Abstract
The beige mouse, C57BL/6 (bg/bg), is an animal model for the Chediak-Higashi syndrome in man, a disease characterized morphologically by giant lysosomes in most cell types. Half-lives for the turnover of [14C]bicarbonate-labeled total soluble liver protein were determined in normal and beige mice. No significant differences were observed between the normal and mutant strain for both rapidly and slowly turning-over classes of proteins. Glucagon treatment during the time-course of protein degradation had similar effects on both normal and mutant strains and led to the conclusion that the rate of turnover of endogenous intracellular protein in the beige mouse liver does not differ from normal.
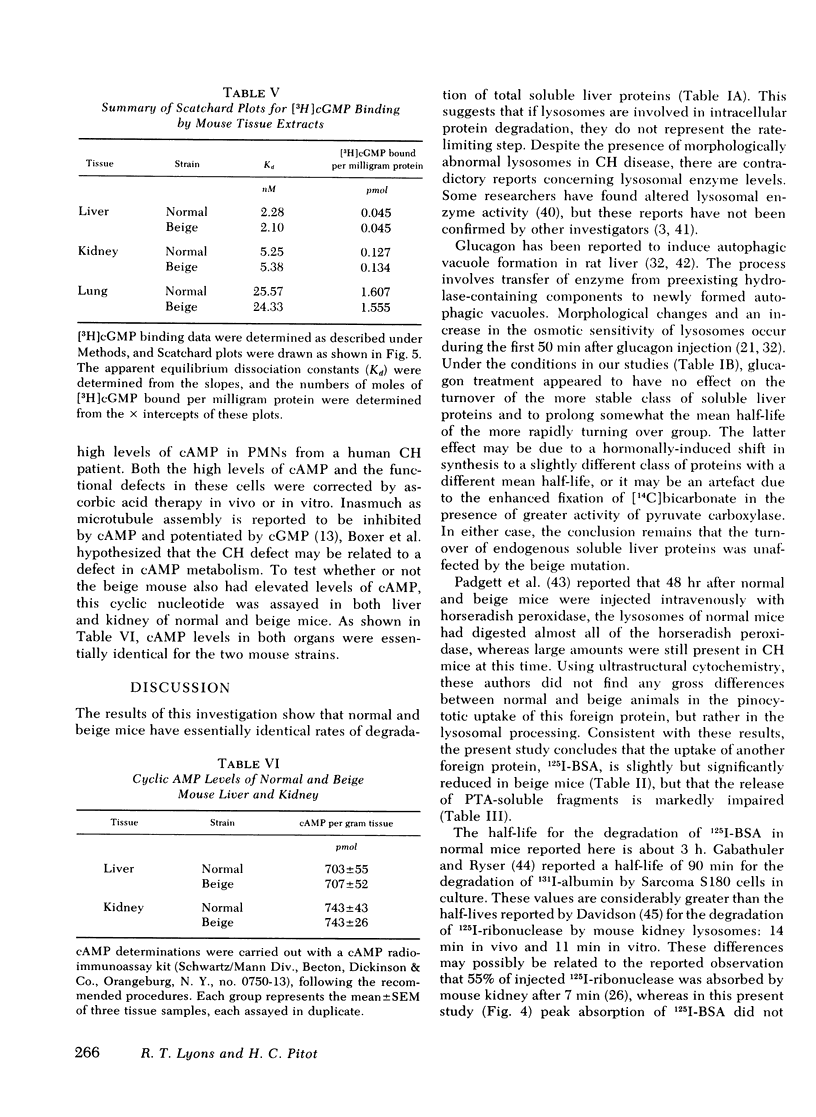
The rates of uptake and degradation of an exogenous protein were determined in normal and beige mice by intravenously injecting 125I-bovine serum albumin and following, in peripheral blood, the loss with time of phosphotungstic acid-insoluble bovine serum albumin and the parallel appearance of phosphotungstic acid-soluble (degraded) material. No significant differences were observed between beige and normal mice in the uptake by liver lysosomes of 125I-bovine serum albumin (t½ = 3.9 and 2.8 h, respectively). However, it was found that lysosomes from livers of beige mice released phosphotungstic acid-soluble radioactivity at a rate significantly slower than normal (t½ = 6.8 and 3.1 h, respectively). This defect in beige mice could be corrected by chronic administration of carbamyl choline (t½ = 3.5 h), a cholinergic agonist which raises intracellular cyclic GMP levels. However, no significant differences between normal and beige mice were observed either in the ability of soluble extracts of liver and kidney to bind [3H]cyclic GMP in vitro or in the basal levels of cyclic AMP in both tissues. The relevance of these observations to the presumed biochemical defect underlying the Chediak-Higashi syndrome is discussed.
Full text
PDF

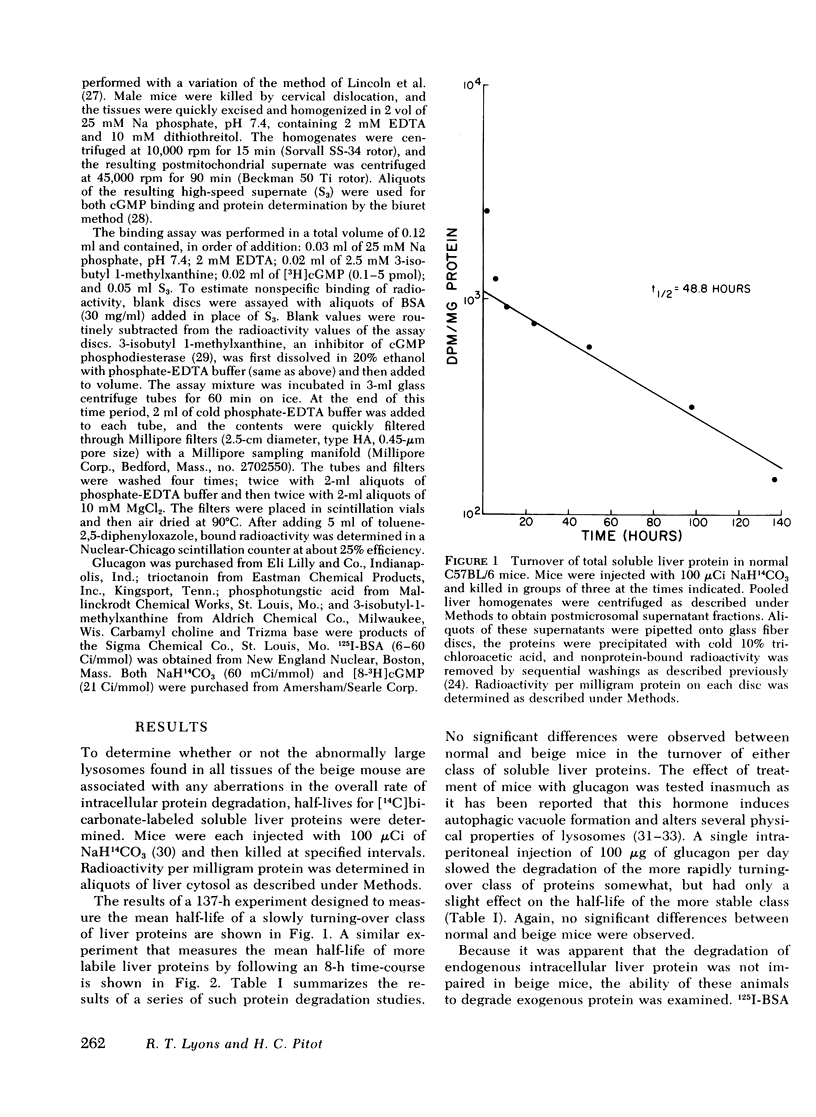
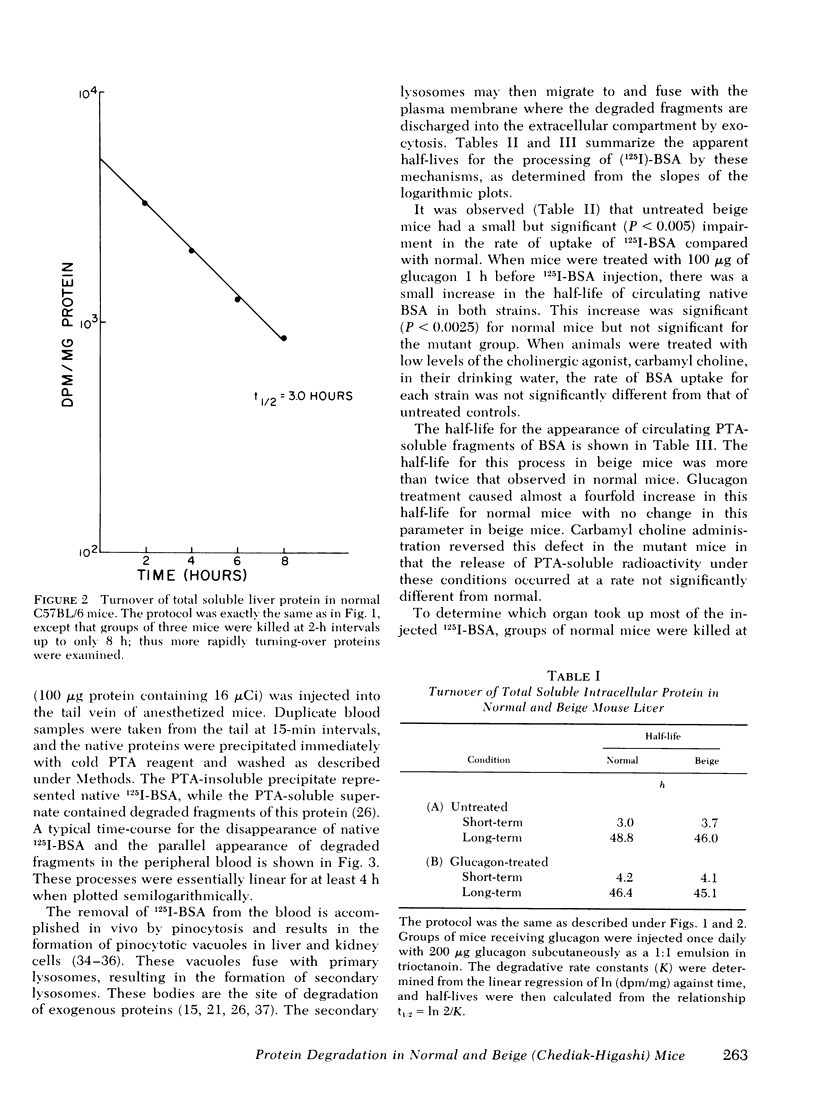
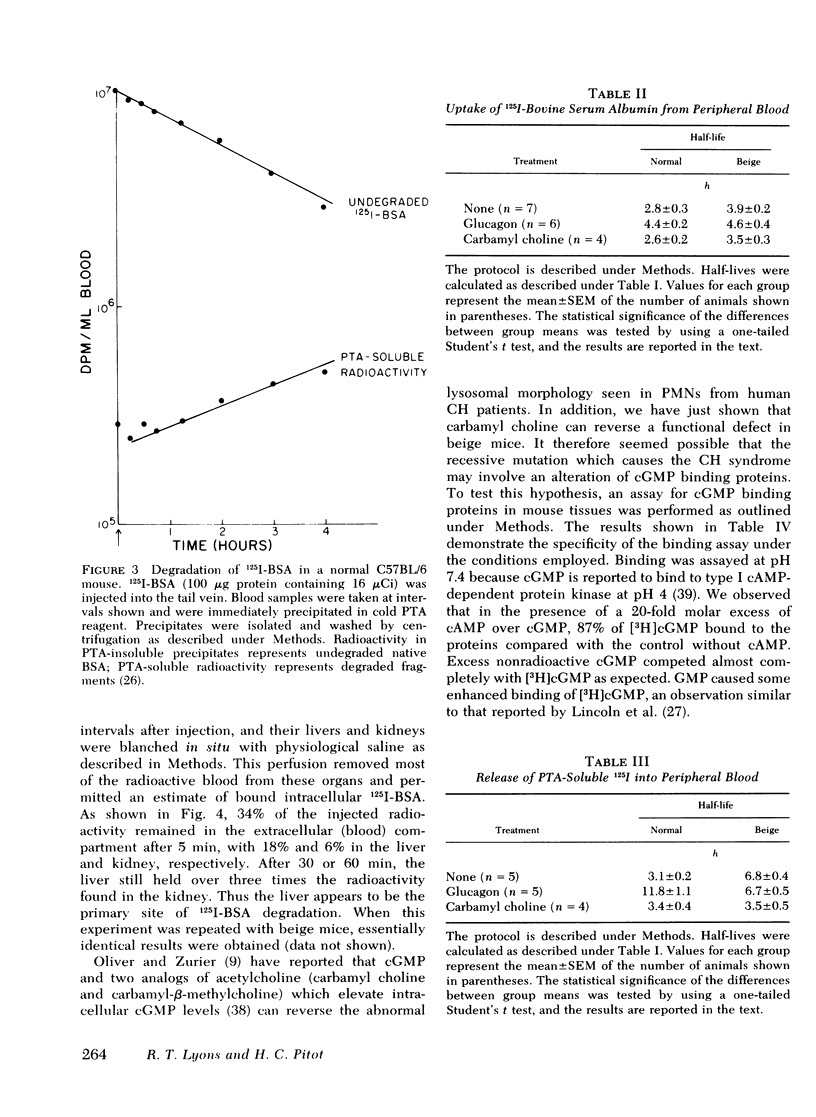
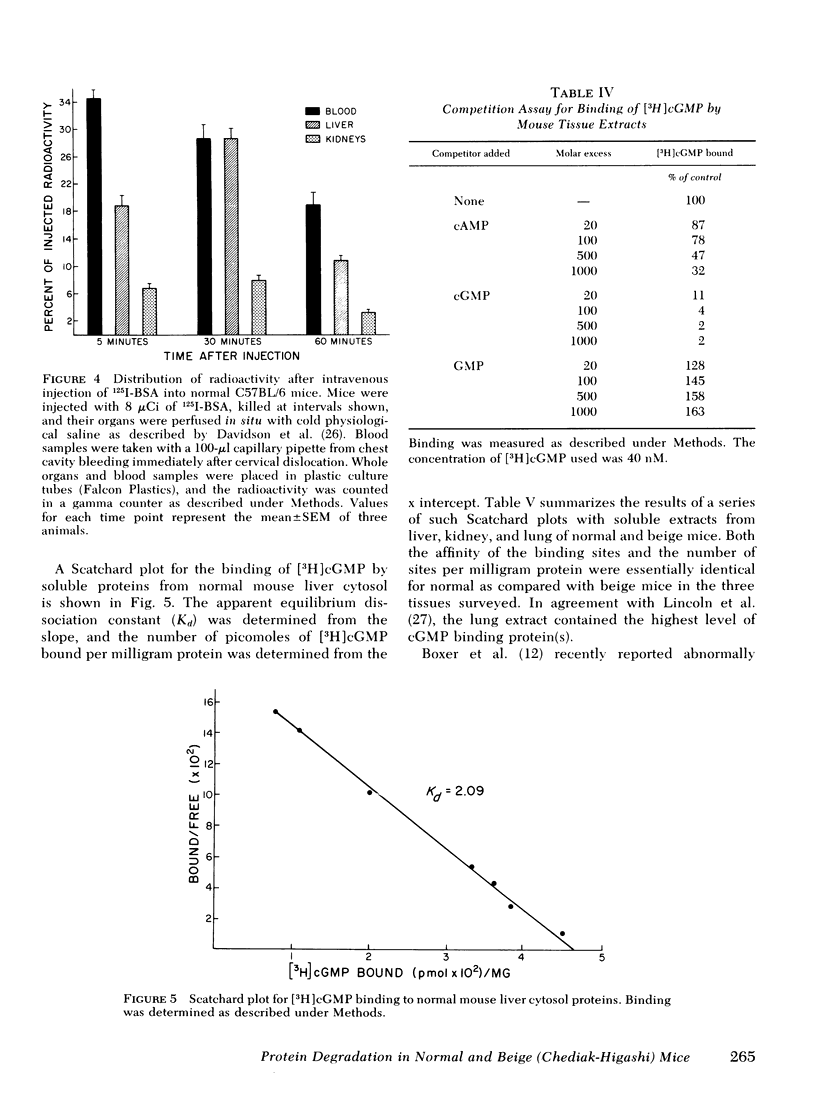


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Watanabe A. M., Rister M., Besch H. R., Jr, Allen J., Baehner R. L. Correction of leukocyte function in Chediak-Higashi syndrome by ascorbate. N Engl J Med. 1976 Nov 4;295(19):1041–1045. doi: 10.1056/NEJM197611042951904. [DOI] [PubMed] [Google Scholar]
- Brandt E. J., Elliott R. W., Swank R. T. Defective lysosomal enzyme secretion in kidneys of Chediak-Higashi (beige) mice. J Cell Biol. 1975 Dec;67(3):774–788. doi: 10.1083/jcb.67.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. W., De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968 Jun 25;243(12):3255–3263. [PubMed] [Google Scholar]
- Davidson S. J., Hughes W. L., Barnwell A. Renal protein absorption into sub-cellular particles. I. Studies with intact kidneys and fractionated homogenates. Exp Cell Res. 1971 Jul;67(1):171–187. doi: 10.1016/0014-4827(71)90633-1. [DOI] [PubMed] [Google Scholar]
- Davidson S. J. Protein absorption by renal cells. II. Very rapid lysosomal digestion of exogenous ribonuclease in vitro. J Cell Biol. 1973 Oct;59(1):213–222. doi: 10.1083/jcb.59.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S. J. Proteolytic activity within lysosomes and turnover of pinocytic vesicles. A kinetic analysis. Biochim Biophys Acta. 1975 Dec 5;411(2):282–290. [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L. Quantitative characterization of dense body, autophagic vacuole, and acid phosphatase-bearing particle populations during the early phases of glucagon-induced autophagy in rat liver. J Cell Biol. 1971 Mar;48(3):473–489. doi: 10.1083/jcb.48.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPasquale A. M., McGuire J., Moellmann G., Wasserman S. J. Microtubule assembly in cultivated Greene melanoma cells is stimulated by dibutyryl adenosine 3':5'-cyclic monophosphate or cholera toxin. J Cell Biol. 1976 Dec;71(3):735–748. doi: 10.1083/jcb.71.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Gabathuler M. P., Ryser H. J. The digestive function of lysosomes as studies by the turnover of ingested foreign macromolecules. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):95–98. doi: 10.1098/rspb.1969.0041. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W., Hepp K. D., Wieland O. The catabolic action of glucagon in rat liver. The influence of age, nutritional state and adrenal function on the effect of glucagon on lysosomal N-acetyl-beta, D-glucosaminidase. Biochim Biophys Acta. 1970 Dec 29;222(3):593–605. [PubMed] [Google Scholar]
- Haddox M. K., Nicol S. E., Goldberg N. D. pH induced increase in cyclic GMP reactivity with cyclic AMP-dependent protein kinases. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1444–1450. doi: 10.1016/0006-291x(73)91148-0. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Estensen R. D., Quie P. G., Hogan N. A., Goldberg N. D. Modulation of human neutrophil chemotactic responses by cyclic 3',5'-guanosine monophosphate and cyclic 3',5'-adenosine monophosphate. Metabolism. 1975 Mar;24(3):447–456. doi: 10.1016/0026-0495(75)90124-9. [DOI] [PubMed] [Google Scholar]
- Kirsch R. E., Frith L. O., Saunders S. J. Albumin catabolism in vitro by cultured peritoneal and pulmonary mononuclear phagocytes. Biochim Biophys Acta. 1972 Aug 18;279(1):87–91. doi: 10.1016/0304-4165(72)90243-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lincoln T. M., Hall C. L., Park C. R., Corbin J. D. Guanosine 3':5'-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2559–2563. doi: 10.1073/pnas.73.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. T., Pitot H. C. The regulation of ornithine aminotransferase synthesis by glucagon in the rat. Arch Biochem Biophys. 1976 May;174(1):262–272. doi: 10.1016/0003-9861(76)90345-3. [DOI] [PubMed] [Google Scholar]
- Oliver C., Essner E. Distribution of anomalous lysosomes in the beige mouse: a homologue of Chediak-Higashi syndrome. J Histochem Cytochem. 1973 Mar;21(3):218–228. doi: 10.1177/21.3.218. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M. Impaired microtubule function correctable by cyclic GMP and cholinergic agonists in the Chediak-Higashi syndrome. Am J Pathol. 1976 Nov;85(2):395–418. [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Krawiec J. A., Berlin R. D. Carbamycholine prevents giant granule-formation in cultured fibroblasts from beige (Chediak-Higashi) mice. J Cell Biol. 1976 Apr;69(1):205–210. doi: 10.1083/jcb.69.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Zurier R. B. Correction of characteristic abnormalities of microtubule function and granule morphology in Chediak-Higashi syndrome with cholinergic agonists. J Clin Invest. 1976 May;57(5):1239–1247. doi: 10.1172/JCI108392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett G. A. Neutrophilic function in animals with the Chediak-Higashi syndrome. Blood. 1967 Jun;29(6):906–915. [PubMed] [Google Scholar]
- Padgett G. A., Reiquam C. W., Gorham J. R., Henson J. B., O'Mary C. C. Comparative studies of the Chediak-Higashi syndrome. Am J Pathol. 1967 Oct;51(4):553–571. [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Davis W. C., Padgett G. A. Defective function of renal lysosomes in mice with the Chediak-Higashi syndrome. Am J Pathol. 1972 May;67(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS W. CYTOCHEMICAL OBSERVATIONS ON THE RELATIONSHIP BETWEEN LYSOSOMES AND PHAGOSOMES IN KIDNEY AND LIVER BY COMBINED STAINING FOR ACID PHOSPHATASE AND INTRAVENOUSLY INJECTED HORSERADISH PEROXIDASE. J Cell Biol. 1964 Mar;20:497–507. doi: 10.1083/jcb.20.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Baird C. E., Sutherland E. W. The importance of calcium ions for the regulation of guanosine 3':5'-cyclic monophosphage levels. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3889–3893. doi: 10.1073/pnas.70.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Possible role of microtubules in thyroid secretion. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1901–1908. doi: 10.1073/pnas.67.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. M. The Chediak-Higashi syndrome: studies of host defenses. Ann Intern Med. 1972 Feb;76(2):293–306. doi: 10.7326/0003-4819-76-2-293. [DOI] [PubMed] [Google Scholar]


