Abstract
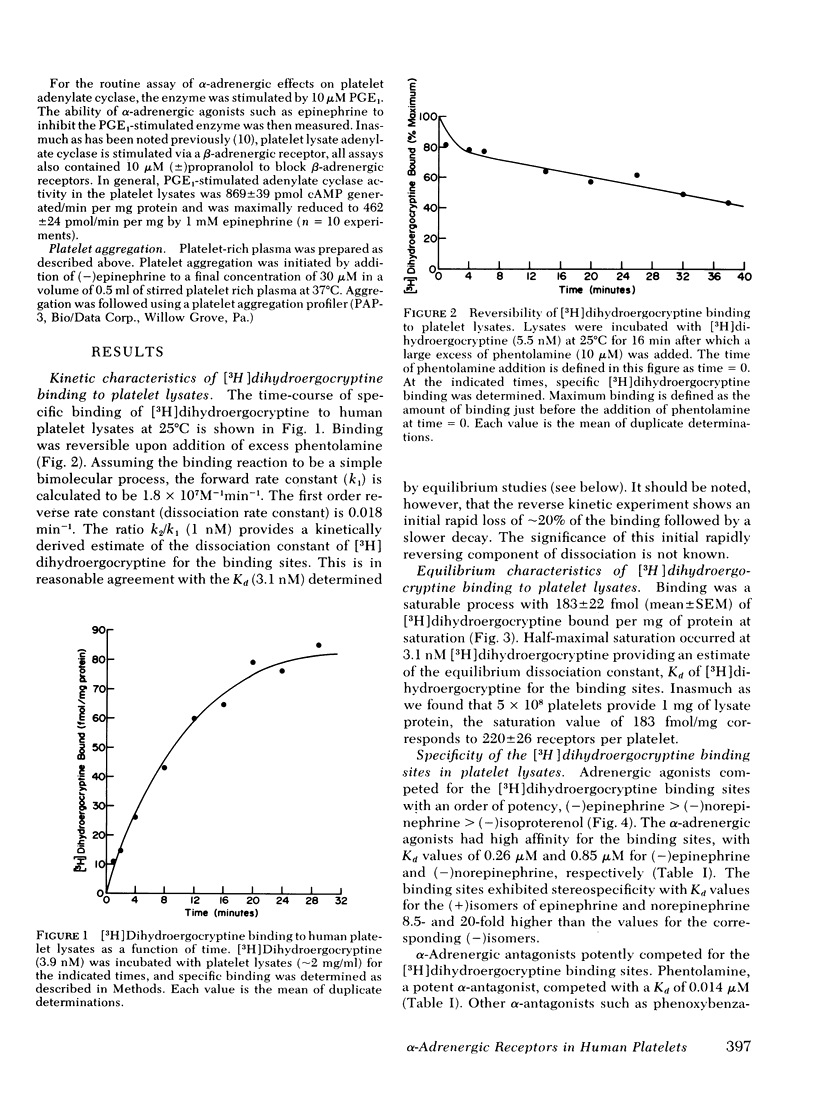
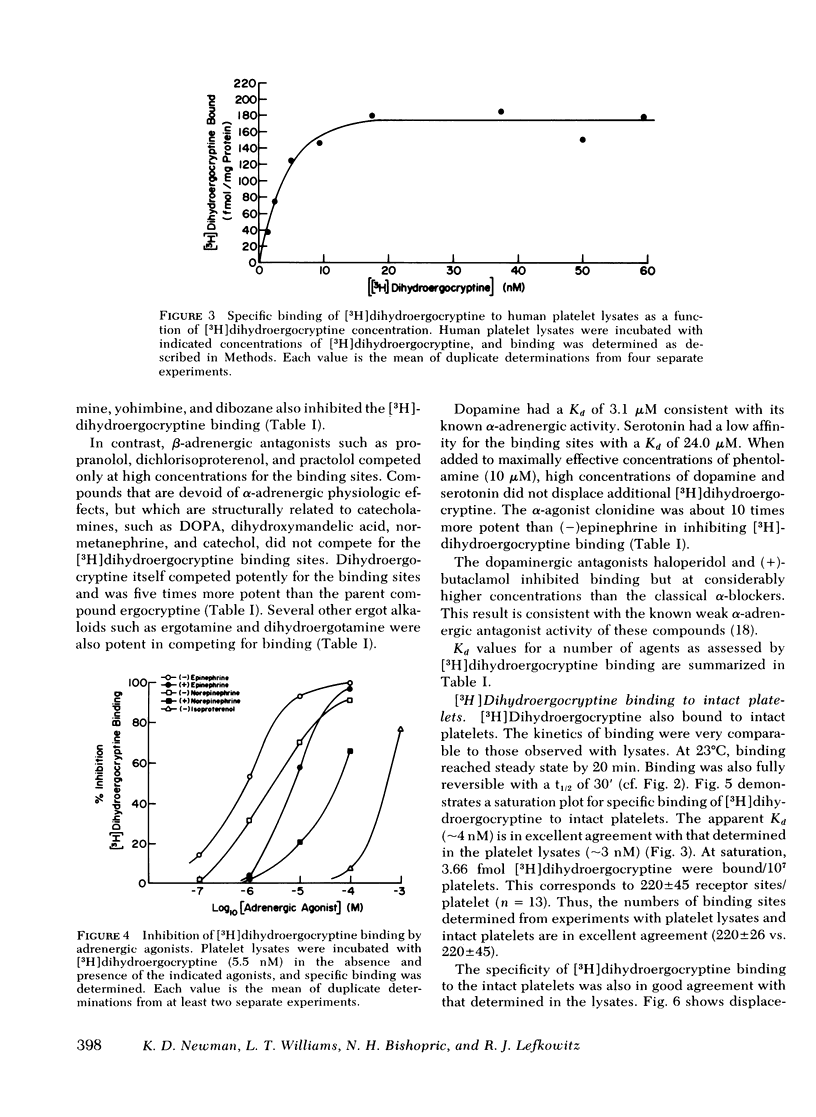
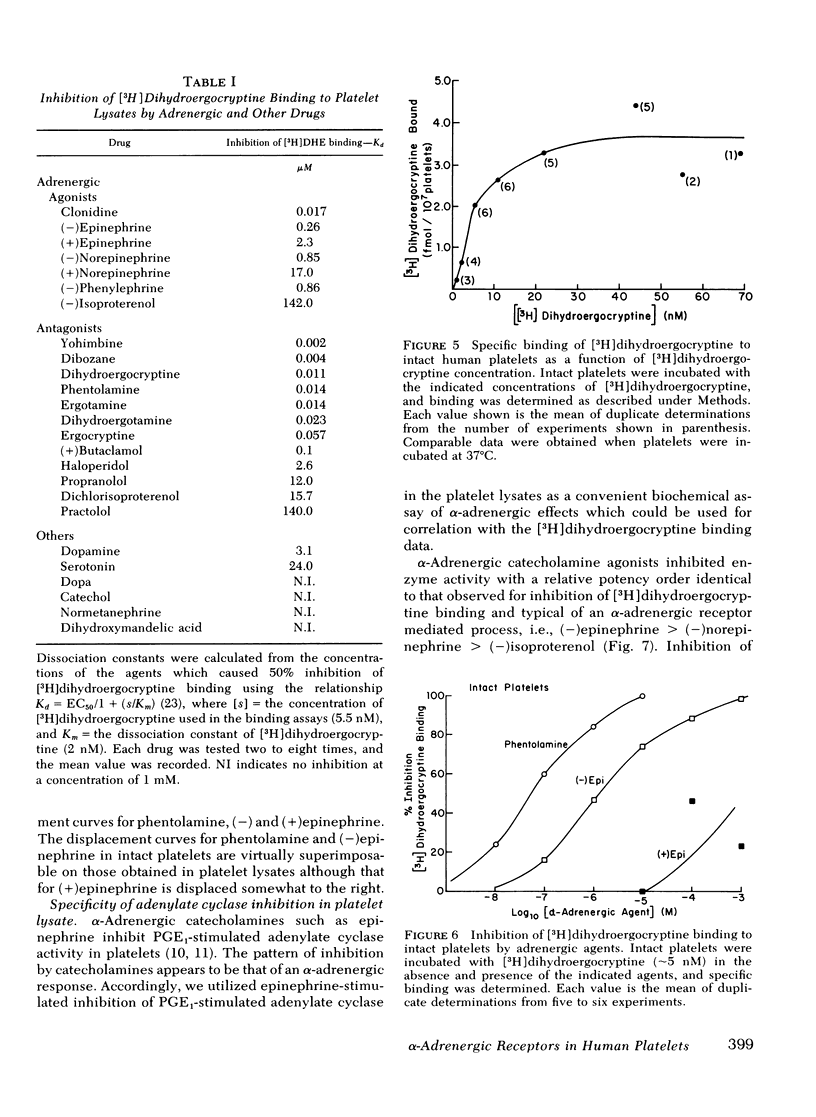
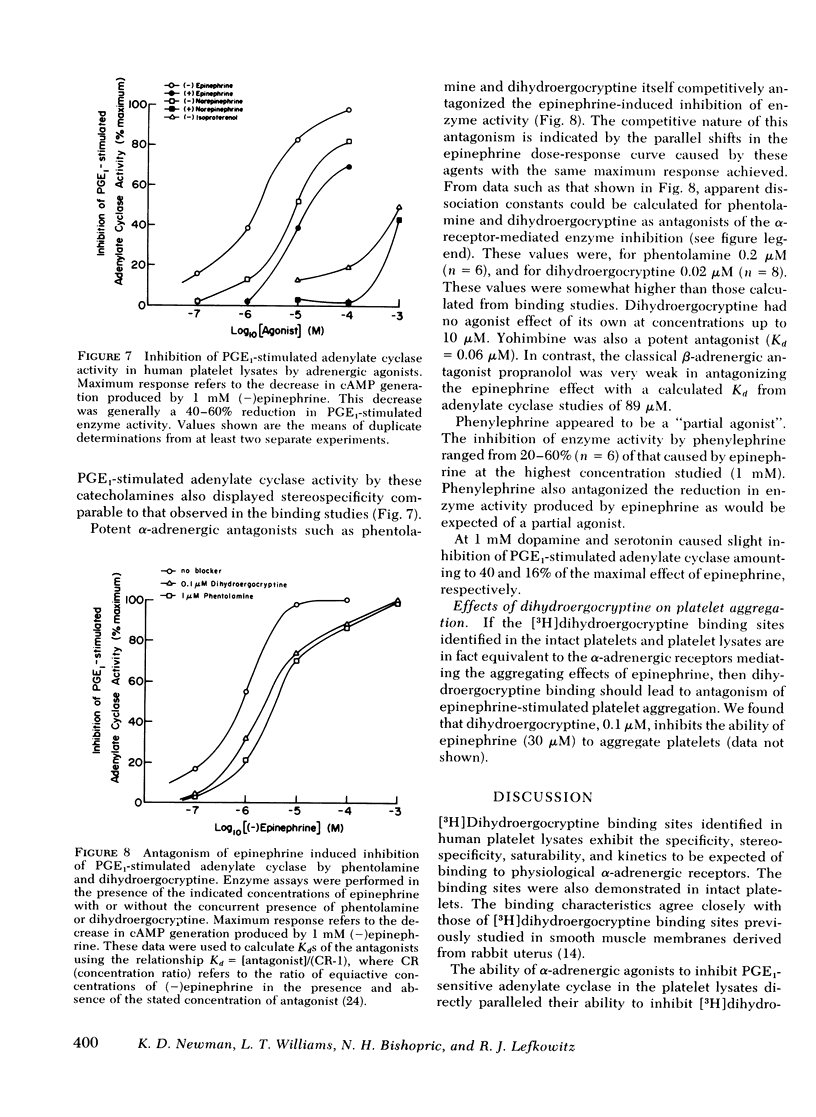
Binding of [3H]dihydroergocryptine to platelet lysates appears to have all the characteristics of binding to α-adrenergic receptors. At 25°C binding reaches equilibrium within 20 min and is reversible upon addition of excess phentolamine. Binding is saturable with 183±22 fmol of [3H]dihydroergocryptine bound per mg of protein at saturation, corresponding to 220±26 sites per platelet. Kinetic and equilibrium studies indicate the dissociation constant of [3H]dihydroergocryptine for the receptors is 1-3 nM. The specificity of the binding sites is typical of an α-adrenergic receptor. Catecholamine agonists compete for occupancy of the [3H]dihydroergocryptine binding sites with an order of potency (−)epinephrine> (−)norepinephrine≫ (−)isoproterenol. Stereospecificity was demonstrated inasmuch as the (+)isomers of epinephrine and norepinephrine were 10-20-fold less potent than the (−)isomers. The potent α-adrenergic antagonists phentolamine, phenoxybenzamine, and yohimbine competed potently for the sites, whereas β-antagonists such as propranolol and dichlorisoproterenol were quite weak. Dopamine and serotonin competed only at high concentrations (0.1 mM).
The [3H]dihydroergocryptine binding sites could also be demonstrated in intact platelets where they displayed comparable specificity, stereospecificity, and saturability. Saturation binding studies with the intact platelets indicated 220±45 receptors per platelet, in good agreement with the value derived from studies with platelet lysates. Ability of α-adrenergic agonists to inhibit adenylate cyclase and of α-adrenergic antagonists to antagonize this inhibitory effect directly paralleled ability to interact with the [3H]dihydroergocryptine binding sites. These data demonstrate the feasibility of directly studying α-adrenergic receptor binding sites in human platelets with [3H]dihydroergocryptine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bygdeman S., Johnsen O. Studies on the effect of adrenergic blocking drugs on catecholamine-induced platelet aggregation and uptake of noradrenaline and 5-hydroxytryptamine. Acta Physiol Scand. 1969 Jan-Feb;75(1):129–138. doi: 10.1111/j.1748-1716.1969.tb04364.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cole B., Robison G. A., Hartmann R. C. The role of cyclic AMP in platelet function. Ann N Y Acad Sci. 1971 Dec 30;185:477–487. doi: 10.1111/j.1749-6632.1971.tb45274.x. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The pharmacological differentiation of adrenergic receptors. Ann N Y Acad Sci. 1967 Feb 10;139(3):553–570. doi: 10.1111/j.1749-6632.1967.tb41229.x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Harwood J. P., Moskowitz J., Krishna G. Dynamic interaction of prostaglandin and norepinephrine in the formation of adenosine 3',5'-monophosphate in human and rabbit platelets. Biochim Biophys Acta. 1971 Feb 28;261(2):444–456. doi: 10.1016/0304-4165(72)90069-4. [DOI] [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Haslam R. J., Taylor A. Effects of catecholamines on the formation of adenosine 3':5'-cyclic monophosphate in human blood platelets. Biochem J. 1971 Nov;125(1):377–379. doi: 10.1042/bj1250377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H., Saur W., Schultz G. Reduction of adenylate cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):381–392. [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Marquis N. R., Becker J. A., Vigdahl R. L. Platelet aggregation. 3. An epinephrine induced decrease in cyclic AMP synthesis. Biochem Biophys Res Commun. 1970 Jun 5;39(5):783–789. doi: 10.1016/0006-291x(70)90391-8. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Roberts G. C. Effects of adrenaline on human blood platelets. J Physiol. 1967 Nov;193(2):443–453. doi: 10.1113/jphysiol.1967.sp008369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BRIEN J. R. SOME EFFECTS OF ADRENALINE AND ANTI-ADRENALINE COMPOUNDS ON PLATELETS IN VITRO AND IN VIVO. Nature. 1963 Nov 23;200:763–764. doi: 10.1038/200763a0. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Feinstein M. B. Interrelationships between Ca2+ and adenylate and guanylate cyclases in the control of platelet secretion and aggregation. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1829–1833. doi: 10.1073/pnas.73.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Neri L. L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. Nature. 1969 Nov 8;224(5219):609–610. doi: 10.1038/224609a0. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Greenberg D. A., Snyder S. H. Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol. 1977 May;13(3):454–473. [PubMed] [Google Scholar]
- Williams L. T., Mullikin D., Lefkowitz R. J. Identification of alpha-adrenergic receptors in uterine smooth muscle membranes by [3H]dihydroergocryptine binding. J Biol Chem. 1976 Nov 25;251(22):6915–6923. [PubMed] [Google Scholar]
- Zieve P. D., Greenough W. B., 3rd Adenyl cyclase in human platelets: activity and responsiveness. Biochem Biophys Res Commun. 1969 May 22;35(4):462–466. doi: 10.1016/0006-291x(69)90368-4. [DOI] [PubMed] [Google Scholar]


