Abstract
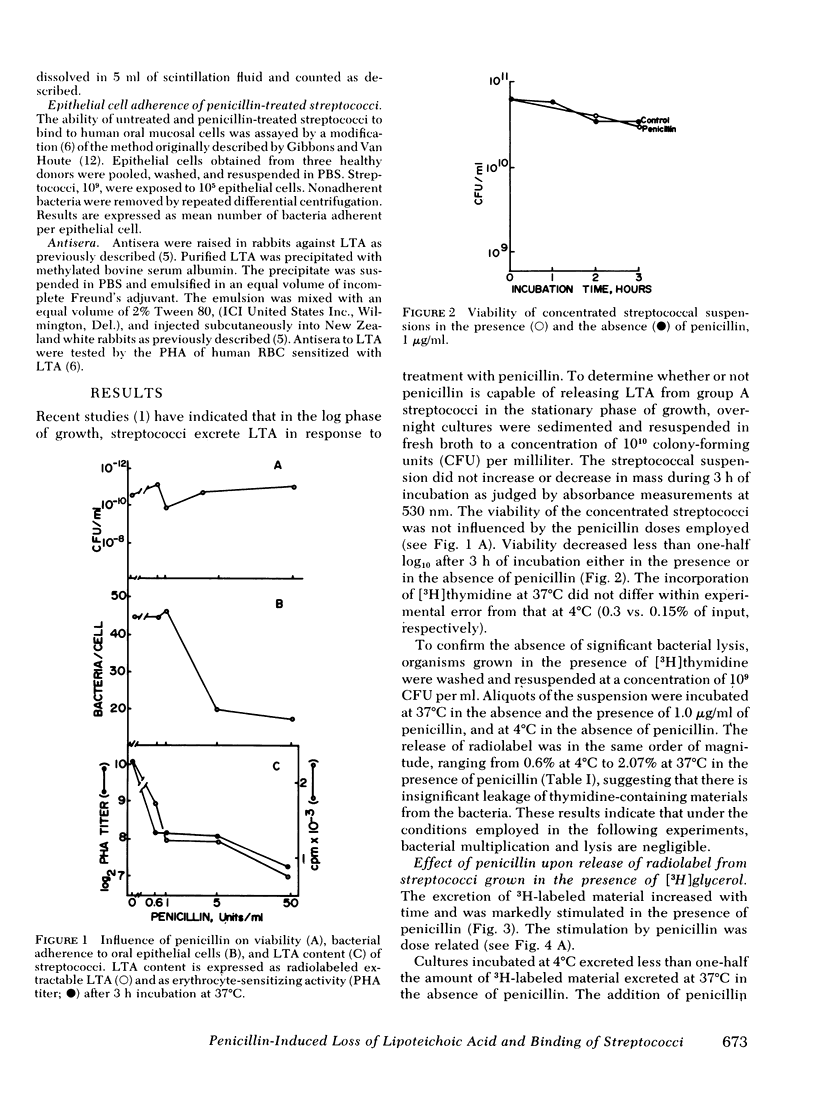
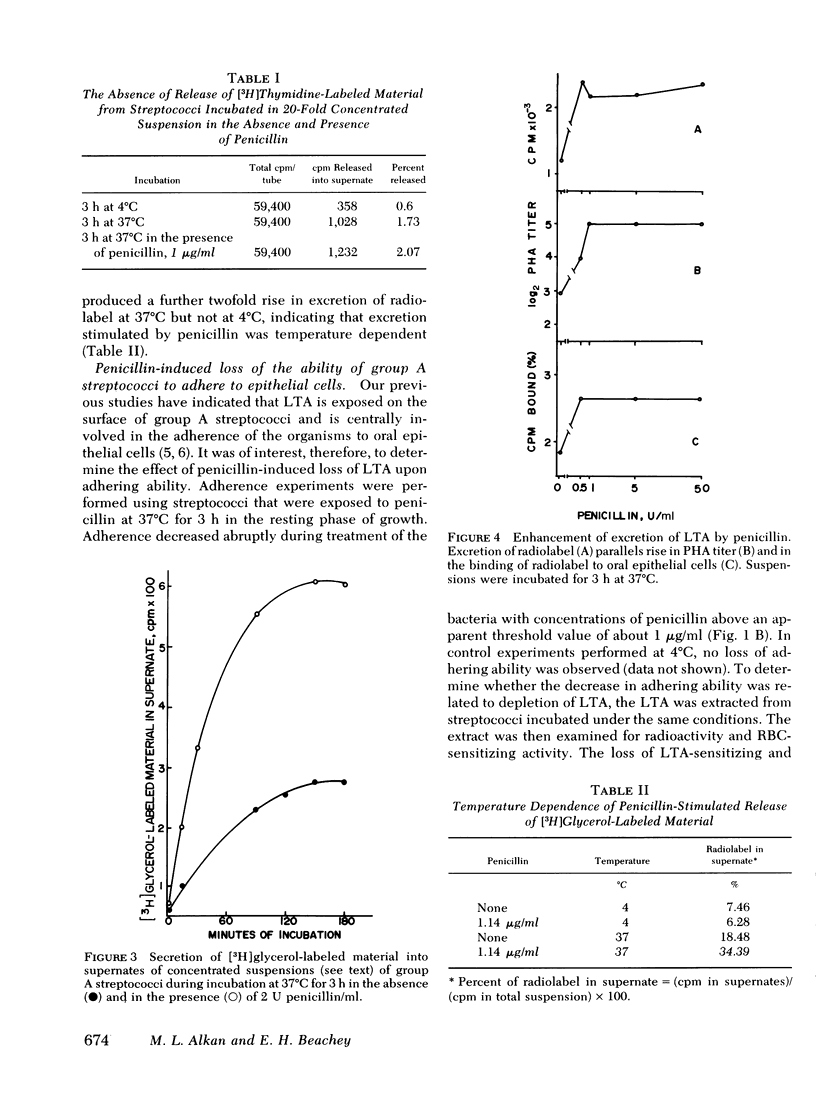
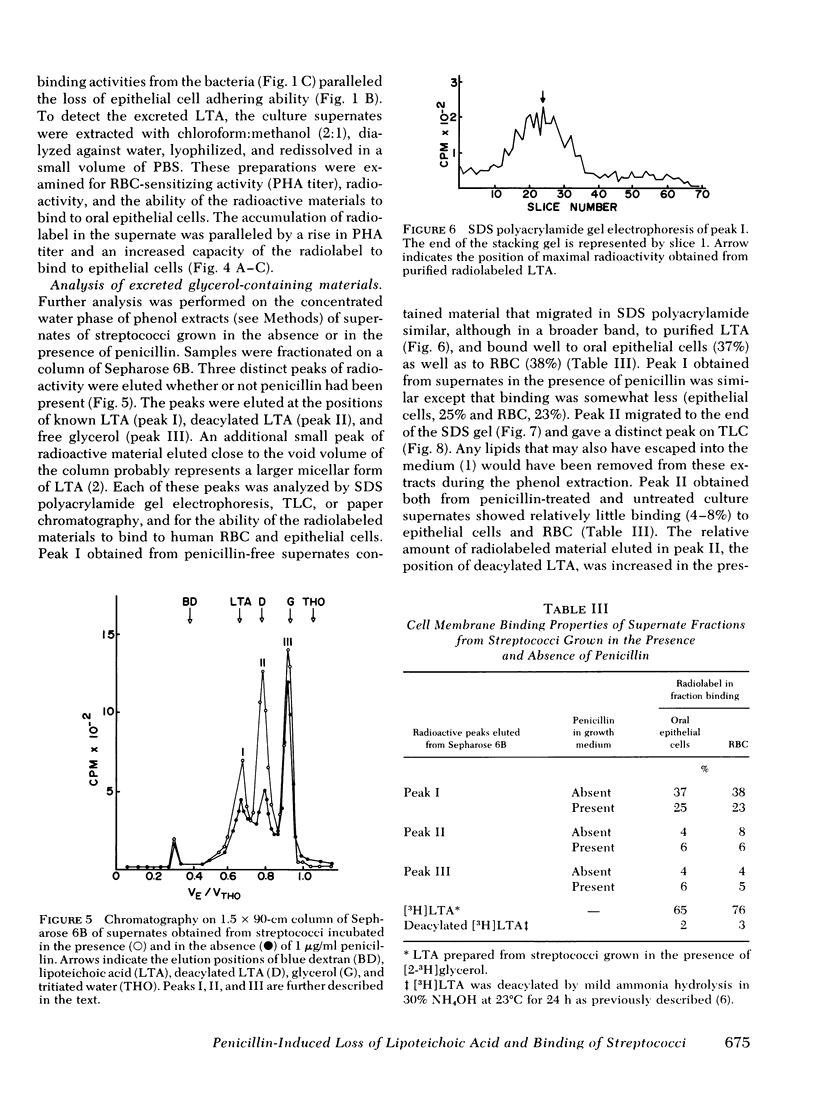
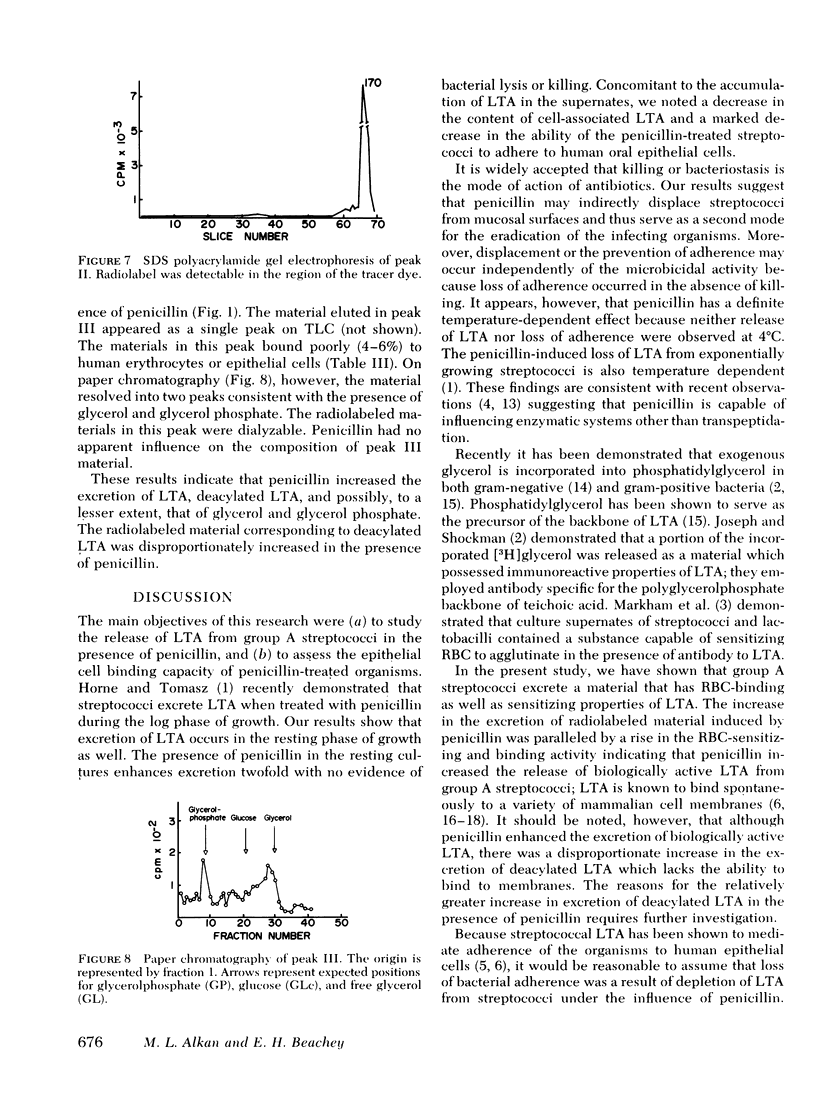
Group A streptococci were grown in the presence of [2-3H]glycerol. Concentrated suspensions of the labeled organisms were incubated with and without penicillin. [3H]Glycerol-labeled material accumulated in the supernates in increasing amounts with increasing concentrations of penicillin, ranging from 0 to 50 U/ml. The excretion of labeled material occurred in the absence of nucleic acid synthesis or bacteriolysis indicating that the phenomenon is independent of cell multiplication or decay. The accumulation of label was paralleled by an accumulation of erythrocyte-sensitizing material measured by passive hemagglutination tests for lipoteichoic acid antigen, indicating that a portion of the labeled material possessed the properties of lipoteichoic acid. Culture supernates were fractionated by column chromatography, and the materials obtained were analyzed by electrophoresis on sodium dodecyl sulfate polyacrylamide, thin-layer chromatography, and paper chromatography. The ability of the same materials to bind to human erythrocytes and epithelial cells was tested. The culture supernate contained lipoteichoic acid, deacylated lipoteichoic acid, glycerol phosphate, and free glycerol. Penicillin caused an increase in the amounts of each of the excreted materials. Streptococci that were stimulated with penicillin to lose their lipoteichoic acid (previously shown to mediate adherence of group A streptococci) lost their ability to adhere to buccal mucosal cells, suggesting that penicillin may influence bacterial ecology by mechanisms other than killing sensitive organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. Toxic effects of streptococcal M protein on platelets and polymorphonuclear leukocytes in human blood. J Exp Med. 1971 Aug 1;134(2):351–365. doi: 10.1084/jem.134.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpenning F. W., Stamper H. B. Spontaneous adsorption of teichoic acid to erythrocytes. Immunochemistry. 1973 Jan;10(1):15–20. doi: 10.1016/0019-2791(73)90245-0. [DOI] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. Separation and properties of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2200–2204. doi: 10.1128/jb.91.6.2200-2204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART F. S., MARTIN W. T. Adsorption of a streptococcal red cell-sensitising antigen to various tissues. J Pathol Bacteriol. 1962 Jul;84:251–253. doi: 10.1002/path.1700840135. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


