Abstract
A 50% reduction in the activity of uroporphyrinogen-I (URO) synthase in liver, erythrocytes, and cultured skin fibroblasts characterizes all patients with clinically active acute intermittent porphyria (AIP). The same enzyme defect has also been demonstrated in the erythrocytes and skin fibroblasts of completely latent gene carriers of this disorder and presumably exists in the liver as well.
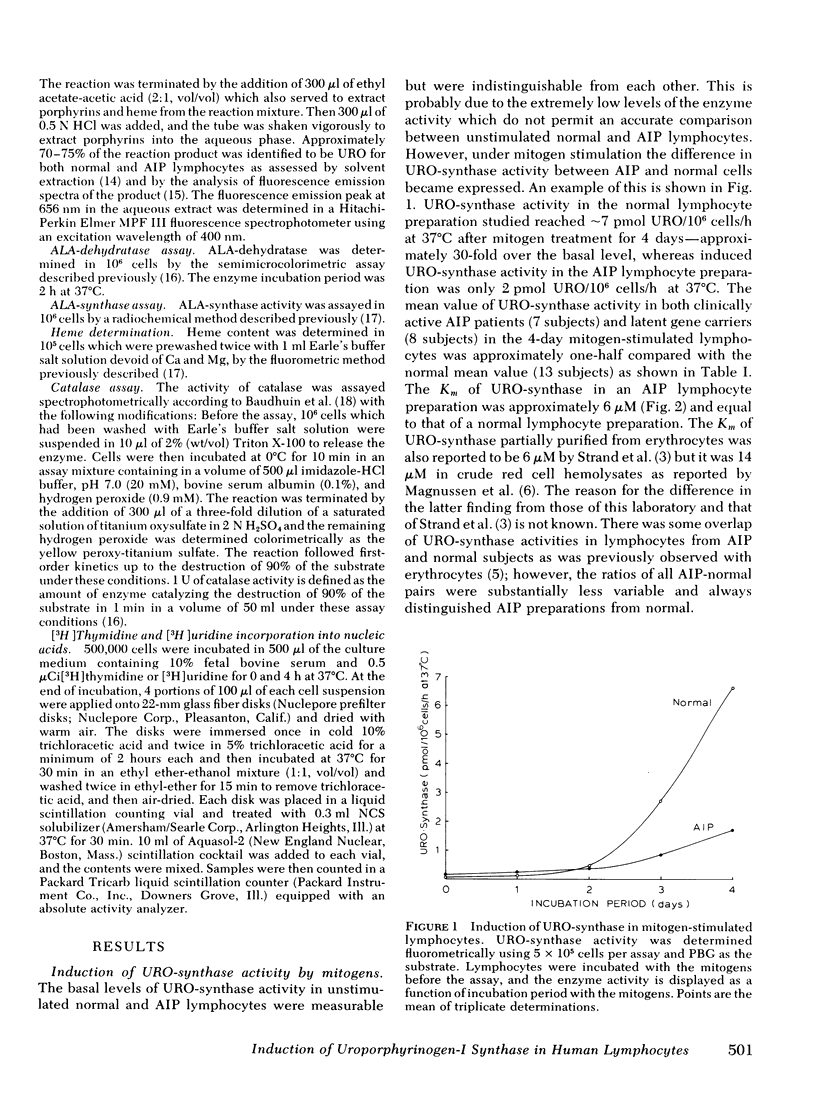
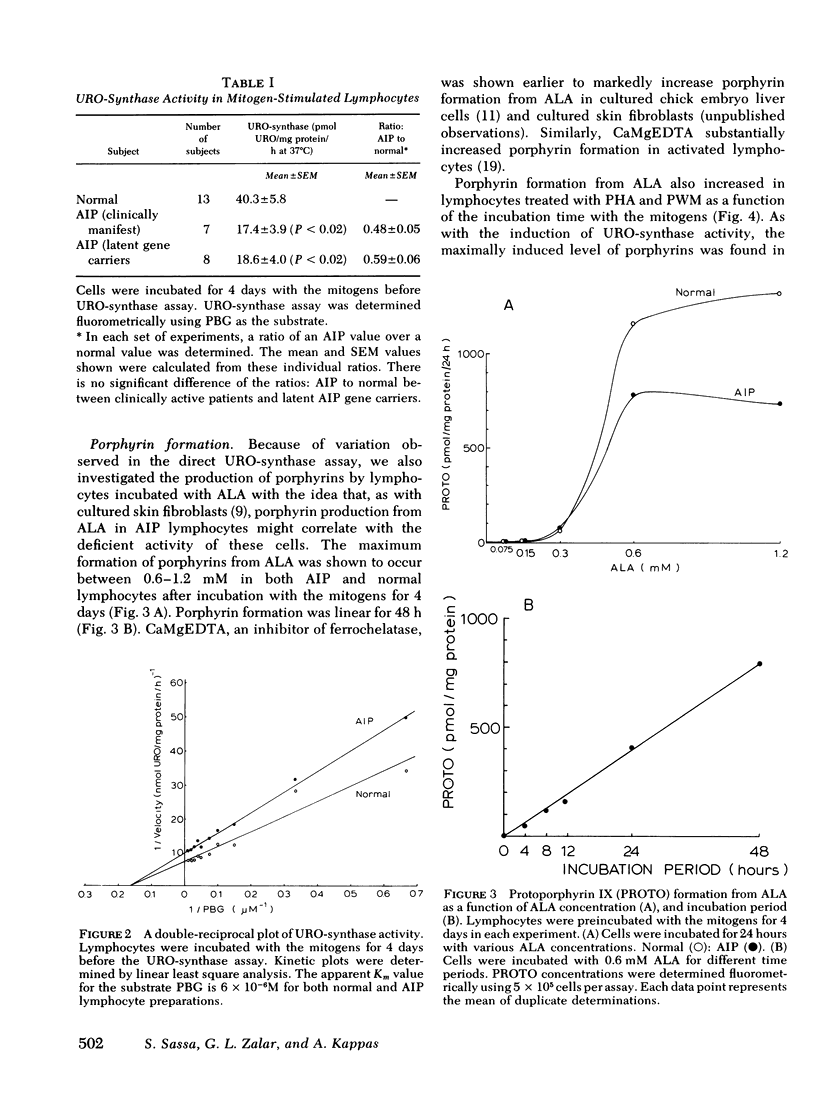
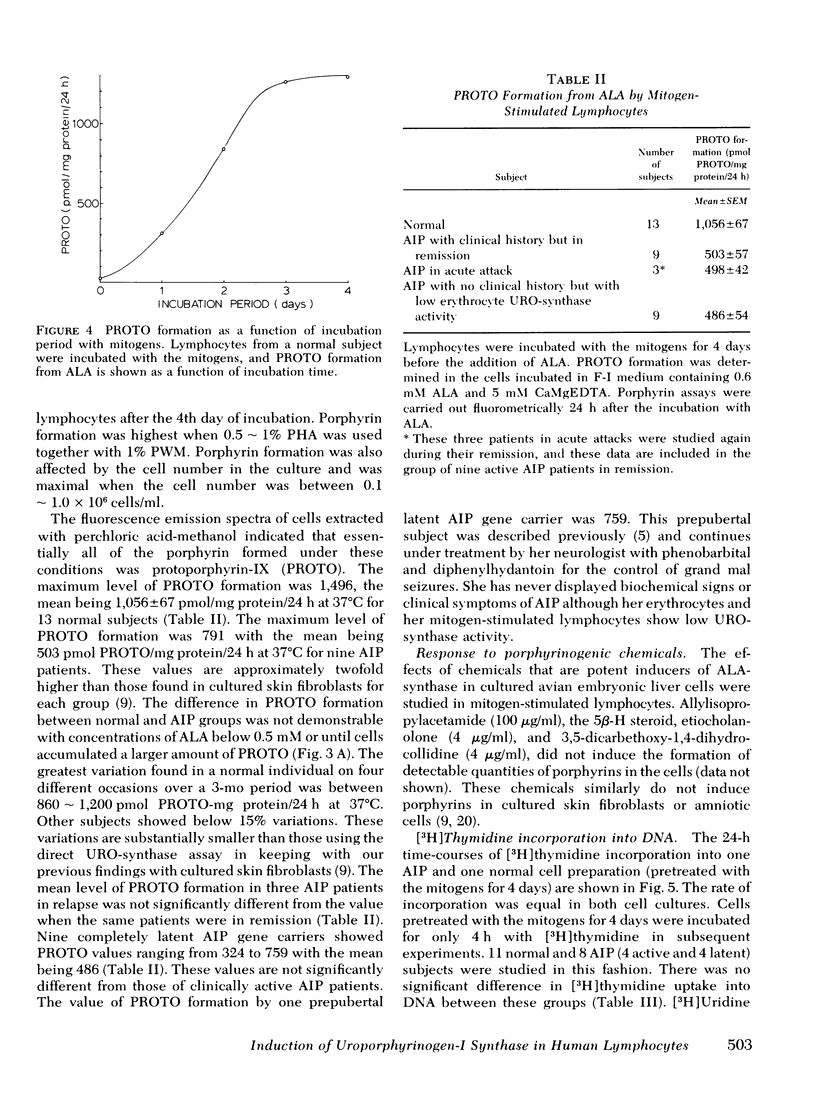
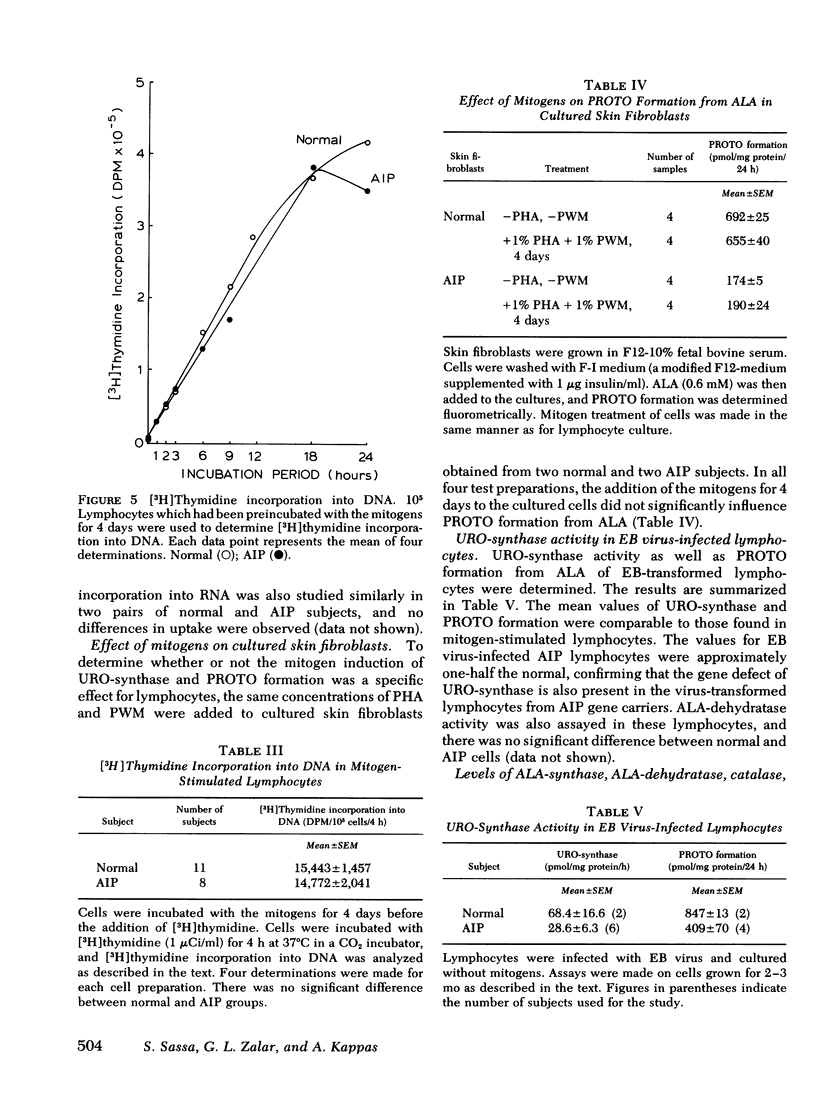
In this study, we examined whether or not the formation of URO-synthase is impaired in AIP cells using lymphocytes treated with mitogens or infected with Epstein-Barr virus. Both mitogens (phytohemagglutinin and pokeweed mitogen) and Epstein-Barr virus induced the synthesis of URO-synthase in lymphocytes, but the induction of URO-synthase in AIP lymphocytes was only 50% as compared with that in normal lymphocytes. The impaired induction of URO-synthase in AIP lymphocytes reflects a specific gene defect because AIP lymphocytes showed normal [3H] thymidine uptake into DNA, [3H] uridine uptake into RNA, and normal δ-aminolevulinic acid (ALA) synthase, ALA-dehydratase, catalase activities, and heme content. Utilizing the same methodology, the ferrochelatase deficiency of hereditary erythropoietic protoporphyria could also be identified. The Km of the induced URO-synthase in AIP cells was identical to that of the enzyme in normal cells. The induced URO-synthase of mitogen-treated AIP lymphocytes was not accompanied by a concurrent enhanced level of ALA-synthase. Moreover, the URO-synthase deficiency in lymphocytes from actively ill AIP patients was not different from the level of enzyme activity when they were in clinical remission, or when compared with the enzyme activity of cells from completely latent AIP gene carriers. The results of this study indicate that the URO-synthase deficiency in AIP may be the result of a gene mutation regulating the rate of synthesis of a normal enzyme rather than a mutation causing a structural abnormality of this enzyme protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. The molecular genetics of the thalassemia syndromes. Prog Hematol. 1975;9:107–155. [PubMed] [Google Scholar]
- Bickers D. R., Keogh L., Rifkind A. B., Harber L. C., Kappas A. Studies in porphyria. VI. Biosynthesis of porphyrins in mammalian skin and in the skin of porphyric patients. J Invest Dermatol. 1977 Jan;68(1):5–9. doi: 10.1111/1523-1747.ep12485121. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Weinbach E. C., Ebert P. S., Doherty J. M. Porphyrin synthesis and mitochondrial respiration in acute intermittent porphyria: studies using cultured human fibroblasts. J Lab Clin Med. 1975 Jan;85(1):93–102. [PubMed] [Google Scholar]
- Bradlow H. L., Gillette P. N., Gallagher T. F., Kappas A. Studies in porphyria. II. Evidence for a deficiency of steroid delta-4-5-alpha-reductase activity in acute intermittent porphyria. J Exp Med. 1973 Oct 1;138(4):754–763. doi: 10.1084/jem.138.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters J. K. The role of zinc ions in the transformation of lymphocytes by phytohaemagglutinin. Biochem J. 1972 Nov;130(1):133–139. doi: 10.1042/bj1300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Decreased lymphocyte coproporphyrinogen III oxidase activity in hereditary coproporphyria. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1089–1095. doi: 10.1016/0006-291x(77)91630-8. [DOI] [PubMed] [Google Scholar]
- Granick S., Sassa S., Granick J. L., Levere R. D., Kappas A. Assays for porphyrins, delta-aminolevulinic-acid dehydratase, and porphyrinogen synthetase in microliter samples of whole blood: applications to metabolic defects involving the heme pathway. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2381–2385. doi: 10.1073/pnas.69.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Hodgson C., Hell E. Failure of phytohaemagglutinin to effect epidermal DNA synthesis. Br J Dermatol. 1966 Oct;78(10):525–527. doi: 10.1111/j.1365-2133.1966.tb12140.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Josephson A. S., Levere R. D., Lowenthal I., Swerdlow F., Ginsberg M. Porphyrin synthesis by cultured lymphocytes. Blood. 1972 Apr;39(4):568–574. [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Bickers D. R., Alvares A. P. Induction of a deficiency of steroid delta 4-5 alpha-reductase activity in liver by a porphyrinogenic drug. J Clin Invest. 1977 Jan;59(1):159–164. doi: 10.1172/JCI108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Studies in porphyria. I. A defect in the reductive transformation of natural steroid hormones in the hereditary liver disease, acute intermittent porphyria. J Exp Med. 1972 Nov 1;136(5):1043–1053. doi: 10.1084/jem.136.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Sassa S., Granick S., Bradlow H. L. Endocrine-gene interaction in the pathogenesis of acute intermittent porphyria. Res Publ Assoc Res Nerv Ment Dis. 1974;53:225–237. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magnussen C. R., Levine J. B., Doherty J. M., Cheesman J. O., Tschudy D. P. A red cell enzyme method for the diagnosis of acute intermittent porphyria. Blood. 1974 Dec;44(6):857–868. [PubMed] [Google Scholar]
- Meyer U. A. Intermittent acute porphyria. Clinical and biochemical studies of disordered heme biosynthesis. Enzyme. 1973;16(1):334–342. [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Miyagi K., Cardinal R., Bossenmaier I., Watson C. J. The serum porphobilinogen and hepatic porphobilinogen deaminase in normal and porphyric individuals. J Lab Clin Med. 1971 Nov;78(5):683–695. [PubMed] [Google Scholar]
- Saillen R., Jéquier E., Vannotti A. Porphyrin synthesis by the phytohemagglutinin-transformed lymphocytes in vitro. J Reticuloendothel Soc. 1969 Apr;6(2):175–183. [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Levere R. D., Kappas A. Studies on the inheritance of human erythrocyte delta-aminolevulinate dehydratase and uroporphyrinogen synthetase. Enzyme. 1973;16(1):326–333. doi: 10.1159/000459397. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. J., Pierach C. A., Bossenmaier I., Cardinal R. Postulated deficiency of hepatic heme and repair by hematin infusions in the "inducible" hepatic porphyrias. Proc Natl Acad Sci U S A. 1977 May;74(5):2118–2120. doi: 10.1073/pnas.74.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]


