Abstract
AIM: To assess the protective effect of berberine administration and the role of nitric oxide (NO) in visceral hypersensitivity.
METHODS: Fifty male Sprague-Dawley rats were randomly assigned to five groups. An inflammatory bowel disease model was induced in rats by intracolonic instillation of 1 mL 4% acetic acid at 8 cm proximal to the anus for 30 s and restraint stress. After subsidence of inflammation on day 7 of the experiment, the rats were subjected to rectal distension, performed by a balloon (6-Fr, 2 mm external diameter, disposable silicon balloon-urethral catheter for pediatric use) which was rapidly inflated with increasing volumes of prewarmed (37 °C) water (0.1, 0.2, 0.3, 0.4, 0.6, 0.8 and 1 mL) for 30 s at four-minute intervals, and then the abdominal withdrawal reflex (AWR) and the level of fecal output were measured, respectively. AWR scores either 0, 1, 2, 3 or 4 were obtained by blinded observers. Rats had been pretreated with berberine or aminoguanidine (NO synthetase inhibitor) or berberine + aminoguanidine before measurement.
RESULTS: The rats in the placebo group showed a hypersensitive response to rectal distension (2.69 ± 0.08 vs 1.52 ± 0.08, P = 0.000) and defecated more frequently than those in the control group (5.0 ± 0.16 vs 0.44 ± 0.16, P = 0.000). Comparing the berberine with placebo group, the AWR scores were reduced for all distension volumes and were significant at 0.2-1 mL (1.90 ± 0.08 vs 2.69 ± 0.08, P = 0.000), while the numbers of hard pellets, soft pellets, formless stools, and total fecal output in the placebo group were significantly larger than in the berberine group (5.0 ± 0.16 vs 2.56 ± 0.16, P = 0.000). Administration of aminoguanidine or berberine + aminoguanidine before VH score measurement reversed the antinociceptive effect of berberine (2.52 ± 0.08 vs 1.90 ± 0.08, P = 0.000; 2.50 ± 0.08 vs 1.90 ± 0.08, P = 0.000). The numbers of hard pellets, soft pellets, formless stool, and total of fecal output in aminoguanidine group were significantly larger than the corresponding values in control group, berberine group, and berberine + aminoguanidine group (4.81 ± 0.16 vs 0.44 ± 0.16, P = 0.000; 4.81 ± 0.16 vs 2.56 ± 0.16, P = 0.000; 4.81 ± 0.16 vs 3.75 ± 0.16, P = 0.000). The berberine and berberine + aminoguanidine groups showed reduced defecation, but aminoguanidine alone did not reduce defecation (2.56 ± 0.16 vs 4.81 ± 0.16, P = 0.000; 3.75 ± 0.16 vs 4.81 ± 0.16, P = 0.000).
CONCLUSION: Berberine had an antinociceptive effect on visceral hypersensitivity, and NO might play a role in this effect.
Keywords: Berberine, Irritable bowel syndrome, Visceral hypersensitivity, Nitric oxide
Core tip: Berberine had an antinociceptive effect on visceral hypersensitivity. This effect was reduced by nitric oxide (NO) synthetase inhibitor, thus NO might play a role in the effect of berberine.
INTRODUCTION
It is believed that chronic visceral hypersensitivity (VH), abnormal gastrointestinal motility and altered central processing may be major pathophysiological mechanisms of irritable bowel syndrome (IBS)[1]. Gut hypersensitivity may lead to alterations in gut motility by disturbing regulatory reflex pathways and secretory function[2]. These abnormalities typically reflect the symptom pattern of IBS, which is characterized by abdominal pain or discomfort, and is associated with alterations in defecation frequency, stool passage, and stool form[3,4].
According to the recent Rome III Criteria[5], IBS can be diagnosed based on at least 3 mo, with onset at least 6 mo, of recurrent abdominal pain or discomfort associated with two or more of the followings: (1) improvement with defecation; (2) onset associated with a change in stool frequency; and (3) onset associated with a change in stool form (appearance). Additionally, IBS patients are further subdivided into IBS with diarrhea, IBS with constipation, mixed IBS with diarrhea and unspecified IBS.
VH is a consistent finding in a large proportion of patients with IBS and provides a physiological basis for the development of IBS symptoms[2,6]. However, the exact mechanism of its action is still unclear. Recently, it has been documented that increasing the nitric oxide (NO) level in the extracellular space of the target tissue is one of the major mechanisms involved and this has been clarified in recent molecular studies[7]. There is growing evidence from previous studies that NO plays an important role in pain transmission and the antinociceptive action on VH or peritoneal pain[2,6,8-10].
It has been discovered that some drugs can be used to attenuate VH in IBS patients[11]. However, many drug treatments have not been satisfactory, with intractable adverse effects. Therefore, it is necessary to seek an effective and low-cost treatment for IBS. Berberine (Coptis chinensis Franch, var. asperma Don, family Ranunculaceae) is a botanical alkaloid isolated from the root and bark of Rhizoma coptidis, an ancient Chinese herb that has been used to treat gastroenteritis for many years, which is preferred for its inexpensiveness and low incidence of adverse effects[12]. It has been demonstrated that berberine has multiple pharmacological activities including anti-inflammatory[13], antimicrobial[14], anticancer[15,16], antidiabetic[17], antiarrhythmic[18], and antiseptic[19] effects. According to former studies, berberine has a significant effect in the treatment of experimental colitis[20-22]. Further evidence has shown that, in relation to the NO pathway, berberine has a significant effect on ethanol-induced gastric ulcers[23], endothelial progenitor cell mobilization and function[24], hyperglycemia-induced cellular injury and endothelial dysfunction[25], the early phase of hepatocarcinogenesis[26], and a rat model of Alzheimer’s disease[27]. To establish whether berberine has a beneficial effect in IBS patients through reversal of VH, we examined the effects of berberine in a validated rodent model in which acute inflammation of the colon was associated with VH. The aim of this study was to evaluate whether berberine treatment prevents progression of VH to colorectal distension (CRD), and the involvement of NO in these effects.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats, weighing 270-300 g, were obtained from the Animal Facility of Southeast Hospital, Zhangzhou, China. The rats were housed individually in an access-restricted room with controlled conditions (22 ± 1 °C and 65%-70% humidity) with free access to standard laboratory food and water. All the experimental protocols in this study were reviewed and approved by the Animal Studies Ethics Committee of Southeast Hospital.
Experiment model
The rats were lightly anesthetized with ether after an overnight fast and colitis was induced by intracolonic instillation of 1 mL 4% acetic acid at 8 cm proximal to the anus for 30 s. Then, 1 mL PBS was instilled to dilute the acetic acid and flush the colon. The control animals were handled identically except that 1 mL saline was instilled instead of 4% acetic acid. Rats were left to recover from colitis for 6 d, and were used for the experiments 7 d after induction of colitis.
Histological examination of inflammation
To examine the extent of colonic inflammation, histological samples were collected at the selected time points (2 and 7 d post-enema in two rats of each group). Sections with a thickness of 5 μm were cut and processed for hematoxylin and eosin staining. The coded slides were analyzed by a pathologist blinded with regard to the treatment group and the time points.
Rectal distension procedure
At 7 d post-enema, eight rats in each group were used for studying progression of VH to CRD. A 6-Fr (2 mm external diameter) disposable silicon balloon-urethral catheter for pediatric use was used. The maximal inflation volume for the balloon was 1 mL and the length of the maximally inflated balloon was 1.2 cm. After an overnight fast, the animals were lightly anesthetized with ether, and the balloon was carefully inserted into the rectum until the premarked line on the catheter (2 cm distal from the end of the balloon) was positioned at the anus. The catheter was taped to the base of the tail to prevent displacement. After this procedure, the rats were placed in a transparent cubicle (20 cm × 8 cm × 8 cm) on a mirror-based, elevated platform while still sedated, and were allowed to recover and adjust for a minimum of 30 min before testing. The catheter was connected to a pressure transducer via a three-way connector. The signals from pressure transducer were processed and recorded on an IBM-compatible computer.
After the animals were fully awake and adjusted to the environment, ascending-limit phasic distension (0.1, 0.2, 0.3, 0.4, 0.6, 0.8 and 1.0 mL) was applied for 30 s every 4 min to induce CRD. The balloon was distended with prewarmed (37 °C) water. We chose this protocol because hypersensitivity was reported to be best elicited by rapid phasic distension. The abdominal withdrawal reflex (AWR) was semiquantitatively scored as previously described[4]. The AWR score was assigned as follows: 0 = no behavioral response to distension; 1 = brief head movements followed by immobility; 2 = contraction of abdominal muscle without lifting of the abdomen; 3 = lifting of the abdomen; and 4 = body arching and lifting of pelvic structure.
After the experiments, the balloon was withdrawn and immersed in 37 °C water. The compliance of balloon was not infinite, therefore, we measured intraballoon pressure at each distension volume in 37 °C water, and digitally subtracted the value from that recorded during the CRD experiment to calculate the intrarectal pressure.
Restraint stress procedure
The rats were housed individually with no restrictions on food intake before testing. At 7 d post-enema, eight rats from each group were placed in restraint cages (5 cm × 5 cm × 20 cm), which could limit their body movement, but not restrict breathing. The rats were in the restraint cages for 3 h at room temperature. The feces excreted during restraint stress were divided into three types: hard pellet, soft pellet, and formless, and counted separately.
Experimental protocol
Ten healthy rats without treatment served as controls. In the placebo group, IBS was induced as described above and eight rats were treated once with physiological saline 1 d after enema. In the berberine group, IBS was induced as described above and eight rats were treated once daily with berberine (50 mg/kg) 1 d after enema. In the aminoguanidine group, eight rats were treated once daily with aminoguanidine (100 mg/kg) via intraperitoneal injection 1 d after enema. In the berberine + aminoguanidine group, eight rats were treated once daily with berberine (50 mg/kg) 1 d post-enema, and then were treated once daily with aminoguanidine (100 mg/kg) via intraperitoneal injection.
Statistical analysis
Data were expressed as mean ± SD. Significant differences between the three groups (AWR score) at each distension volume were statistically analyzed using ANOVA. The relationship between the intraballoon volume and intrarectal pressure was determined by linear regression analysis, and the estimated slope coefficients and intercepts were compared between groups using ANOVA. The level of fecal output was compared using ANOVA and further analyzed using Bonferroni’s or Tamhane’s T2 test. Differences with P < 0.05 were considered to be significant. Multiple comparisons between the groups were corrected by SPSS version 13.0 software.
RESULTS
Histology of colonic tissue
Figure 1 shows the histology of the distal colon at days 2 and 7 after acetic acid instillation in the control group, placebo group, berberine group, aminoguanidine group, and berberine + aminoguanidine group. Mucosal hemorrhage with an inflammatory infiltrate in the lamina propria and the edematous submucosa were observed in the IBS model group, berberine group, aminoguanidine group and berberine + aminoguanidine group 2 d after acetic acid instillation. At day 7 after induction of colitis, all signs of inflammation had disappeared. No remarkable inflammatory features were detected at 7 d after acetic acid instillation in each group.
Figure 1.
Photomicrographs (hematoxylin and eosin stain, × 100) of distal colon at 2 and 7 d, respectively. A, B: Control group; C, D: Placebo group; E, F: Berberine group; G, H: Aminoguanidine group; I, J: Berberine + aminoguanidine group. At 2 d, histological inflammatory features including mucosal hemorrhage, submucosal edema, and inflammatory infiltration in the lamina propria and the submucosa were observed in the placebo, berberine, aminoguanidine, and berberine + aminoguanidine groups (A, C, E, G, I). At 7 d, there was no marked inflammatory feature compared with the control group (B, D, F, H, J).
Detection of VH
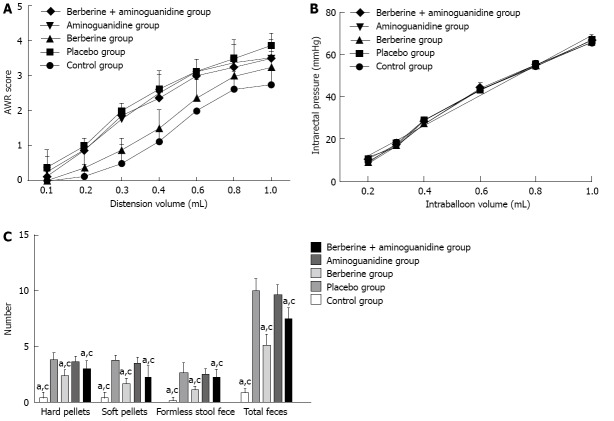
The AWR scores were recorded in at least eight rats in each group after CRD was induced. The changes in AWR score paralleled the balloon volume in CRD, which confirmed that the AWR score reflected the intensity of distension. Comparing the berberine with placebo group, the AWR scores were reduced for all distension volumes and were significant at 0.2-1.0 mL (1.90 ± 0.08 vs 2.69 ± 0.08, P = 0.000). These data indicated that progression of VH to CRD was attenuated by berberine (Figure 2A). As shown in Figure 2A, rats in the placebo group showed a hypersensitive response to the ascending-limit phasic rectal distension, and berberine effectively reduced VH (2.69 ± 0.08 vs 1.90 ± 0.08, P = 0.000). However, VH was not effectively reduced in the aminoguanidine and berberine + aminoguanidine groups. The pain threshold (minimal volume to induce AWR 2) was measured in rats that underwent CRD. As shown in Figure 2, berberine significantly increased the nociceptive threshold in rats. Administration of aminoguanidine or berberine + aminoguanidine before VH score measurement reversed the antinociceptive effect of berberine (2.52 ± 0.08 vs 1.90 ± 0.08, P = 0.000; 2.50 ± 0.08 vs 1.90 ± 0.08, P = 0.000). In order to examine whether VH in rats was related to changes in rectal compliance, we compared the intraballoon volume-intrarectal pressure relationship in the five groups. A distension volume of 0.2-1.0 mL and the corresponding intrarectal pressure were plotted for regression analysis. Intrarectal pressure increased linearly as the balloon was inflated (r = 0.9758, P < 0.001, in the control group; r = 0.9842, P < 0.001, in the placebo group; r = 0.9822, P < 0.001, in the berberine group; r = 0.9773, P < 0.001 in the aminoguanidine group; and r = 0.9827, P < 0.001 in the berberine + aminoguanidine group). The fitted functions of the five groups were not significantly different (Figure 2B).
Figure 2.
Summarized plots. A: Colorectal distension-induced abdominal withdrawal reflex (AWR) in each group. Comparing the berberine and placebo groups, the AWR scores were reduced at all distension volumes and were significant at 0.2-1.0 mL. The AWR score in the berberine group was significantly lower than in the aminoguanidine and berberine + aminoguanidine groups; B: The relationship between intraballoon volume and intrarectal pressure in each group. The distension volume from 0.2 to 1.0 mL and the corresponding intrarectal pressure were plotted for regression analysis. Intrarectal pressure was linearly increased as the balloon was inflated. The fitted functions of the five groups were not significantly different; C: Restraint-stress-induced defecation in each group. Defecation in the placebo and aminoguanidine groups was significantly more frequent than in the control, berberine and berberine + aminoguanidine groups (aP < 0.05 vs placebo group; cP < 0.05 vs aminoguanidine group). The berberine and berberine + aminoguanidine groups showed reduced defecation, but aminoguanidine group alone did not effectively reduce defecation.
Restraint-stress-induced defecation
As demonstrated in Figure 2C, the numbers of hard pellets, soft pellets, formless stools, and total fecal output in the placebo were significantly larger than in the berberine groups (5.0 ± 0.16 vs 2.56 ± 0.16, P = 0.000). As shown in Figure 2C, the number of hard pellets, soft pellets, formless stool,and total of fecal output in aminoguanidine group were significantly larger than the corresponding values in control group, berberine group, and berberine + aminoguanidine group (4.81 ± 0.16 vs 0.44 ± 0.16, P = 0.000; 4.81 ± 0.16 vs 2.56 ± 0.16, P = 0.000; 4.81 ± 0.16 vs 3.75 ± 0.16, P = 0.000). The berberine and berberine + aminoguanidine groups showed reduced defecation, but aminoguanidine alone did not reduce defecation (2.56 ± 0.16 vs 4.81 ± 0.16, P = 0.000; 3.75 ± 0.16 vs 4.81 ± 0.16, P = 0.000).
DISCUSSION
This study was performed in order to clarify the effects of berberine administration on VH in a rat model of IBS. Berberine effectively attenuated the heightened visceral nociceptive response, that is, an increase in AWR score to CRD, in rats recovering from experimental colitis. In the placebo group, 7 d after instillation of acetic acid when there was no sign of inflammation in the colon, these rats still had VH and a high frequency of defecation in response to restraint stress. Stool form in the placebo group was softer and more shapeless than in the control group. These findings are in accordance with the clinical symptoms in IBS patients, but there is a great dilemma in using this model to investigate the effect of drugs on IBS.
To avoid the ambiguity that any sign of improvement can be interpreted as a drug effect on colitis, histopathological parameters of inflammation in each group, 2 and 7 d after acetic acid instillation, were evaluated and there was no difference. Therefore, we concluded that berberine administration, at least at the dose used in our study, had neither a positive nor negative effect on the histopathological parameters of inflammation in the colon, and did not impair establishment of the postinflammatory model. Considering all these factors, we elucidated the effects of berberine administration at a dose that showed a beneficial effect on the inflammatory response. This indicated that berberine significantly reduced VH and stool frequency and increased stool consistency. We investigated the role of NO in the protective effects of berberine using an experimental model with the NO synthetase (NOS) inhibitor aminoguanidine. We demonstrated that aminoguanidine significantly reduced the effect of berberine on VH, which suggests this effect of berberine is at least partly mediated through NOS.
Berberine has been used in the treatment of gastroenteritis and infectious diarrhea in Chinese traditional medicine for thousands of years. Some recent studies have indicated that it has various pharmacological effects, including anti-inflammatory[20] and antimicrobial[28] effects; each of which may contribute to the antidiarrheal effect. The fact that berberine has low bioavailability and shows poor absorption through the colon wall (< 5%)[29] support the thesis that it may exert its antidiarrheal effect on the intestinal epithelial cells before absorption. However, until now the effect of berberine on HV has not been confirmed.
NO is a key neurotransmitter in both short- and long-acting inhibitory motor neurons[7] and plays a critical part in mediating gastrointestinal motility. Some studies have revealed that NOS neuronal activity considerably changes after inflammation and is responsible for some acute postinflammatory consequences in bowel-like ileus[30,31]. Apart from its major role in the peripheral nervous system, such as in the enteric inhibitory nerves of the myenteric plexus, NO is believed to be an intracellular messenger or neurotransmitter in the central nervous system (CNS). It has been verified that due to its free diffusibility, NO acts as a retrograde transmitter in the CNS, mediating some nervous paradigms, for instance, long-term potentiation, and is the key neurotransmitter in descending inhibitory neurons modulating nociception at the spinal level[32]. Furthermore, it proves that NO is involved in the modulation of visceral perception, for example, intraperitoneal injection of acetic acid in rats increases nitrergic neurons in specific regions of the brain, and NOS immunoreactivity has been confirmed in lumbosacral afferents and preganglionic neurons innervating the pelvic viscera[33]. Therefore, some hypotheses can be performed based on our results, which interpret the protective effects of berberine through NO on VH in IBS at the myenteric plexus level, CNS, and smooth muscles. Our results could be interpreted at the myenteric plexus level.
Our findings were similar to a recent study that identified no significant difference in NO-containing neurons of the colonic myenteric plexus between IBS rats and controls[34]. Thus, we hypothesize that basal NO synthesis is not significantly decreased in IBS, and it might be that the positive effects of berberine decrease VH through increasing NO levels. Therefore, we speculate that the positive effects of berberine on VH in the postinflammatory rat model are at least partly exerted through NO synthesis potentiation.
We also examined the effect of aminoguanidine (NOS inhibitor) on stool form (hard pellets, soft pellets, and shapeless) in IBS rats under restraint stress. Aminoguanidine had no noticeable effect on stool form, but it did diminish the protective effects of berberine on stool form. NO plays a major role in mediating gastrointestinal motility, which is critical for stool formation. As a result of the short time between acute drug administration and defecation measurement, aminoguanidine did not produce any change in the stool consistency pattern of rats. The effects of aminoguanidine on stool frequency were more sophisticated and hard to explain. The protective effects of berberine on stool frequency were diminished by aminoguanidine, but the specific mechanism was not established. Further study will be needed to explore the specific mechanism. However, it should be noted that this study investigated only the antinociceptive effect of berberine on VH. One of the limitations of this study is the lack of measurement of NOS inhibitors.
In conclusion, we indicated that berberine administration prevented progression of VH to CRD. It is possible that NO released by berberine may affect colonic hypersensitivity. All of our data suggest that berberine may be of interest in the treatment of visceral hyperalgesia, particularly in IBS.
COMMENTS
Background
It is believed that chronic visceral hypersensitivity (VH), abnormal gastrointestinal motility, and altered central processing may be major pathophysiological mechanisms of irritable bowel syndrome (IBS). Berberine is an ancient Chinese herb that has been used to treat gastroenteritis for many years, which is preferred for its low cost and low incidence of adverse effects. Recently, berberine has been shown to have a considerable effect in the treatment of experimental colitis. However, the mechanism remains unknown.
Research frontiers
Berberine was used to treat rats with VH induced by 4% acetic acid. Berberine administration significantly increased the nociceptive threshold in rats, whereas the administration of aminoguanidine or berberine + aminoguanidine before VH measurement reversed the antinociceptive effect of berberine. The mechanism underlying the effect of berberine on VH of rats appears to be partly mediated by nitric oxide (NO).
Innovations and breakthroughs
Recently, it has been demonstrated that berberine has multiple pharmacological activities. In this study, authors indicated that berberine may be of interest in the treatment of VH, particularly in IBS.
Applications
The present study demonstrated that berberine administration prevented progression of VH to colorectal distension in a rat model of IBS. The effect of berberine is mediated by NO pathways, thus providing evidence for the treatment of VH in IBS.
Terminology
IBS is a common gastrointestinal disorder characterized by chronic visceral pain and bloating in association with altered gut movements. Berberine is an ancient Chinese herb that might play a role in the treatment of IBS.
Peer review
This paper describes positive effects of berberine on VH in rats with IBS. It is reported that berberine administration significantly increased the nociceptive threshold in rats, whereas administration of aminoguanidine or berberine + aminoguanidine before VH measurement reversed the antinociceptive effect of berberine. The results presented are crucial for clinicians and for the fundamental scientific community as well.
Footnotes
P- Reviewers Kanazawa M, Masclee AAM S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Kuiken SD, Klooker TK, Tytgat GN, Lei A, Boeckxstaens GE. Possible role of nitric oxide in visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:115–122. doi: 10.1111/j.1365-2982.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Heading RC, Thompson WG. Clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407–1430. doi: 10.1046/j.1365-2036.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- 4.La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol. 2005;11:237–241. doi: 10.3748/wjg.v11.i2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51 Suppl 1:i67–i71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho SV, Gebhart GF. A role for spinal nitric oxide in mediating visceral hyperalgesia in the rat. Gastroenterology. 1999;116:1399–1408. doi: 10.1016/s0016-5085(99)70504-4. [DOI] [PubMed] [Google Scholar]
- 9.Shamshiri H, Paragomi P, Paydar MJ, Moezi L, Bahadori M, Behfar B, Ardalan FA, Dehpour AR. Antinociceptive effect of chronic lithium on visceral hypersensitivity in a rat model of diarrhea-predominant irritable bowel syndrome: The role of nitric oxide pathway. J Gastroenterol Hepatol. 2009;24:672–680. doi: 10.1111/j.1440-1746.2008.05652.x. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Guandalini S, Zhao DH, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem. 2012;362:43–53. doi: 10.1007/s11010-011-1126-5. [DOI] [PubMed] [Google Scholar]
- 11.Kuiken SD, Tytgat GN, Boeckxstaens GE. Review article: drugs interfering with visceral sensitivity for the treatment of functional gastrointestinal disorders--the clinical evidence. Aliment Pharmacol Ther. 2005;21:633–651. doi: 10.1111/j.1365-2036.2005.02392.x. [DOI] [PubMed] [Google Scholar]
- 12.Kong DX, Li XJ, Tang GY, Zhang HY. How many traditional Chinese medicine components have been recognized by modern Western medicine? A chemoinformatic analysis and implications for finding multicomponent drugs. ChemMedChem. 2008;3:233–236. doi: 10.1002/cmdc.200700291. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, You YO. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 15.Letasiová S, Jantová S, Cipák L, Múcková M. Berberine-antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006;239:254–262. doi: 10.1016/j.canlet.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Piyanuch R, Sukhthankar M, Wandee G, Baek SJ. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007;258:230–240. doi: 10.1016/j.canlet.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–412. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Gu L, Li N, Li Q, Zhang Q, Wang C, Zhu W, Li J. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia. 2009;80:241–248. doi: 10.1016/j.fitote.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Niu L, Qiao W, Hu Z, Li N, Huang Q, Gong J, Li Q, Zhu W, Li J. Berberine attenuates lipopolysaccharide-induced impairments of intestinal glutamine transport and glutaminase activity in rat. Fitoterapia. 2011;82:323–330. doi: 10.1016/j.fitote.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;294:822–829. [PubMed] [Google Scholar]
- 21.Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur J Pharmacol. 2010;648:162–170. doi: 10.1016/j.ejphar.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Long Y, Sun Y, Wang Y, Li Q, Wu H, Guo Z, Li Y, Niu Y, Li C, et al. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur J Pharmacol. 2011;651:187–196. doi: 10.1016/j.ejphar.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Pan LR, Tang Q, Fu Q, Hu BR, Xiang JZ, Qian JQ. Roles of nitric oxide in protective effect of berberine in ethanol-induced gastric ulcer mice. Acta Pharmacol Sin. 2005;26:1334–1338. doi: 10.1111/j.1745-7254.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology. 2009;112:279–286. doi: 10.1159/000157336. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Zhang JJ, Wang X, Bu XY, Lou YQ, Zhang GL. Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed Pharmacother. 2008;62:567–572. doi: 10.1016/j.biopha.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cernáková M, Kostálová D. Antimicrobial activity of berberine--a constituent of Mahonia aquifolium. Folia Microbiol (Praha) 2002;47:375–378. doi: 10.1007/BF02818693. [DOI] [PubMed] [Google Scholar]
- 29.Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91:193–197. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]
- 30.Kreiss C, Toegel S, Bauer AJ. Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents. Am J Physiol Gastrointest Liver Physiol. 2004;287:G658–G666. doi: 10.1152/ajpgi.00526.2003. [DOI] [PubMed] [Google Scholar]
- 31.Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2004;286:G225–G233. doi: 10.1152/ajpgi.00447.2002. [DOI] [PubMed] [Google Scholar]
- 32.Fritz E, Hammer J, Schmidt B, Eherer AJ, Hammer HF. Stimulation of the nitric oxide-guanosine 3’, 5’-cyclic monophosphate pathway by sildenafil: effect on rectal muscle tone, distensibility, and perception in health and in irritable bowel syndrome. Am J Gastroenterol. 2003;98:2253–2260. doi: 10.1111/j.1572-0241.2003.07661.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuiken SD, Vergeer M, Heisterkamp SH, Tytgat GN, Boeckxstaens GE. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut. 2002;51:212–218. doi: 10.1136/gut.51.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu JR, Luo JY, Shang L, Kong WM. Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin J Dig Dis. 2006;7:89–96. doi: 10.1111/j.1443-9573.2006.00248.x. [DOI] [PubMed] [Google Scholar]