Abstract
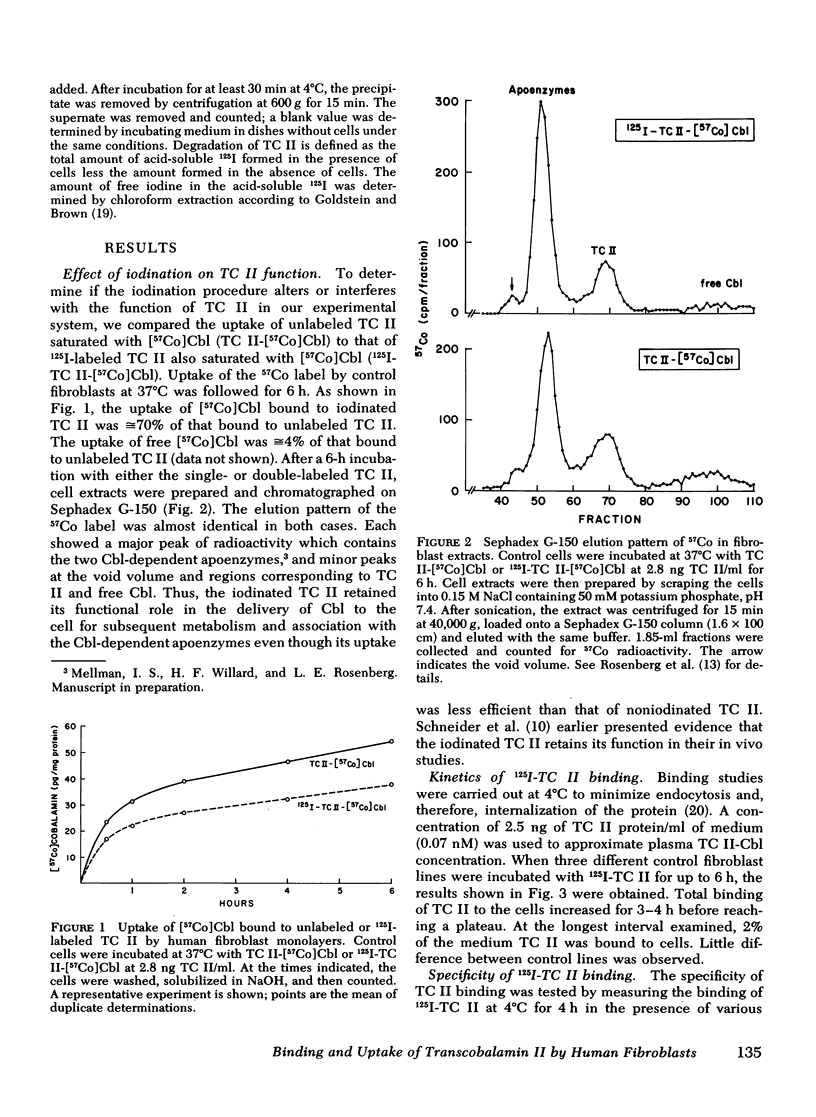
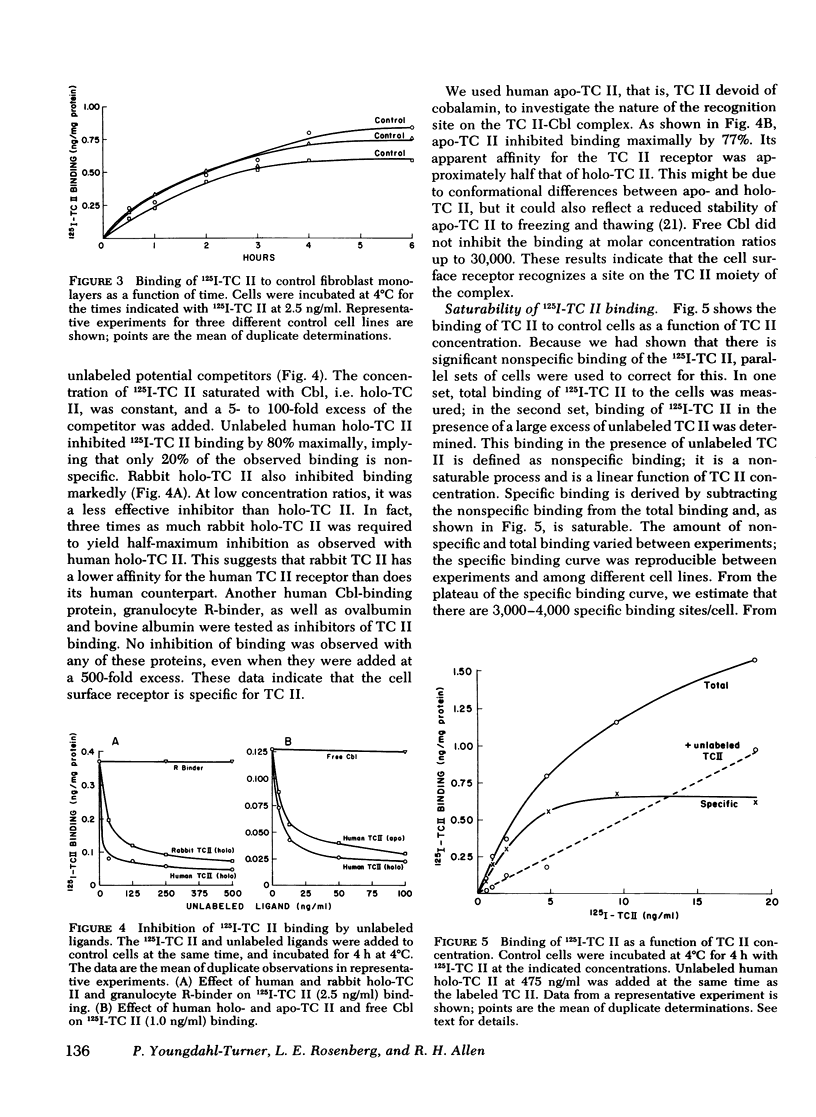
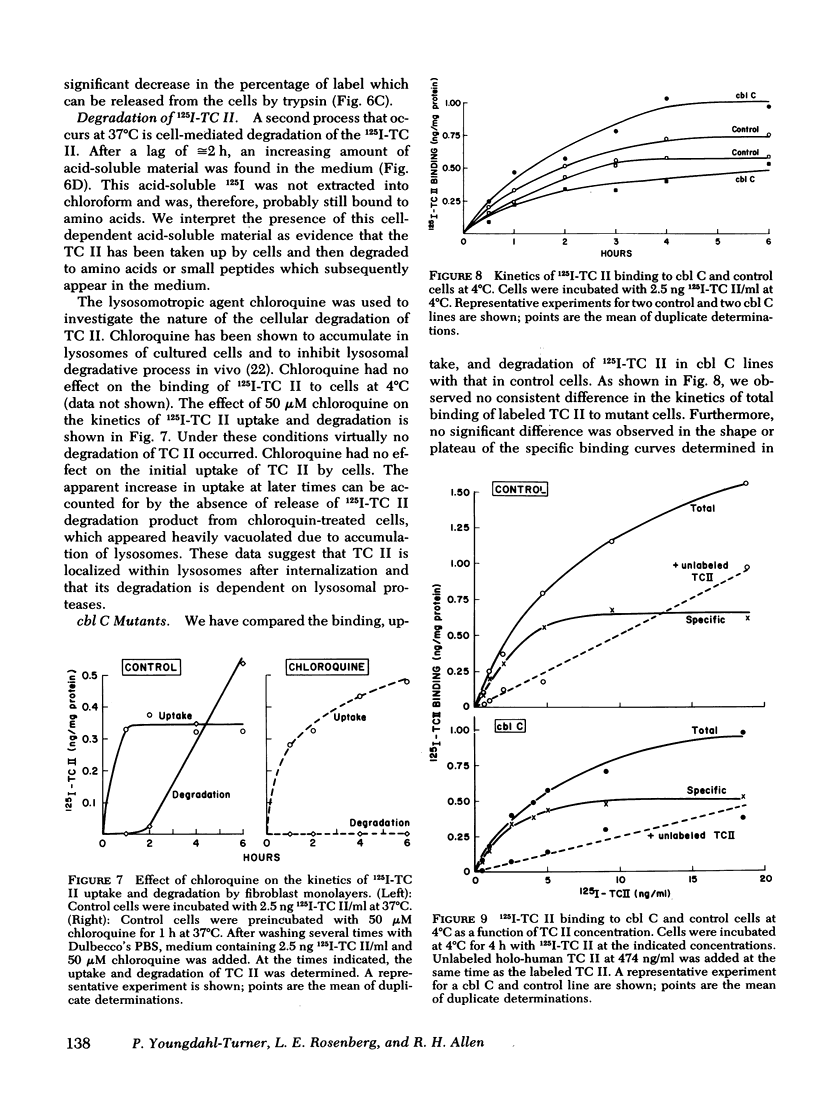
We have used purified, 125I-labeled human transcobalamin II (TC II), saturated with cobalamin (Cbl), to study the uptake process for the TC II-Cbl complex by intact normal cultured human skin fibroblasts. We have also investigated the possibility that a defect in one step of this process underlies that inborn error of Cbl metabolism—designated cbl C—in which mutant cells are unable to retain Cbl intracellularly or convert it to its coenzyme forms. TC II-Cbl binding at 4°C reached a plateau after 3-4 hr; 95% of the bound 125I was releasable with trypsin. Binding of TC II-Cbl at 4°C could be inhibited by human and rabbit TC II-Cbl and human TC II devoid of Cbl but not by other Cbl-binding proteins, albumin, or free Cbl. Specific binding reached saturation at ≅5 ng TC II/ml (0.13 nM) and could be inhibited by ethylene glycol-bis (β-aminoethyl ether) N,N,N′,N′- tetraacetic acid. At 37°C, the TC II-Cbl complex was internalized as shown by a progressive decrease in the trypsin-releasable fraction of bound 125I. After 2 h at 37°C, increasing amounts of acid-soluble 125I were found in the incubation medium indicating that the labeled TC II was being degraded. Chloroquine, an inhibitor of lysosomal proteolysis, prevented this degradation. The binding, internalization, and degradation of TC II-Cbl by cbl C cells was indistingusihable from that by control cells. Our studies provide additional support for the concepts: (a) that the TC II-Cbl complex binds to a specific cell surface receptor through a site on the TC II; (b) that the interaction between the receptor and TC II is calcium dependent; (c) that the TC II-Cbl is internalized via endocytosis; (d) that the degradation of TC II and release of Cbl from the complex occurs in lysosomes. We also conclude that the defect in cbl C must reside at some step beyond this receptor-mediated uptake process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H., Majerus P. W. Isolation of vitamin B12-binding proteins using affinity chromatography. 3. Purification and properties of human plasma transcobalamin II. J Biol Chem. 1972 Dec 10;247(23):7709–7717. [PubMed] [Google Scholar]
- Allen R. H. The plasma transport of vitamin B12. Br J Haematol. 1976 Jun;33(2):161–171. doi: 10.1111/j.1365-2141.1976.tb03527.x. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Burger R. L., Mehlman C. S., Allen R. H. Human plasma R-type vitamin B12-binding proteins. I. Isolation and characterization of transcobalamin I. TRANSCOBALAMIN III. and the normal granulocyte vitamin B12-binding protein. J Biol Chem. 1975 Oct 10;250(19):7700–7706. [PubMed] [Google Scholar]
- DiGirolamo P. M., Huennekens F. M. Transport of vitamin B12 into mouse leukemia cells. Arch Biochem Biophys. 1975 Jun;168(2):386–393. doi: 10.1016/0003-9861(75)90267-2. [DOI] [PubMed] [Google Scholar]
- Fiedler-Nagy C., Rowley G. R., Coffey J. W., Miller O. N. Binding of vitamin B12--rat transcobalamin II and free vitamin B12 to plasma membranes isolated from rat liver. Br J Haematol. 1975 Nov;31(3):311–321. doi: 10.1111/j.1365-2141.1975.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Shia M. A., Wallace J. K. A saturable high affinity binding site for transcobalamin II-vitamin B12 complexes in human placental membrane preparations. J Clin Invest. 1977 Jan;59(1):51–58. doi: 10.1172/JCI108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The LDL pathway in human fibroblasts: a receptor-mediated mechanism for the regulation of cholesterol metabolism. Curr Top Cell Regul. 1976;11:147–181. doi: 10.1016/b978-0-12-152811-9.50011-0. [DOI] [PubMed] [Google Scholar]
- Green P. D., Savage C. R., Jr, Hall C. A. Mouse transcobalamin II: biosynthesis and uptake by L-929 cells. Arch Biochem Biophys. 1976 Oct;176(2):683–689. doi: 10.1016/0003-9861(76)90212-5. [DOI] [PubMed] [Google Scholar]
- Hall C. A. Transcobalamins I and II as natural transport proteins of vitamin B12. J Clin Invest. 1975 Nov;56(5):1125–1131. doi: 10.1172/JCI108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Recognition of two intracellular cobalamin binding proteins and their identification as methylmalonyl-CoA mutase and methionine synthetase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):921–925. doi: 10.1073/pnas.74.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnell J. C., Matthews D. M., Mudd S. H., Uhlendorf B. W., Wise I. J. Cobalamins in fibroblasts cultured from normal control subjects and patients with methylmalonic aciduria. Pediatr Res. 1976 Mar;10(3):179–183. doi: 10.1203/00006450-197603000-00007. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E., Mudd S. H., Uhlendorf B. W. Defective metabolism of vitamin B 12 in fibroblasts from children with methylmalonicaciduria. Biochem Biophys Res Commun. 1971 Jul 16;44(2):375–381. doi: 10.1016/0006-291x(71)90610-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Youngdahl-Turner P., Willard H. F., Rosenberg L. E. Intracellular binding of radioactive hydroxocobalamin to cobalamin-dependent apoenzymes in rat liver. Proc Natl Acad Sci U S A. 1977 Mar;74(3):916–920. doi: 10.1073/pnas.74.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark P., Newman G. E., O'Brien J. R. Vitamin B12 in the rat kidney. Evidence for an association with lysosomes. Arch Biochem Biophys. 1970 Nov;141(1):121–130. doi: 10.1016/0003-9861(70)90114-1. [DOI] [PubMed] [Google Scholar]
- Peirce K., Abe T., Cooper B. A. Incorporation and metabolic conversion of cyanocobalamin by Ehrlich ascites carcinoma cells in vitro and in vivo. Biochim Biophys Acta. 1975 Feb 13;381(2):348–358. doi: 10.1016/0304-4165(75)90240-8. [DOI] [PubMed] [Google Scholar]
- Pletsch Q. A., Coffey J. W. Properties of the proteins that bind vitamin B 12 in subcellular fractions of rat liver. Arch Biochem Biophys. 1972 Jul;151(1):157–167. doi: 10.1016/0003-9861(72)90484-5. [DOI] [PubMed] [Google Scholar]
- Retief F. P., Gottlieb C. W., Herbert V. Mechanism of vitamin B12 uptake by erythocytes. J Clin Invest. 1966 Dec;45(12):1907–1915. doi: 10.1172/JCI105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. E., Patel L., Lilljeqvist A. C. Absence of an intracellular cobalamin-binding protein in cultured fibroblasts from patients with defective synthesis of 5'-deoxyadenosylcobalamin and methylcobalamin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4617–4621. doi: 10.1073/pnas.72.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Burger R. L., Mehlman C. S., Allen R. H. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. J Clin Invest. 1976 Jan;57(1):27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


