Abstract
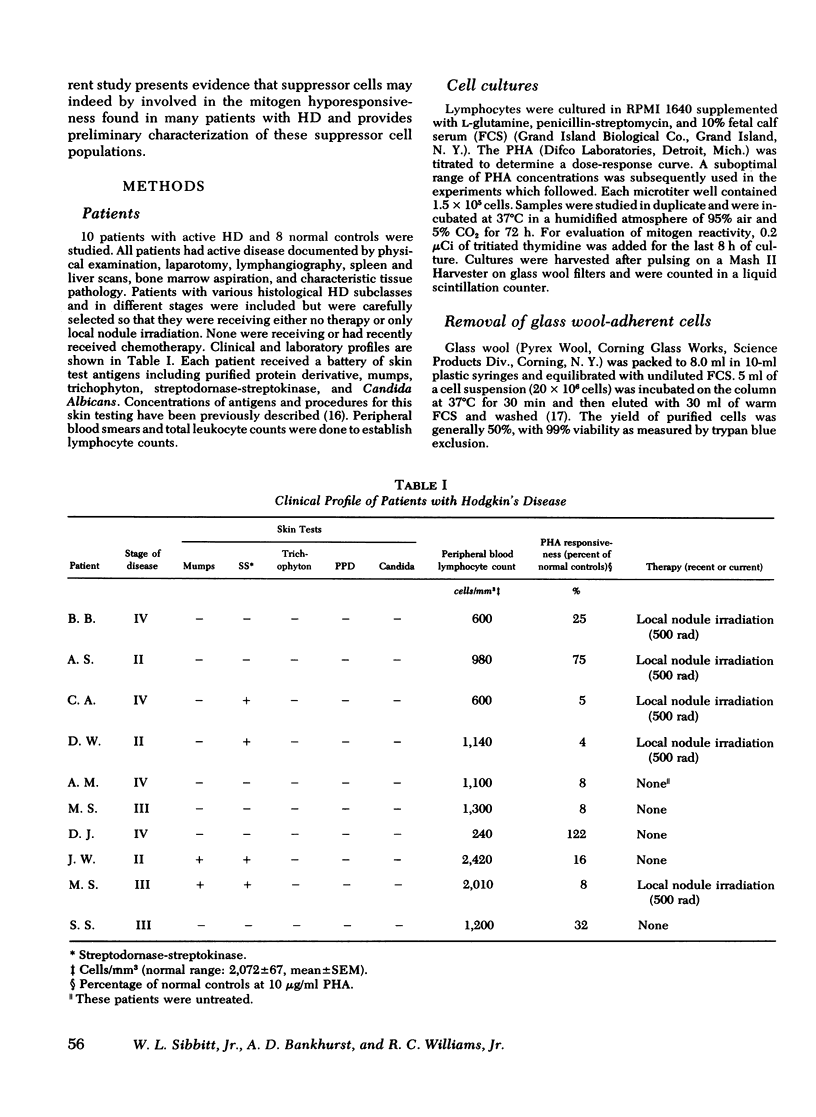
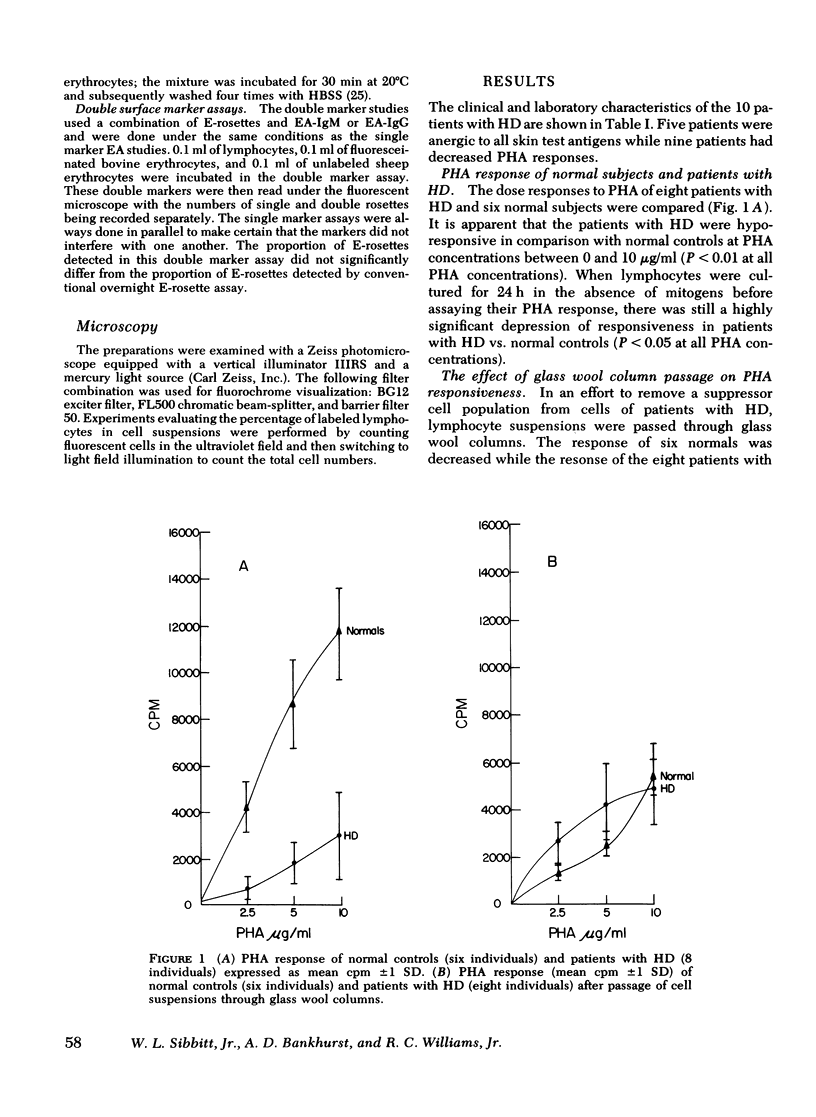
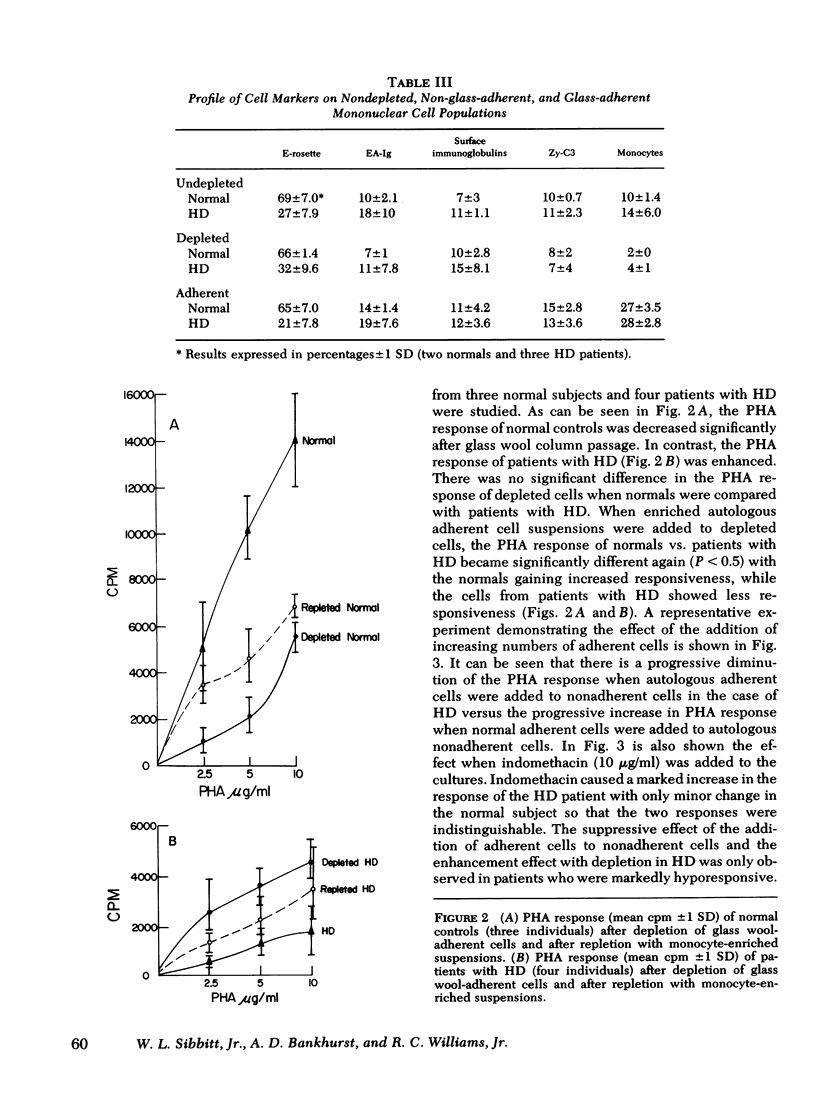
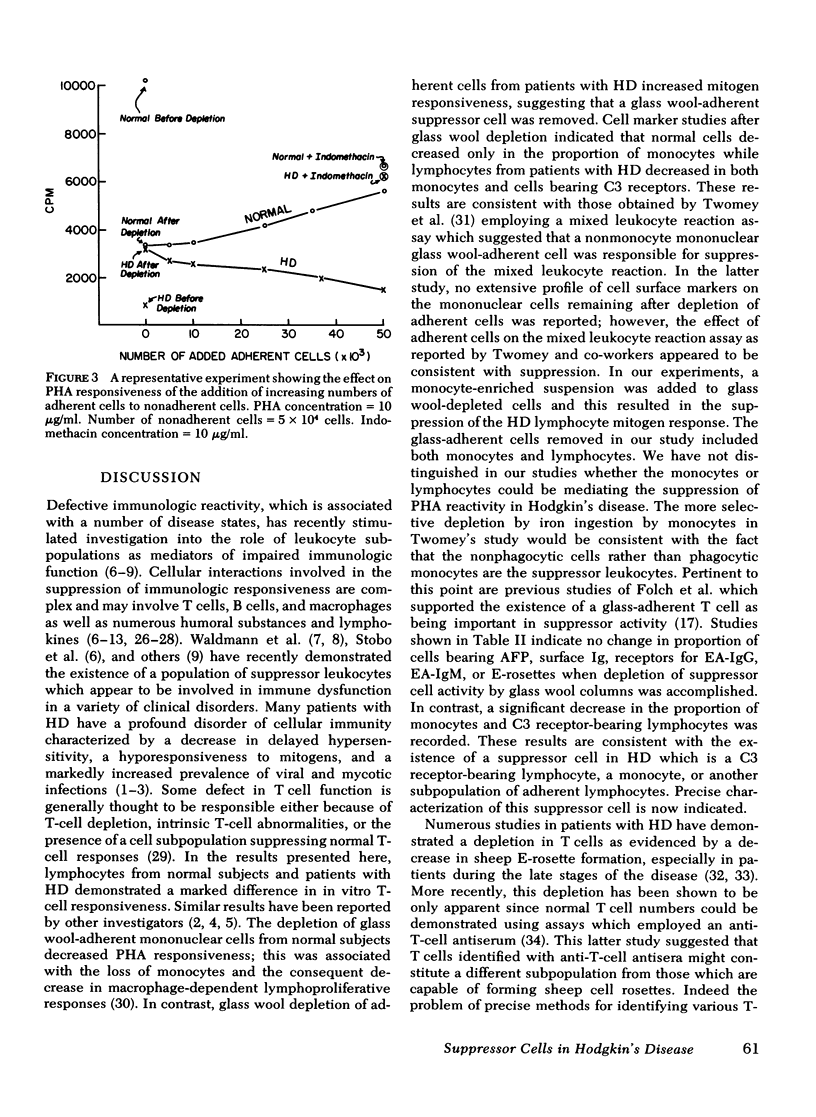
Hodgkin's disease (HD) is associated with a deficit in T-cell immunity characterized by skin test anergy and decreased lymphocyte responses to phytohemagglutinin (PHA). To investigate this mitogen hyporesponsiveness in HD, we separated peripheral blood mononuclear cells on Ficoll-Hypaque gradients and determined their response to various suboptimal concentrations of PHA. As was expected, patients with HD demonstrated marked mitogen hyporesponsiveness relative to normal controls; however, if the cell suspensions were first passed through glass wool columns to remove adherent cells, the PHA responsiveness of the hyporesponsive HD cells was markedly increased. In contrast, the responsiveness of normal controls was decreased so that the responses of nonadherent normal and HD cells were statistically indistinguishable. Evidently, a glass wool-adherent suppressor cell had been removed from patients with HD, while a glass wool-adherent cell which enhanced mitogenic responses had been removed from normal controls during column passage. Previous to column depletion, patients with HD had decreased proportions of E-rosettes and increased proportions of cells with surface α-fetoprotein; however, the proportion of these cells was not changed after column passage. Significant changes with column depletion of glass wool-adherent cells in HD were recorded in the proportions of monocytes (13.2 vs 5.8%) and lymphocytes with C-3 receptors (12.6 vs. 7.8%). The only significant change in normal controls was a decrease in the proportion of monocytes (10 vs. 1.7%). To determine if glass-adherent cells would have a suppressor effect, HD-adherent cells were added in progressively increasing numbers to mononuclear cell suspensions depleted of glass wool-adherent cells. PHA responsiveness returned toward predepletion levels. In summary, patients with HD possess a glass wool-adherent suppressor cell which is responsible at least in part for in vitro mitogen hyporesponsiveness.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C. Manifestations of immunologic unresponsiveness in Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1152–1164. [PubMed] [Google Scholar]
- Beavers E. M. Letter: Lung cancer in chloromethyl methyl ether workers. N Engl J Med. 1974 Apr 25;290(17):971–972. doi: 10.1056/NEJM197404252901718. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M. W. Delayed-type hypersensitivity and the immunology of Hodgkin's disease, with a parallel examination of sarcoidosis. Cancer Res. 1966 Jun;26(6):1097–1120. [PubMed] [Google Scholar]
- Ferrarini M., Tonda G. P., Risso A., Viale G. Lymphocyte membrane receptors in human lymphoid leukemias. Eur J Immunol. 1975 Feb;5(2):89–93. doi: 10.1002/eji.1830050204. [DOI] [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H. Role of the thymus in tolerance. X. "Suppressor" activity of antigen-stimulated rat thymocytes transferred to normal recipients. J Immunol. 1973 May;110(5):1290–1299. [PubMed] [Google Scholar]
- Hersh E. M., Irvin W. S. Blastogenic responses of lymphocytes from patients with untreated and treated lymphomas. Lymphology. 1969 Dec;2(4):150–160. [PubMed] [Google Scholar]
- Huber Ch, Wigzell H. A simple rosette assay for demonstration of complement receptor sites using complement-coated zymosan beads. Eur J Immunol. 1976 Jun;5(6):432–435. doi: 10.1002/eji.1830050616. [DOI] [PubMed] [Google Scholar]
- Husby G., Strickland R. G., Caldwell J. L., Williams R. C., Jr Localization of T and B cells and alpha fetoprotein in hepatic biopsies from patients with liver disease. J Clin Invest. 1975 Nov;56(5):1198–1209. doi: 10.1172/JCI108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelfinger F. J. Editorial: The University of Massachusetts Medical School comes of age. N Engl J Med. 1976 Apr 15;294(16):899–900. doi: 10.1056/NEJM197604152941610. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Möller G. Effect of B-cell mitogens on lymphocyte subpopulations possesing C'3 and Fc receptors. J Exp Med. 1974 Apr 1;139(4):969–982. doi: 10.1084/jem.139.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960 Dec 9;132(3441):1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- Ng R. P., Moran C. J., Alexopoulos C. G., Bellingham A. J. Transfer factor in Hodgkin's disease. Lancet. 1975 Nov 8;2(7941):901–903. doi: 10.1016/s0140-6736(75)92131-5. [DOI] [PubMed] [Google Scholar]
- Order S. E., Hellman S. Pathogenesis of Hodgkin's disease. Lancet. 1972 Mar 11;1(7750):571–573. doi: 10.1016/s0140-6736(72)90360-1. [DOI] [PubMed] [Google Scholar]
- Papac R. J. Lymphocyte transformation in malignant lymphomas. Cancer. 1970 Aug;26(2):279–286. doi: 10.1002/1097-0142(197008)26:2<279::aid-cncr2820260206>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliata F., Lawrence V. J., Phillips-Quagliata J. M. Prostaglandin E 1 as a regulator of lymphocyte function. Selective action on B lymphocytes and synergy with procarbazine in depression of immune responses. Cell Immunol. 1973 Mar;6(3):457–465. doi: 10.1016/0008-8749(73)90044-0. [DOI] [PubMed] [Google Scholar]
- Ramot B., Many A., Biniaminov M., Aghai E. Thymus-derived lymphocyte (T-cell) depletion in Hodgkin's disease. Isr J Med Sci. 1973 May;9(5):657–659. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausser H. R., Humes J. L. Prostaglandin synthesis inhibition: effect on bone changes and sarcoma tumor induction in balb/c mice. Int J Cancer. 1975 May 15;15(5):724–730. doi: 10.1002/ijc.2910150503. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Sharkey O., Jr, Brown J. A., Laughter A. H., Jordan P. H., Jr Cellular requirements for the mitotic response in allogeneic mixed leukocyte cultures. J Immunol. 1970 Apr;104(4):845–853. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Palmer D. L., Williams R. C., Jr Characterization of serum inhibitors of neutrophil chemotaxis associated with anergy. J Immunol. 1974 Jul;113(1):189–200. [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Jr, Jamieson T. Control of mitogen-induced transformation: characterization of a splenic suppressor cell and its mode of action. Cell Immunol. 1976 Jun 1;24(1):45–57. doi: 10.1016/0008-8749(76)90130-1. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


