consumption and growth hormone production are regulated by T3 and l-thyroxine.
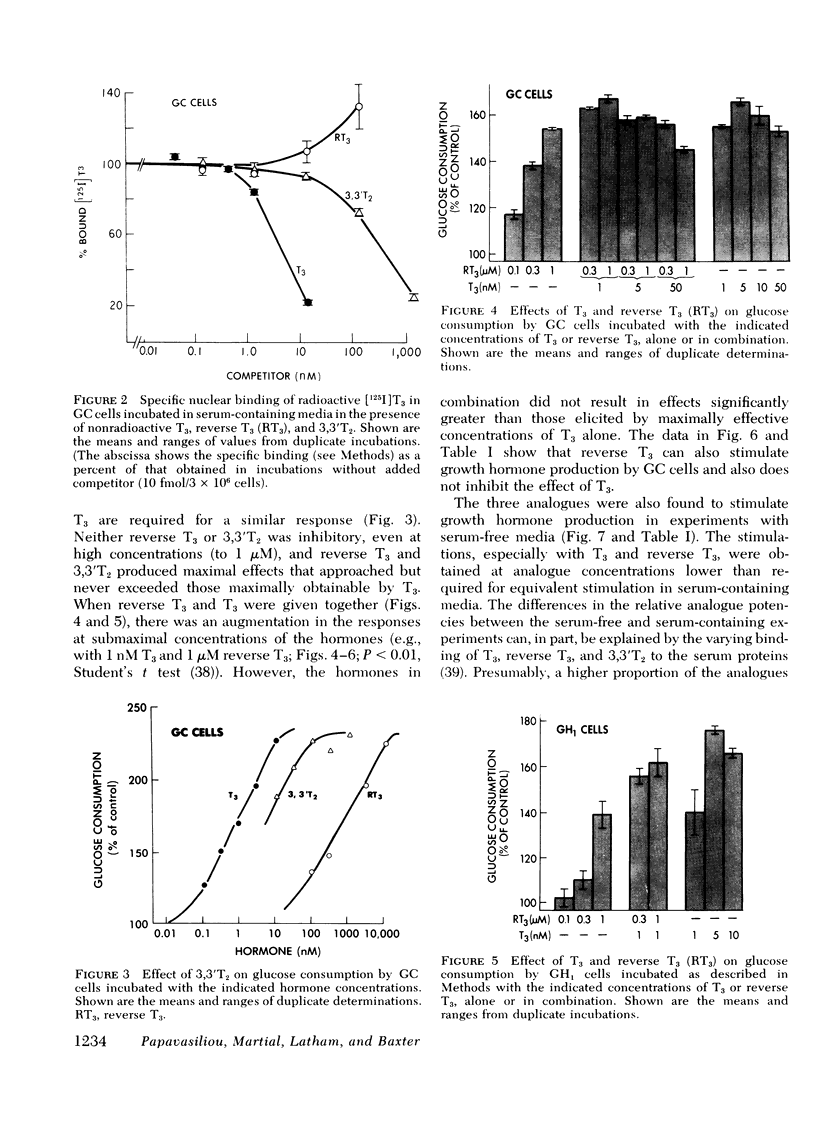
Reverse T3 and 3,3′T2 stimulated growth hormone production and glucose consumption and inhibited nuclear binding of radioactive T3. Either metabolite produced maximal effects that equaled those of T3, and neither inhibited the T3 response. Further, additive effects were observed when reverse T3 was combined with submaximal concentrations of T3.
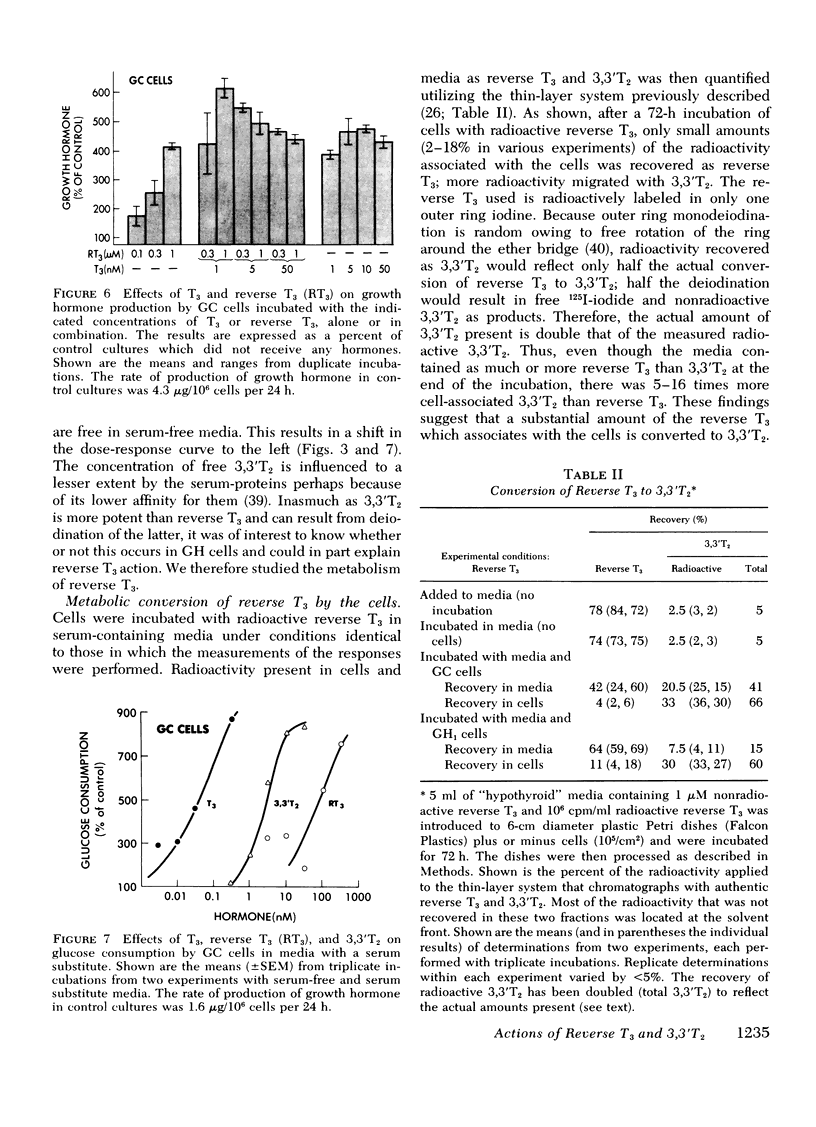
In serum-free and serum-containing media, concentrations of 3,3′T2 50- to 70- and 10- to 100-fold greater, respectively, than those of T3 were required for equivalent stimulations and for inhibition of nuclear binding by T3. The relative activity differences under the two conditions can be attributed to weaker serum protein binding of 3,3′T2 than T3. With cells in serum-free media, reverse T3 was a less avid competitor than 3,3′T2 for T3 binding by the nuclear receptors, and was less potent than 3,3′T2 (0.001 the potency of T3) in inducing growth hormone production or glucose oxidation. In incubations with serum-containing media, reverse T3 was an ineffective competitor for T3 binding, and had only 0.1 the inducing potency of 3,3′T2 (0.001 the potency of T3). The weaker activity of reverse T3 relative to 3,3′T2 in serum-containing media could be explained by stronger serum binding of reverse T3 than 3,3′T2. In addition, after long-term incubation of cells with radioactive reverse T3, much of the cell-associated radioactivity was recovered as 3,3′T2.
These studies suggest that reverse T3 and 3,3′T2 can stimulate thyroid hormone-regulated functions as weak agonists by acting via the same receptors that mediate T3 actions. Moreover, some of the effects of reverse T3 may be due to 3,3′T2 produced by deiodination of reverse T3.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson L. F., Ingbar S. H. Some properties of the stimulatory effect of thyroid hormones on amino acid transport by embryonic chick bone. Endocrinology. 1967 Dec;81(6):1372–1378. doi: 10.1210/endo-81-6-1372. [DOI] [PubMed] [Google Scholar]
- Bancroft F. C. Measurement of growth hormone synthesis by rat pituitary cells in culture. Endocrinology. 1973 Apr;92(4):1014–1021. doi: 10.1210/endo-92-4-1014. [DOI] [PubMed] [Google Scholar]
- Bauer R. F., Arthur L. O., Fine D. L. Propagation of mouse mammary tumor cell lines and production of mouse mammary tumor virus in a serum-free medium. In Vitro. 1976 Aug;12(8):558–563. doi: 10.1007/BF02797439. [DOI] [PubMed] [Google Scholar]
- Block P., Jr Synthesis of 3-iodo-L-thyronine and its iodinated derivatives. J Med Chem. 1976 Aug;19(8):1067–1069. doi: 10.1021/jm00230a018. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Nicod P., Suter P., Vallotton M. B., Vagenakis P., Braverman L. Reduced active thyroid hormone levels in acute illness. Lancet. 1976 Mar 27;1(7961):653–655. doi: 10.1016/s0140-6736(76)92774-4. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Read J., Dimond R. C., Strum D., Wright F. D., Patow W., Earll J. M., Wartofsky L. Measurement of 3,3',5'-Triiodothyroinine (reverse T3), 3,3'-L-diiodothyronine, T3 and T4 in human amniotic fluid and in cord and maternal serum. J Clin Endocrinol Metab. 1976 Dec;43(6):1351–1359. doi: 10.1210/jcem-43-6-1351. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A radioimmunoassay for measurement of 3,3',5'-triiodothyronine (reverse T3). J Clin Invest. 1974 Sep;54(3):583–592. doi: 10.1172/JCI107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Chopra U., Smith S. R., Reza M., Solomon D. H. Reciprocal changes in serum concentrations of 3,3',5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab. 1975 Dec;41(06):1043–1049. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Crandall B. F. Thyroid hormones and thyrotropin in amniotic fluid. N Engl J Med. 1975 Oct 9;293(15):740–743. doi: 10.1056/NEJM197510092931503. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Ho R. S., Lam R. An improved radioimmunoassay of triiodothyronine in serum: its application to clinical and physiological studies. J Lab Clin Med. 1972 Nov;80(5):729–739. [PubMed] [Google Scholar]
- Fisher D. A., Chopra I. J., Dussault J. H. Extrathyroidal conversion of thyroxine to triiodothyronine in sheep. Endocrinology. 1972 Oct;91(4):1141–1144. doi: 10.1210/endo-91-4-1141. [DOI] [PubMed] [Google Scholar]
- HEIDER J. G., BRONK J. R. A RAPID SEPARATION OF THYROXINE AND SOME OF ITS ANALOGUES BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1965 Feb 8;95:353–355. doi: 10.1016/0005-2787(65)90500-9. [DOI] [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Kollman P. A., Murray W. J., Nuss M. E., Jorgensen E. C., Rothenberg S. Molecular orbital studies of thyroid hormone analogs. J Am Chem Soc. 1973 Dec 26;95(26):8518–8525. doi: 10.1021/ja00807a004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- MICHEL R., ROCHE J. Nature and metabolism of thyroid hormones. Recent Prog Horm Res. 1956;12:1-22; discussion, 22-6. [PubMed] [Google Scholar]
- PITMAN C. S., BARKER S. B. Inhibition of thyroxine action by 3,3',5'-triiodothyronine. Endocrinology. 1959 Mar;64(3):466–468. doi: 10.1210/endo-64-3-466. [DOI] [PubMed] [Google Scholar]
- ROCHE J., MICHEL R., NUNEZ U., WOLF W. Sur deux constituants hormonaux nouveaux du corps thyroïde: la 3:3'-diiodothyronine et la 3:3':5'-triiodothyronine. Biochim Biophys Acta. 1955 Sep;18(1):149–150. doi: 10.1016/0006-3002(55)90027-5. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974 Oct;20(10):1255–1270. [PubMed] [Google Scholar]
- STASILLI N. R., KROC R. L., MELTZER R. I. Antigoitrogenic and calorigenic activities of thyroxine analogues in rats. Endocrinology. 1959 Jan;64(1):62–82. doi: 10.1210/endo-64-1-62. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. M., Cavalieri R. R., Goldfine I. D., Ingbar S. H., Jorgensen E. C. Binding of thyroid hormones and their analogues to thyroxine-binding globulin in human serum. J Biol Chem. 1976 Nov 10;251(21):6489–6494. [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: stimulation of growth hormone and inhibition of prolactin secretion in cultured GH1 cells. Biochem Biophys Res Commun. 1974 Jul 10;59(1):420–428. doi: 10.1016/s0006-291x(74)80223-8. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Chopra I. J., Nakamura Y., Solomon D. H., Bennett L. R. A radioimmunoassay for measurement of 3,3'-L-diiodothyronine (T2). J Clin Endocrinol Metab. 1976 Sep;43(3):682–685. doi: 10.1210/jcem-43-3-682. [DOI] [PubMed] [Google Scholar]


