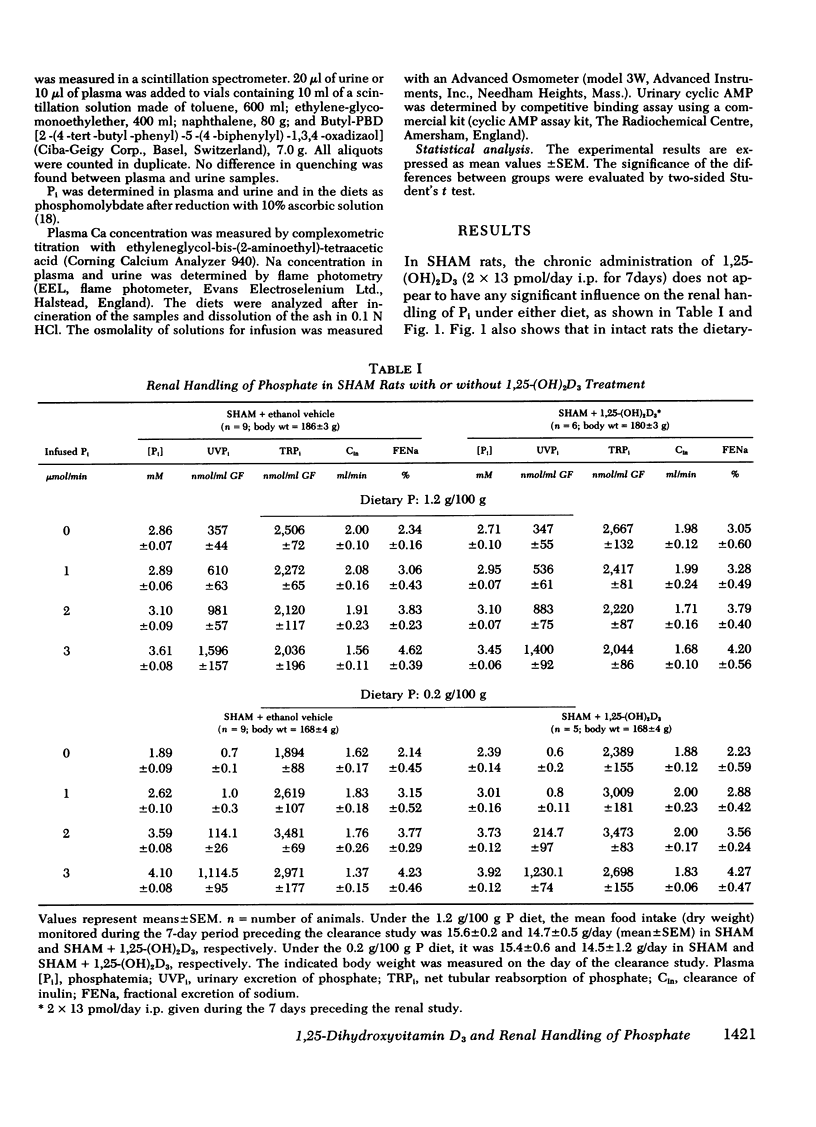
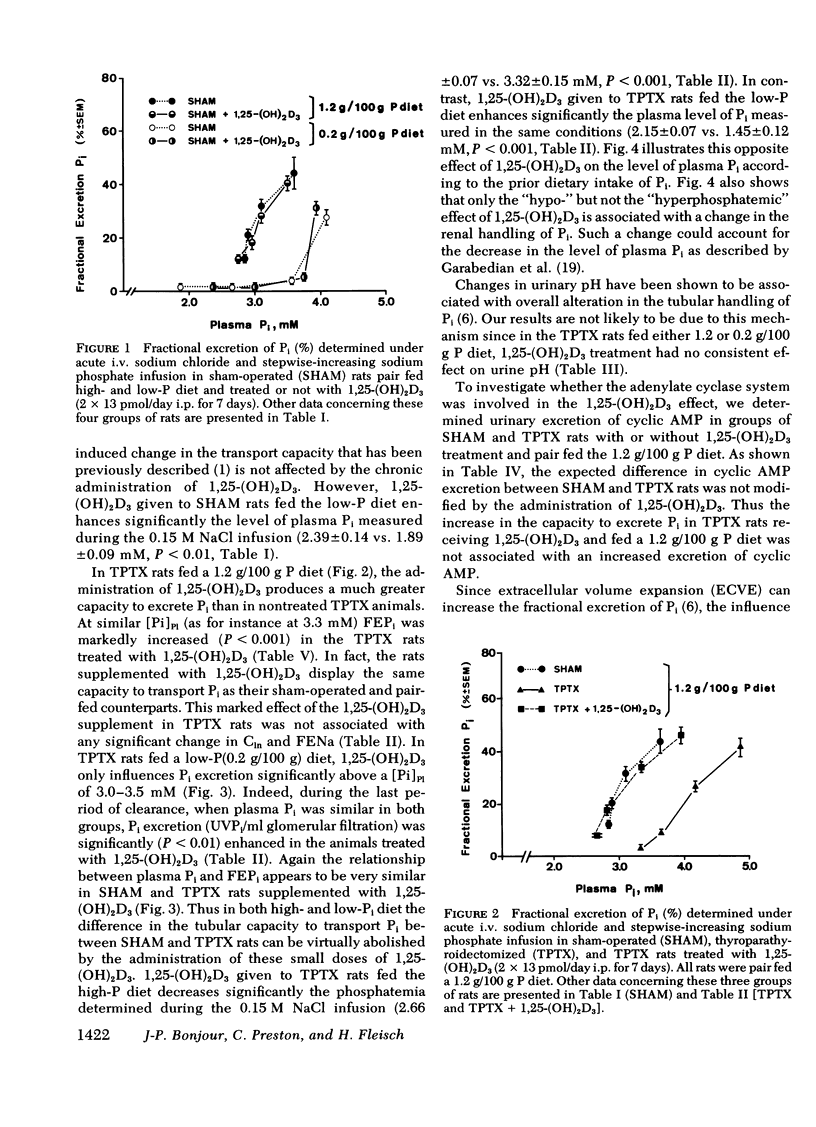
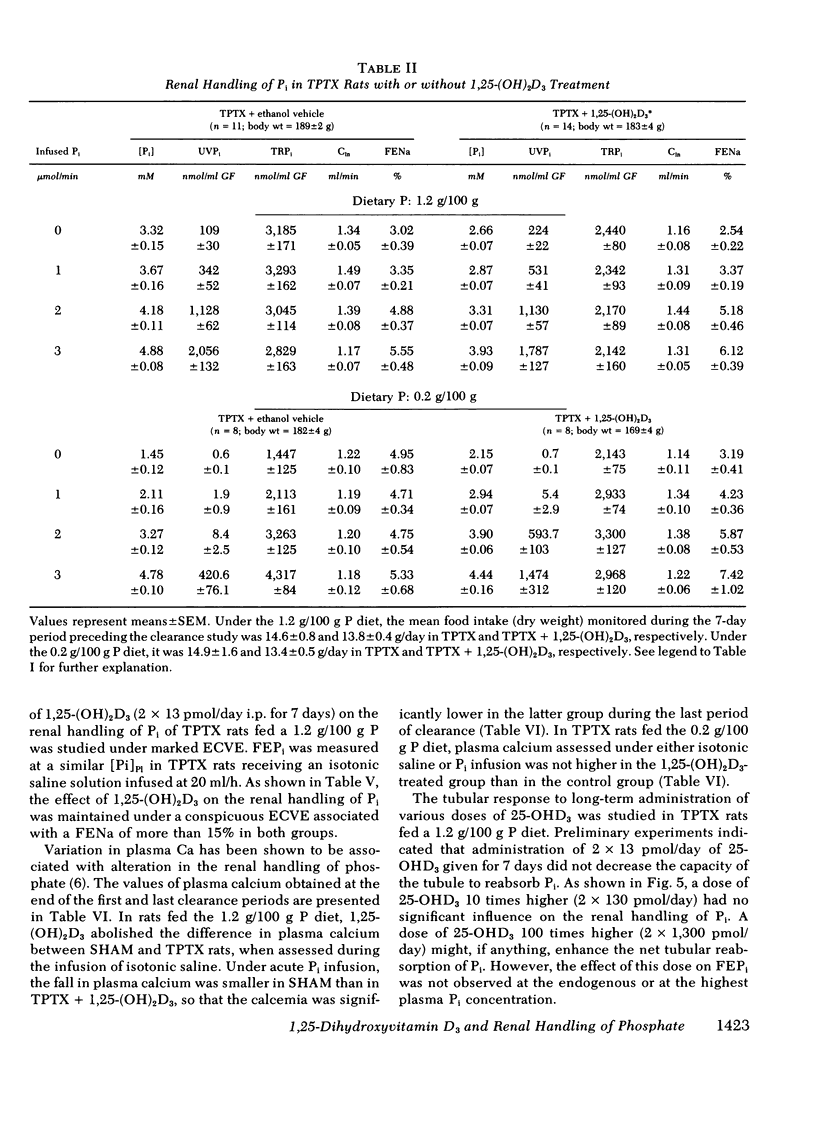
Abstract
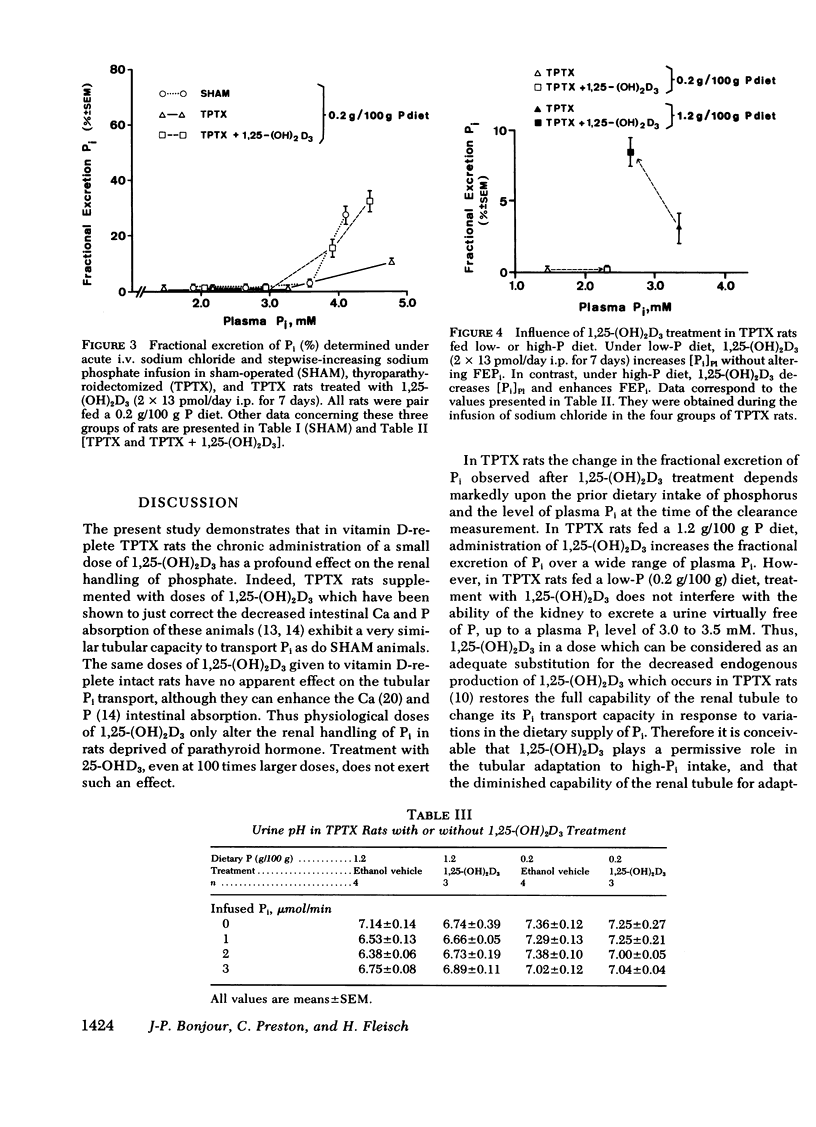
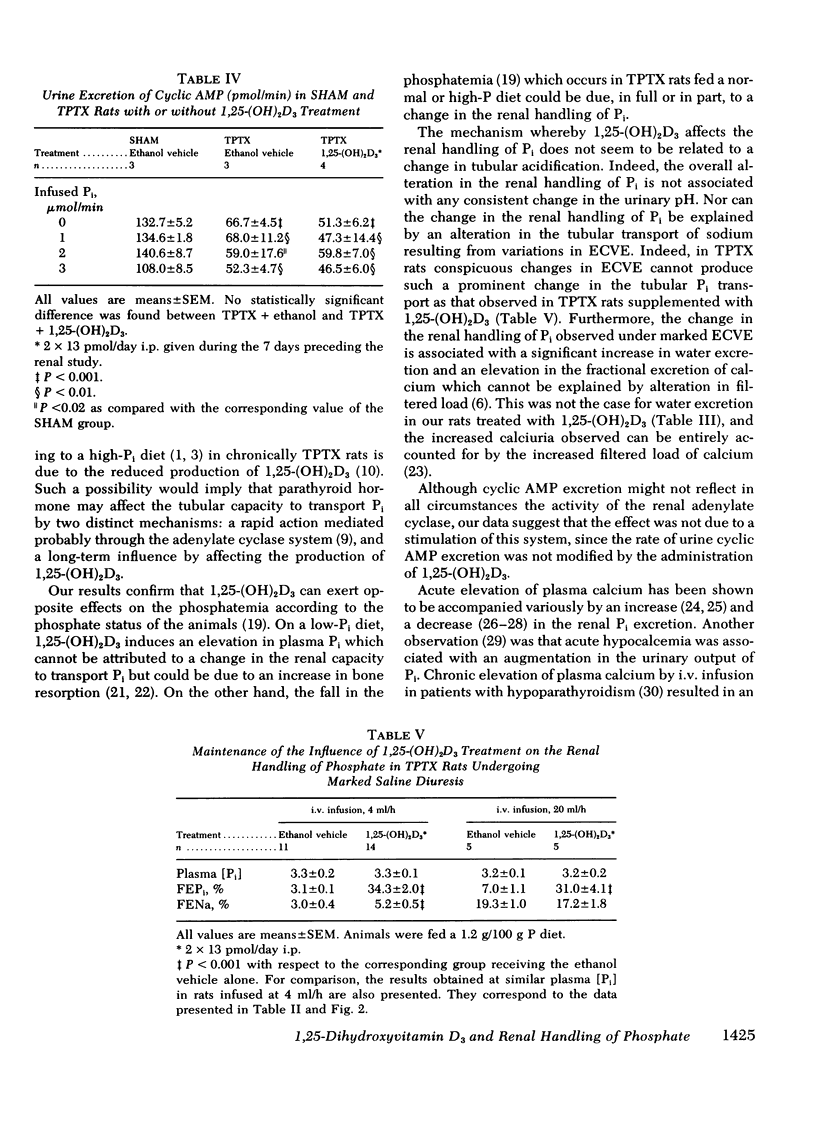
The kidney adapts its tubular capacity to transport inorganic phosphate (Pi) according to the dietary supply of Pi in both intact and thyropara-thyroidectomized (TPTX) rats. However, in TPTX rats the capability of the renal tubule to adapt to a high Pi diet is diminished. In TPTX rats the production of the active vitamin D3 metabolite, 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], is also reduced. 1,25-(OH)2D3 has been shown to have a marked effect on Pi metabolism. Therefore the question arises whether the deficient production of 1,25-(OH)2D3 contributes to the alteration of the tubular transport of Pi observed in chronically TPTX rats. In the present investigation, vitamin D-replete rats were sham operated (SHAM) or thyroparathyroidectomized and then pair fed diets containing either 0.2 or 1.2 g/100 g P for 7 days. During this period, groups of SHAM and TPTX rats received i.p. 2 × 13 pmol/day of 1,25-(OH)2D3, a dose which was shown to just normalize the decreased intestinal absorption of Ca and Pi in TPTX rats. The capacity of tubular Pi transport was then assessed by measuring the fractional excretion of Pi (FEPi) at increasing plasma Pi concentration ([Pi]Pl) obtained by acute infusion of Pi. The results show that in SHAM rats fed either P diet, 1,25-(OH)2D3 has no effect on the renal handling of Pi. In TPTX rats fed 1.2 g/100 g P diet, 1,25-(OH)2D3 increases FEPi over a wide range of [Pi]Pl. In TPTX rats fed a 0.2 g/100 g P diet, 1,25-(OH)2D3 does not alter FEPi up to a [Pi]Pl of 3.0-3.5 mM, but does increase it at higher [Pi]Pl. In fact, on both diets TPTX rats supplemented with 1,25-(OH)2D3 appear to have the same renal handling of Pi as SHAM counterparts. The effect of 1,25-(OH)2D3 was not associated with a change in urine pH or in urinary excretion of cyclic AMP and was maintained under marked extracellular volume expansion. It was associated with a rise in plasma calcium in the TPTX rats fed the high, but not the low, P diet. In TPTX rats fed 1.2 g/100 g P diet, 25-hydroxyvitamin D3 in doses of 2 × 130 or 2 × 1,300 pmol/day i.p. did not increase FEPi.
In conclusion, 1,25-(OH)2D3 administered in physiological amounts to TPTX rats restores to normal the capability of the renal tubule to excrete Pi and to adapt to large variation in dietary Pi. The results suggest that 1,25-(OH)2D3 plays an important role in the regulation of the renal handling of Pi and that the chronic change in the tubular capacity to transport Pi after TPTX may be due to the decreased formation of 1,25-(OH)2D3.
Full text
PDF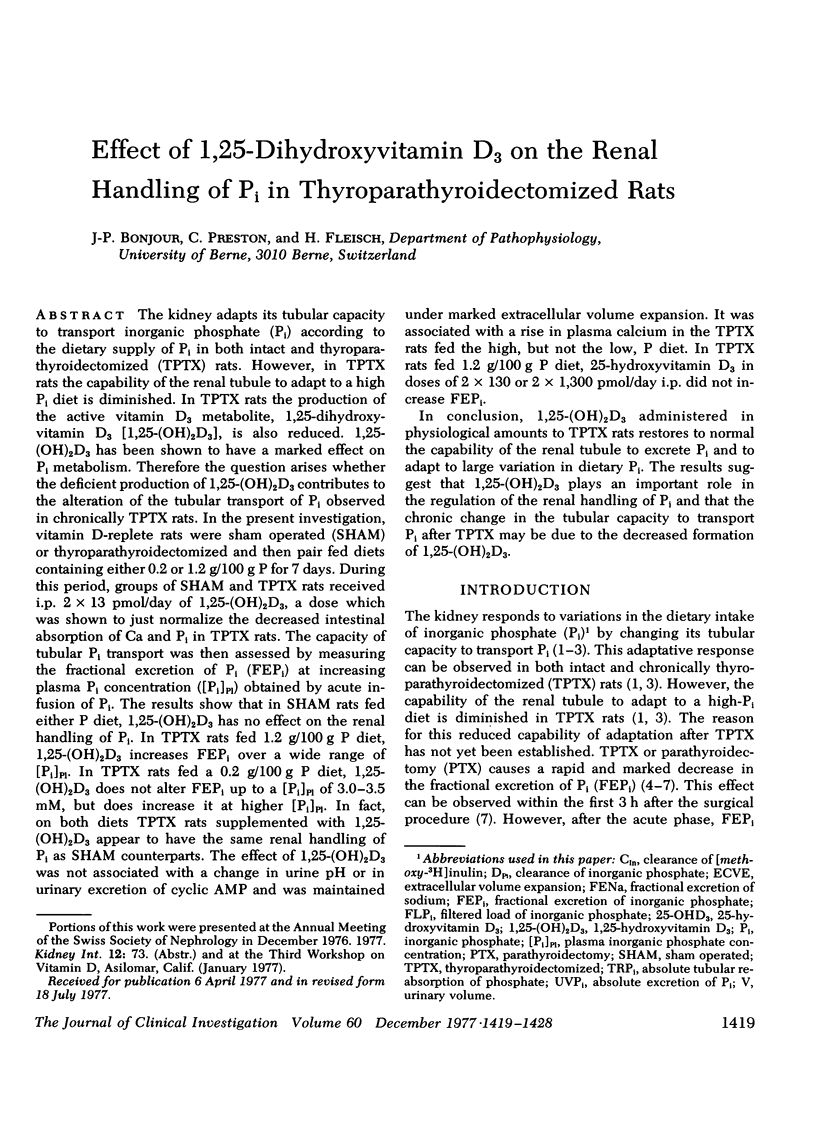



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel C., Kuntziger H., Couette S., Coureau C., Bergounioux N. Evidence for a parathyroid hormone-independent calcium modulation of phosphate transport along the nephron. J Clin Invest. 1976 Feb;57(2):256–263. doi: 10.1172/JCI108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel C., Kuntziger H., Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflugers Arch. 1970;317(2):93–109. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- Bonjour J. P., Fleisch H., Trechsel U. Calcium absorption in diphosphonate-treated rats: effect of parathyroid function, dietary calcium and phosphorus. J Physiol. 1977 Jan;264(1):125–139. doi: 10.1113/jphysiol.1977.sp011660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour J. P., Trechsel U., Fleisch H., Schenk R., DeLuca H. F., Baxter L. A. Action of 1,25-dihydroxyvitamin D3 and a diphosphonate on calcium metabolism in rats. Am J Physiol. 1975 Aug;229(2):402–408. doi: 10.1152/ajplegacy.1975.229.2.402. [DOI] [PubMed] [Google Scholar]
- CRAWFORD J. D., GRIBETZ D., TALBOT N. B. Mechanism of renal tubular phosphate reabsorption and the influence thereon of vitamin D in completely parathyroidectomized rats. Am J Physiol. 1955 Jan;180(1):156–162. doi: 10.1152/ajplegacy.1954.180.1.156. [DOI] [PubMed] [Google Scholar]
- Castillo L., Tanaka Y., DeLuca H. F. The mobilization of bone mineral by 1,25-dihydroxyvitamin D3 in hypophosphatemic rats. Endocrinology. 1975 Oct;97(4):995–999. doi: 10.1210/endo-97-4-995. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Cuche J. L., Ott C. E., Marchand G. R., Diaz-Buxo J. A., Knox F. G. Intrarenal calcium in phosphate handling. Am J Physiol. 1976 Mar;230(3):790–796. doi: 10.1152/ajplegacy.1976.230.3.790. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- EISENBERG E. EFFECTS OF SERUM CALCIUM LEVEL AND PARATHYROID EXTRACTS ON PHOSPHATE AND CALCIUM EXCRETION IN HYPOPARATHYROID PATIENTS. J Clin Invest. 1965 Jun;44:942–946. doi: 10.1172/JCI105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Pezant E., Miravet L., Fellot C., Balsan S. 1,25-Dihydroxycholecalciferol effect on serum phosphorus homeostasis in rats. Endocrinology. 1976 Mar;98(3):794–799. doi: 10.1210/endo-98-3-794. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Tanaka Y., Holick M. F., Deluca H. F. Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathyroidectomized rats. Endocrinology. 1974 Apr;94(4):1022–1027. doi: 10.1210/endo-94-4-1022. [DOI] [PubMed] [Google Scholar]
- Glorieux F., Scriver C. R. Loss of a parathyroid hormone-sensitive component of phosphate transport in X-linked hypophosphatemia. Science. 1972 Mar 3;175(4025):997–1000. doi: 10.1126/science.175.4025.997. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Lavender A. R., Pullman T. N. Changes in inorganic phosphate excretion induced by renal arterial infusion of calcium. Am J Physiol. 1963 Nov;205(5):1025–1032. doi: 10.1152/ajplegacy.1963.205.5.1025. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Friedler R. M., Coburn J. W. Excretion of phosphate and calcium. Physiology of their renal handling and relation to clinical medicine. Arch Intern Med. 1973 Jun;131(6):828–859. doi: 10.1001/archinte.131.6.828. [DOI] [PubMed] [Google Scholar]
- Ney R. L., Kelly G., Bartter F. C. Actions of vitamin D independent of the parathyroid glands. Endocrinology. 1968 Apr;82(4):760–766. doi: 10.1210/endo-82-4-760. [DOI] [PubMed] [Google Scholar]
- Popovtzer M. M., Robinette J. B., DeLuca H. F., Holick M. F. The acute effect of 25-hydroxycholecalciferol on renal handling of phosphorus. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest. 1974 Mar;53(3):913–921. doi: 10.1172/JCI107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Fernandez P. C., Boyle I. T., Gray R. W., Omdahl J. L., DeLuca H. F. The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1972 Oct;141(1):379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Moranz J., Kurnick W. S. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972 Feb;51(2):373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Anast C., Arnaud C. Thyrocalcitonin, EGTA, and urinary electrolyte excretion. J Clin Invest. 1967 May;46(5):746–752. doi: 10.1172/JCI105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R., Fleisch H., Bonjour J. P. Effect of thyroparathyroidectomy of calcium metabolism in rats: role of 1,25-dihydroxyvitamin D3. Am J Physiol. 1977 Sep;233(3):E160–E164. doi: 10.1152/ajpendo.1977.233.3.E160. [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Fleisch H., Bonjour J. P. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest. 1977 Sep;60(3):639–647. doi: 10.1172/JCI108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H., DeLuca H. F. Influence of dietary phosphorus on renal phosphate reabsorption in the parathyroidectomized rat. J Clin Invest. 1976 Apr;57(4):867–874. doi: 10.1172/JCI108363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H., Engle J. E., Tanaka Y., Lorenc R. S., Dudgeon K. L., DeLuca H. F. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975 Aug;229(2):489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- Troehler U., Bonjour J. P., Fleisch H. Renal secretion of diphosphonates in rats. Kidney Int. 1975 Jul;8(1):6–13. doi: 10.1038/ki.1975.70. [DOI] [PubMed] [Google Scholar]
- Trohler U., Bonjour J. P., Fleisch H. Renal tubular adaptation to dietary phosphorus. Nature. 1976 May 13;261(5556):145–146. doi: 10.1038/261145a0. [DOI] [PubMed] [Google Scholar]
- Tröhler U., Bonjour J. P., Fleisch H. Inorganic phosphate homeostasis. Renal adaptation to the dietary intake in intact and thyroparathyroidectomized rats. J Clin Invest. 1976 Feb;57(2):264–273. doi: 10.1172/JCI108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S. F. Micropuncture studies of phosphate transport in the proximal tubule of the dog. The relationship to sodium reabsorption. J Clin Invest. 1974 Jan;53(1):143–153. doi: 10.1172/JCI107532. [DOI] [PMC free article] [PubMed] [Google Scholar]


