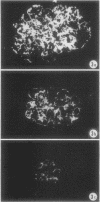
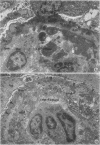
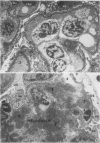
Abstract
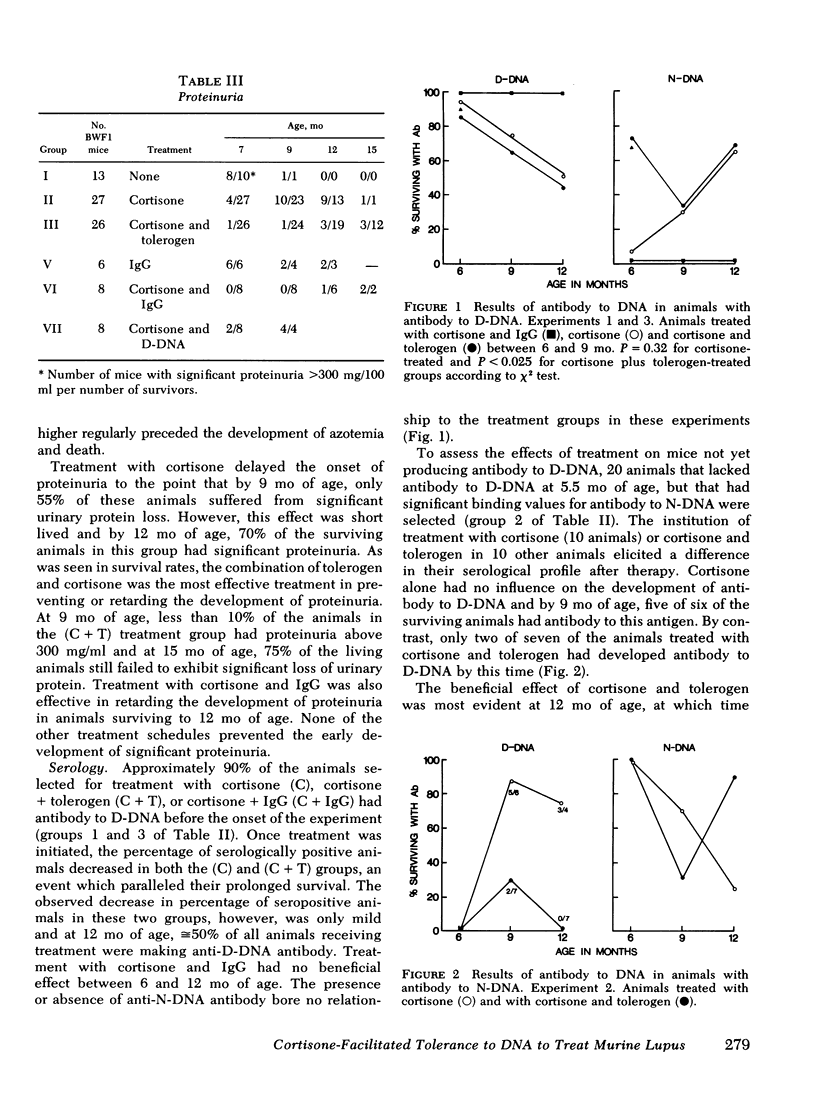
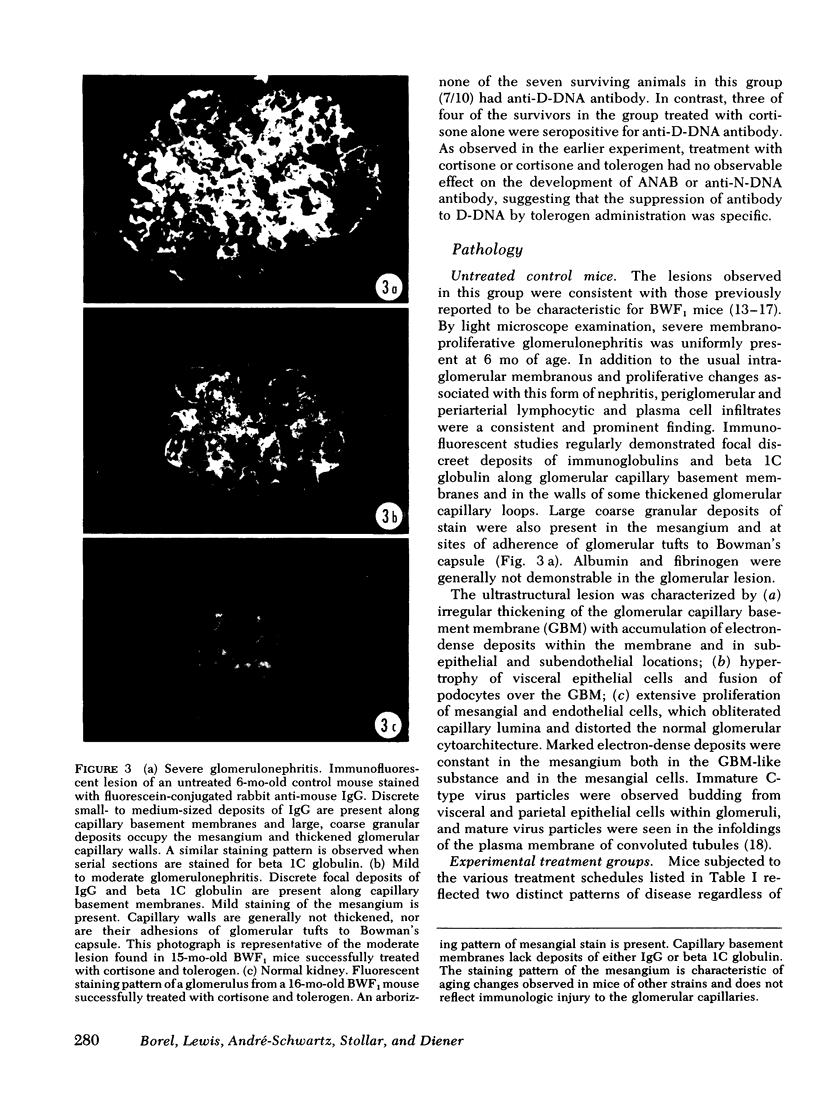
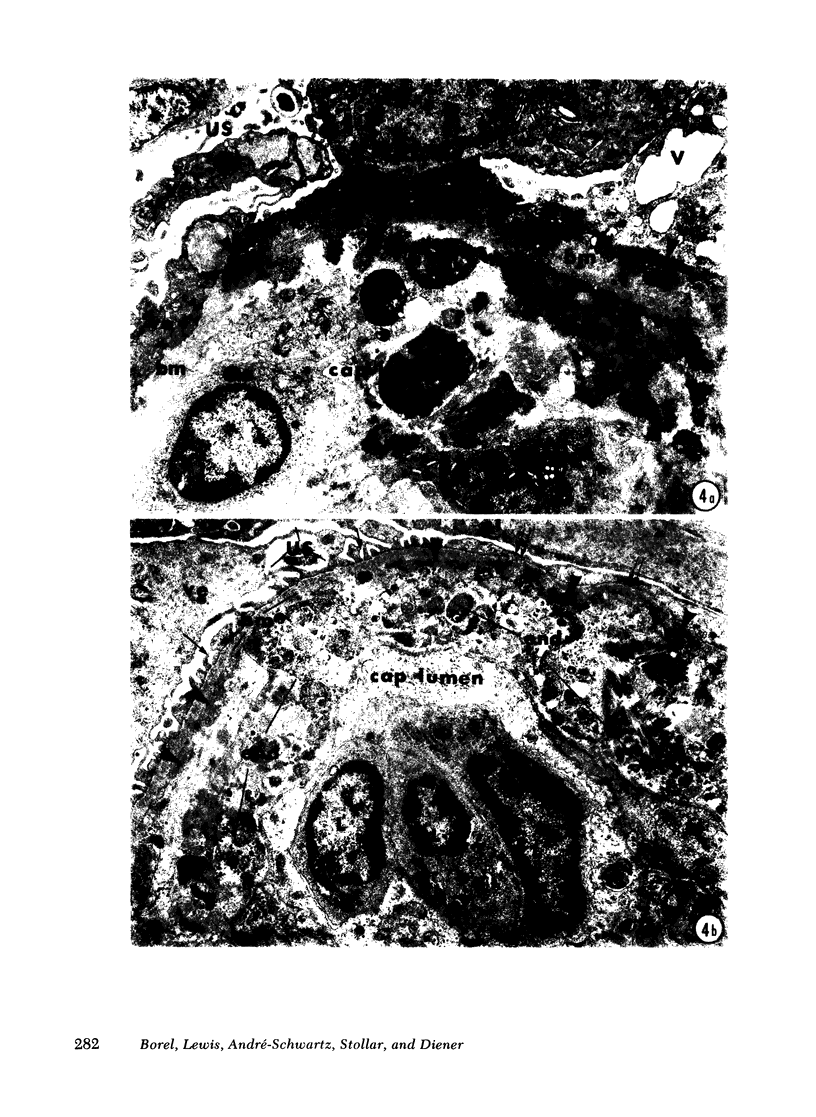
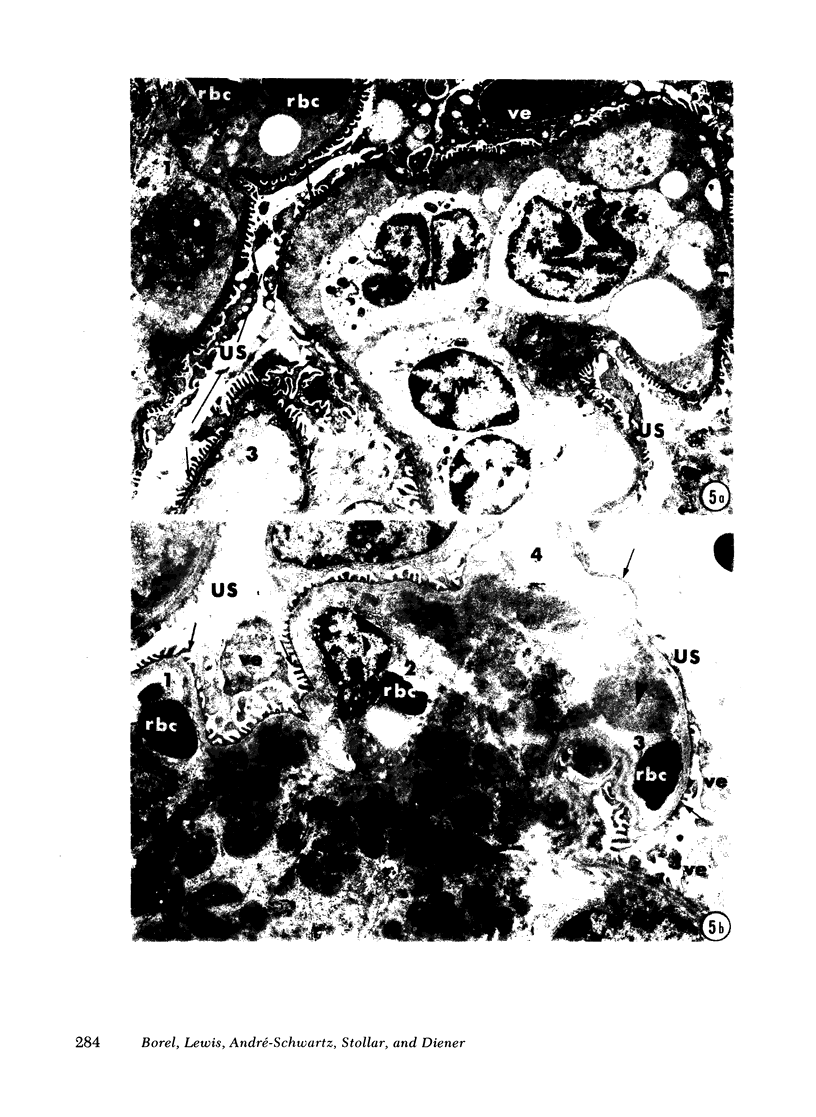
Adult female (NZB + NZW)F1 mice were treated with cortisone, cortisone with tolerogen (isologous NZB IgG-nucleosides conjugates) or cortisone with isologous IgG free of nucleosides. Other treatments also included tolerogen or isologous IgG alone, and cortisone together with denatured DNA. All untreated mice died by 10 mo of age. Cortisone prolonged the survival rate. This effect was further improved by combined treatment of cortisone and tolerogen. Prolonged survival was accompanied by a decrease in proteinuria. Other treatments failed to influence either survival or proteinuria. Although cortisone did not prevent the appearance of antibody to denatured DNA, cortisone and tolerogen suppressed them in most of the animals. Preexisting antibody to denatured DNA was reduced by cortisone and cortisone and tolerogen, but not by cortisone and IgG. In contrast, antibody to native DNA bore no relationship to therapy. Animals living beyond 1 yr of age, regardless of the treatment, fall into three histopathological categories: (a) severe nephritis, as in untreated animals, (b) moderate nephritis (with absence of severe alteration of the glomerular basement membrane, i.e. the histological counterpart of prolonged survival), (c) minimal nephritis. In a small number of animals treated with cortisone or cortisone and IgG and in 6/20 animals treated with cortisone and tolerogen, minimal lesions as judged by light, fluorescent, and electron microscopy were found. These last mice were in good health at 15-16 mo of age, twice the life-span of untreated mice. In conclusion, these data suggest that tolerance to nucleic acid antigens facilitated by cortisone offers a promising new approach to treat established murine lupus nephritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for a small pool of immunocompetent cells in the mouse thymus. Its role in the humoral antibody response against sheep erythrocytes, bovine serum albumin, ovalbumin and the NIP determinant. Cell Immunol. 1970 Oct;1(4):362–371. doi: 10.1016/0008-8749(70)90014-6. [DOI] [PubMed] [Google Scholar]
- Borel Y. Isologous IgG-induced immunologic tolerance to haptens: a model of self versus non-self recognition. Transplant Rev. 1976;31:3–22. doi: 10.1111/j.1600-065x.1976.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Borel Y., Lewis R. M., Stollar B. D. Prevention of murine lupus nephritis by carrier-dependent induction of immunologic tolerance to denatured DNA. Science. 1973 Oct 5;182(4107):76–78. doi: 10.1126/science.182.4107.76. [DOI] [PubMed] [Google Scholar]
- Casey T. P. Systemic lupus erythematosus in NZB x NZW hybrid mice treated with the corticosteroid drug betamethasone. J Lab Clin Med. 1968 Mar;71(3):390–399. [PubMed] [Google Scholar]
- Channing A. A., Kasuga T., Horowitz R. E., Dubois E. L., Demopoulos H. B. An ultrastructural study of spontaneous lupus nephritis in the NZB-BL-NZW mouse. Am J Pathol. 1965 Oct;47(4):677–694. [PMC free article] [PubMed] [Google Scholar]
- Dixon F. J., Oldstone M. B., Tonietti G. Pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1971 Sep 1;134(3 Pt 2):65s–71s. [PubMed] [Google Scholar]
- Germuth F. G., Jr, Valdes A. J., Senterfit L. B., Pollack A. D. A unique influence of cortisone on the transit of specific macromolecules across vascular walls in immune complex disease. Johns Hopkins Med J. 1968 Mar;122(3):137–153. [PubMed] [Google Scholar]
- Golan D. T., Borel Y. Nonantigenicity and immunologic tolerance: the role of the carrier in the induction of tolerance to the hapten. J Exp Med. 1971 Oct 1;134(4):1046–1061. doi: 10.1084/jem.134.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. D., Burnet F. M. Renal lesions in the "auto-immune" mouse strains NZB and F1 NZBxNZW. J Pathol Bacteriol. 1966 Apr;91(2):467–476. doi: 10.1002/path.1700910221. [DOI] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Imamura M., Mellors R. C., Strand M., August J. T. Murine Type C viral envelope glycoprotein gp69/71 and lupus-like glomerulonephritis of New Zealand mice. An immunoperoxidase study. Am J Pathol. 1977 Feb;86(2):375–386. [PMC free article] [PubMed] [Google Scholar]
- Koffler D. Immunopathogenesis of systemic lupus erythematosus. Annu Rev Med. 1974;25:149–164. doi: 10.1146/annurev.me.25.020174.001053. [DOI] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Langman R. E., Paetkau V. H., Diener E. The cellular basis of cortisone-induced immunosuppression of the antibody response studied by its reversal in vitro. Cell Immunol. 1975 Jun;17(2):405–417. doi: 10.1016/s0008-8749(75)80044-x. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Armstrong M. Y., André-Schwartz J., Muftuoglu A., Beldotti L., Schwartz R. S. Chronic allogeneic disease. I. Development of glomerulonephritis. J Exp Med. 1968 Oct 1;128(4):653–679. doi: 10.1084/jem.128.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. M., Stollar B. D., Goldberg E. B. A rapid, sensitive test for the detection of antibodies to DNA. J Immunol Methods. 1973 Dec;3(4):365–374. doi: 10.1016/0022-1759(73)90038-0. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Tannenberg W., Smith C., Schwartz R. S. C-type viruses in systemic lupus erythematosus. Nature. 1974 Nov 1;252(5478):78–79. doi: 10.1038/252078a0. [DOI] [PubMed] [Google Scholar]
- McGiven A. R., Lynraven G. S. Glomerular lesions in NZB/NZW mice. Electron microscopic study of development. Arch Pathol. 1968 Mar;85(3):250–261. [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Shirai T., Aoki T., Huebner R. J., Krawczynski K. Wild-type Gross leukemia virus and the pathogenesis of the glomerulonephritis of New Zealand mice. J Exp Med. 1971 Jan 1;133(1):113–132. doi: 10.1084/jem.133.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D., Borel Y. Nucleoside specificity in the carrier IgG-dependent induction of tolerance. J Immunol. 1976 Oct;117(4):1308–1313. [PubMed] [Google Scholar]
- Stollar B. D., Borel Y. Nucleoside-specific tolerance suppresses anti-nucleoside antibody forming cells. Nature. 1977 May 12;267(5607):158–160. doi: 10.1038/267158a0. [DOI] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D. The pathogenesis of autoimmunity in New Zealand black mice. Curr Top Microbiol Immunol. 1974;64(0):79–103. doi: 10.1007/978-3-642-65848-8_3. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]