Abstract
Inflammation and tissue degeneration play key roles in numerous rheumatic diseases, including osteoarthritis (OA). Efforts to reduce and effectively repair articular cartilage damage in an osteoarthritic environment are limited in their success due to the diseased environment. Treatment strategies focused on both reducing inflammation and increasing tissue production are necessary to effectively treat OA from a tissue-engineering perspective. In this work, we investigated the anti-inflammatory and tissue production capacity of a small molecule 3,4,6-O-tributanoylated-N-acetylglucosamine (3,4,6-O-Bu3GlcNAc) previously shown to inhibit the nuclear factor κB (NFκB) activity, a key transcription factor regulating inflammation. To mimic an inflammatory environment, chondrocytes were stimulated with interleukin-1β (IL-1β), a potent inflammatory cytokine. 3,4,6-O-Bu3GlcNAc exposure decreased the expression of NFκB target genes relevant to OA by IL-1β-stimulated chondrocytes after 24 h of exposure. The capacity of 3,4,6-O-Bu3GlcNAc to stimulate extracellular matrix (ECM) accumulation by IL-1β-stimulated chondrocytes was evaluated in vitro utilizing a three-dimensional hydrogel culturing system. After 21 days, 3,4,6-O-Bu3GlcNAc exposure induced quantifiable increases in both sulfated glycosaminoglycan and total collagen. Histological staining for proteoglycans and type II collagen confirmed these findings. The increased ECM accumulation was not due to the hydrolysis products of the small molecule, n-butyrate and N-acetylglucosamine (GlcNAc), as the isomeric 1,3,4-O-tributanoylated N-acetylglucosamine (1,3,4-O-Bu3GlcNAc) did not elicit a similar response. These findings demonstrate that a novel butanoylated GlcNAc derivative, 3,4,6-O-Bu3GlcNAc, has the potential to stimulate new tissue production and reduce inflammation in IL-1β-induced chondrocytes with utility for OA and other forms of inflammatory arthritis.
Introduction
Osteoarthritis (OA) is the most common form of arthritis, affecting an estimated 27 million adults in the United States,1 with an increasing incidence as the population ages. OA is associated with considerable morbidity as a consequence of joint damage, resulting in pain, loss of joint range of motion, and disability. The disease is marked by considerable articular cartilage damage. Due to the low mitotic activity of the cells and the avascularity of the tissue, cartilage does not effectively self-repair. Treatment for OA remains largely related to pain relief, with additional use of injectable viscosupplements and corticosteroids, which may provide transient periods of improvement, but do not inhibit joint damage. Surgical interventions with joint replacements, while ultimately curative of OA are associated with morbidity and considerable cost. There is thus a considerable need to develop new tissue-engineering strategies that may help to both induce tissue regeneration in a diseased joint environment, while decreasing joint inflammation and pain.
In normal cartilage homeostasis, there is a balance of extracellular matrix (ECM) production and breakdown. During cartilage disease, the balance is disrupted, which results in ECM loss and tissue degradation. It is well known that diseased chondrocytes synthesize less ECM than normal chondrocytes contributing to the net loss of ECM.2,3 Matrix metalloproteinase-2, -9, and -13 (MMP2, MMP9, and MMP13), a disintegrin and metalloproteinase with thrombospondin motifs-4 and -5 (ADAMTS-4 and ADAMTS-5), inducible nitric oxide synthase (iNOS), and both cyclooxygenases and prostaglandin E synthases (PGES) are increased in the OA joint facilitating ECM degradation or inhibit the synthesis of a new ECM.4 Inflammation is believed to play a key role in this matrix imbalance5 with the nuclear factor κB (NFκB) playing a pivotal role in transcriptionally regulating the expression of MMPs, proinflammatory cytokines, and transcription factors and their regulators.6 Moreover, the inflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α, which initiate the mitogen-activated protein kinase signaling cascade resulting in activation of transcription factors, including NFκB, are found in OA joint tissue, including the cartilage and synovial lining.5,7 Therefore, targeting the NFκB activity is a potential effective therapeutic target for engineering cartilage in a diseased environment.
In this work, we investigated the ability of short-chain fatty acid (SCFA)-hexosamine hybrid molecules to improve outcomes in an in vitro model of inflammation and ECM loss modeling osteoarthritis (OA), in part, through these compounds recently reported the NFκB inhibitory activity.8 This approach builds on an emerging paradigm in drug design, where simple carbohydrates function as novel drug candidates wherein derivation of the core sugar results in defined spatial three-dimensional (3D) structures that can engage proteins to alter the biological activity of a cell.9 To date, this concept has been demonstrated with hexosamines through the ability of n-butanoylated N-acetylmannosamine (ManNAc) analog 3,4,6-O-Bu3ManNAc to suppress NFκB and associated metastatic oncogenes in human cancer cell lines.8 Furthermore, the n-butanoylated GlcNAc analog 3,4,6-O-Bu3GlcNAc also suppressed the NFκB activity in those studies.8 An attractive feature of this class of compounds is that they minimize toxic non-natural secondary metabolites, but instead are degraded through decomposition to natural byproducts. For example, in the case of butanoylated ManNAc, n-butyrate and ManNAc are generated, which can function as histone deacetylase inhibitors or be used for sialic acid biosynthesis, respectively. In general, while the biological impact of these hydrolysis products is modest compared to the “whole molecule” effects,10 to the extent that they do occur, however, they are expected to contribute favorably to disease outcomes.
Here we report that the structure–activity relationships that endowed 3,4,6-O-Bu3ManNAc with potential anti-inflammatory properties in cancer cells were retained in the corresponding 3,4,6 GlcNAc-based analog (3,4,6-O-Bu3GlcNAc, Fig. 1) when tested in an in vitro model of inflammation consisting of IL-1β-stimulated chondrocytes.11 Furthermore, we show that 3,4,6-O-Bu3GlcNAc exposure to IL-1β-stimulated chondrocytes resulted in an increase in ECM accumulation and ECM-specific gene expression with limited effects on unstimulated chondrocytes demonstrating the utility of this small molecule-based tissue-engineering strategy to potentially reduce and repair the detrimental effects of inflammation on cartilage as a potential therapeutic for OA. Furthermore, this strategy allows tissue engineers to consider building cartilage in a diseased environment or using cells from a less than perfect environment.
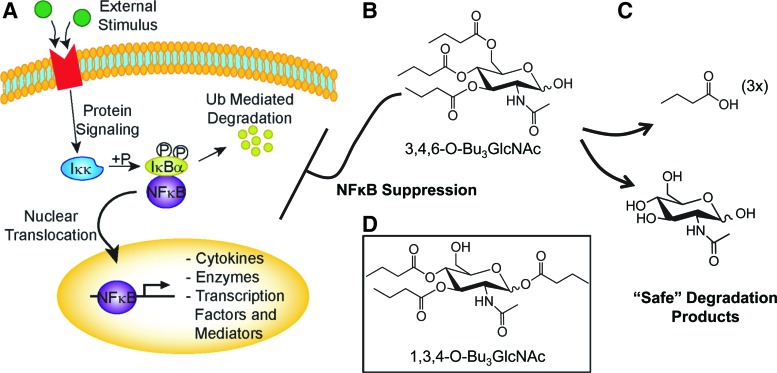
FIG. 1.
Impact of short-chain fatty acid-hexosamine drug candidates on pathways connected to the nuclear factor κB (NFκB). (A) External stimuli with inflammatory cytokines result in an intracellular phosphorylation cascade activating NFκB signaling. (B) 3,4,6-O-tributanoylated-N-acetylglucosamine (3,4,6-O-Bu3GlcNAc) has a whole molecule structure–activity relationship that decreases the NFκB activity. (C) Degradation products of 3,4,6-O-Bu3GlcNAc are n-butyrate and GlcNAc are two natural metabolites that are safely and efficiently metabolized. (D) 1,3,4-O-Bu3GlcNAc,the chemical isomer of 3,4,6-O-Bu3GlcNAc, generates the same metabolites, but has no effect on cells in these studies. Color images available online at www.liebertpub.com/tea
Materials and Methods
Synthesis of monosaccharide hybrid molecules
The chemical structures of 3,4,6-O-Bu3GlcNAc and 1,3,4-O-Bu3GlcNAc are shown in Figure 1 B and D, respectively. Both molecules were synthesized and characterized based on the previously reported methods.12 3,4,6-O-Bu3GlcNAc was synthesized from 2-acetamido-1,3,4,6-tetra-O-butanoyl-2-deoxy-α,β-D-glucopyranose (1,3,4,6-O-Bu4GlcNAc)13 using one-step selective deacylation reaction. Briefly, a mixture of butyric anhydride (15.6 mmol) and 4-(dimethylamino) pyridine (cat.) were added to a stirred solution of GlcNAc,(2.2 mmol) in pyridine (2.0 mL) at 21°C. After 24 h, the mixture was concentrated under vacuum and coconcentrated with toluene (25 mL). The residue was dissolved in methylene chloride (100 mL), washed with cold aqueous HCl (0.5 N, 100 mL), water (100 mL), and saturated NaHCO3 (100 mL). The organic layer was filtered and concentrated. Column chromatography of the residue (hexanes/ethyl acetate) on silica gel provided 1,3,4,6-O-Bu4GlcNAc in the form of syrups that crystallized upon standing. To synthesize 3,4,6-O-Bu3GlcNAc, the 2-acetamido-1,3,4,6-tetra-O-butyl-2-deoxy-α,β-D-glucopyranose (2.0 mmol) was mixed with activated and powdered molecular sieves 4Å (4.0 g) in methanol (100 mL) and stirred at 22°C. The completion of reaction was monitored by thin layer chromatography (TLC) (hexanes:ethyl acetate) to maximize conversion to the hemiacetal, while minimizing deacylation at positions other than C1. After ∼7–12 h, the reaction mixture was filtered through a pad of celite, washed twice with methanol (10 mL), and the combined filtrate was concentrated. Column chromatography of the residue (hexanes:ethyl acetate) was performed to obtain pure 3,4,6-O-Bu3GlcNAc.
Synthesis of 1,3,4-O-Bu3GlcNAc was performed as follows. To a stirring mixture of GlcNAc (0.835 mmol) in pyridine (2.7 mL) was added triphenylmethyl chloride (3.0 g, 1.07 mmol) at 22°C. After 48 h, the reaction mixture was heated at 60°C for 1.0 h and monitored by TLC (ethyl acetate). The reaction mixture was concentrated with toluene (3×20 mL). The residue was dissolved in ethyl acetate and washed with water. The organic layers were collected, dried over anhydrous Na2SO4, filtered, and concentrated to obtain 2-acetamido-2-deoxy-6-O-triphenylmethyl-α,β-D-glucopyranose as a crude product that was taken to the next step without further purification. To the crude product (2.16 mmol) in pyridine (1.46 mL, 18 mmol) at 0°C (ice-water bath), butyric anhydride (12 mmol) was added. The reaction mixture was allowed to warm to 22°C and monitored by TLC (hexanes:ethyl acetate 3:1). After 24 h, the mixture was concentrated with toluene (3×10 mL), and extracted using a mixture of dichloromethane (100 mL) and water (50 mL). The organic layer was collected, dried over anhydrous Na2SO4, filtered, and concentrated. Column chromatography of the residue in hexanes:ethyl acetate gave 2-acetamido-1,3,4-tri-O-butanoyl-2-deoxy-6-O-triphenylmethyl-α,β-D-glucopyranose.A stirred mixture of 2-acetamido-1,3,4-tri-O-butanoyl-2-deoxy-6-O-triphenylmethyl-α,β-D-glucopyranose (0.743 mmol) in 80% aqueous acetic acid (10 mL) was heated at 60°C and monitored by TLC (hexanes:ethyl acetate). After ∼4 h, the reaction mixture was concentrated with toluene (3×10 mL). Column chromatography of the residue in hexanes:ethyl acetate gave 1,3,4-O-Bu3GlcNAc as a mixture of anomers. Compounds were stored at −20°C after lyophilization. Stock solutions used for experiments were made periodically by dissolving analog in 100% ethanol at a concentration of 100 mM; stock solutions were stored at 4°C for up to 3 months.
Isolation of bovine articular chondrocytes
Articular cartilage was dissected from the patellofemoral groove and femoral condyles of 5–8-week-old bovine legs (Research 87, Marlboro, MA) as previously described.14 Tissue was minced into small pieces (∼1 mm3) and digested for 18 h at 37°C in a high-glucose Dulbecco's modified Eagles' medium (DMEM; Gibco, Grand Island, NY) containing 2 mg/mL type II collagenase (Worthington Biochemical Corp., Lakewood, NJ), 5% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin on an orbital shaker. The cell suspension was passed through a 70-μm cell strainer and cell centrifuged to form a pellet. Cells were washed three times with sterile phosphate-buffered saline (PBS). Subsequent cell culturing was performed at 37°C in a water-saturated, 5% CO2 incubator in the high-glucose DMEM supplemented with 10 mM HEPES, 0.4 mM L-proline, 50 μg/mL ascorbic acid, 10% FBS, 0.1 mM nonessential amino acid, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Monolayer cell culture
Cells were plated in six-well plates at 1×106 cells/well and allowed to adhere for 24 h in the presence or absence of 10 ng/mL IL-1β. After 24 h, the medium was changed to a medium supplemented with or without 50 μM 3,4,6-O-Bu3GlcNAc with the respective IL-1β concentration and incubated for an additional 4 or 24 h.
Three-dimensional cell culture
One hundred milligrams of poly(ethylene glycol)-diacrylate (PEGDA; SunBio PEG Shop, Seoul, Korea) was dissolved in 1 mL sterile PBS. The photoinitiator Irgacure 2959® (BASF, Wilmington, MA) was dissolved in 70% ethanol at a concentration of 10% and 5 μL of the photoinitiator added to the 1 mL PEGDA solution. Cells were suspended in the PEGDA precursor solution at a density of 2×107 cells/mL and 100 μL of the cell suspension transferred into sterile cylindrical molds. Polymer crosslinking was initiated via UV-A exposure (365 nm, 3.2 mW/cm2) for 5 min. Cell-laden hydrogels were transferred into 24-well plates and cultured in a 1 mL medium with or without 10 ng/mL IL-1β. After 3 days, the medium was changed to a medium containing indicated hexosamine analog concentrations with or without 10 ng/mL IL-1β. Hydrogels were cultured for an additional 21 days with medium changes three times per week.
Biochemical analysis
The cell-laden hydrogels (n=3 per group) were lyophilized for 48 h. Samples were then homogenized in 125 μg/mL papainase (Worthington Biochemical Corp.) and digested for 16 h at 60°C. The DNA content was determined fluorometrically using Hoechst 33342 dye15 and calf thymus DNA as a standard. The sulfated glycosaminoglycan (sGAG) content was determined by measuring the absorbance at 525 nm with dimethyl methylene blue using chondroitin sulfate as a standard.16 The total collagen content was determined via the hydroxyproline content after acid hydrolysis and the subsequent reaction with p-dimethylaminobenzaldehyde and chloramine-T.17 Absorbance was read at 550 nm using hydroxyproline as a standard.
Gene expression
Total RNA was extracted from chondrocytes using the Trizol reagent and reverse transcribed to cDNA using SuperScript™ II reverse transcriptase following the manufacturer's protocol (Invitrogen, Carlsbad, CA). Real-time PCRs were performed using StepOnePlus™ Real Time PCR System (Applied Biosystems, Carlsbad, CA). All genes were normalized to β-actin, and the level of expression was calculated using the 2-ΔΔCt method as previously described.18 The PCR primers are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Histology and immunohistochemistry
Samples were fixed in 4% paraformaldehyde overnight followed by dehydration in increasing concentrations of ethanol and embedded in paraffin. Five-micrometer-thick sections were collected onto glass slides. Slides were stained with Safranin-O and fast green used as a counter stain. For immunohistochemistry, AEC Broad Spectrum Histostain-SP Kit (Invitrogen) was used following the manufacturer's instructions. Primary antibodies for type I and type II collagen (Fitzgerald, San Diego, CA) using a 1:100 dilution factor in 1% bovine serum albumin dissolved in PBS.
Statistical analysis
Data are expressed as mean±standard deviation. Statistical significance was determined by one-way analysis of variance followed by the Tukey HSD test using SPSS 18.0 software (SPSS, Inc., Chicago, IL) or the two-tailed t-test. Significance was determined at p<0.05.
Results
Early regulation of NFκB target genes by exposure of chondrocytes to 3,4,6-O-Bu3GlcNAc
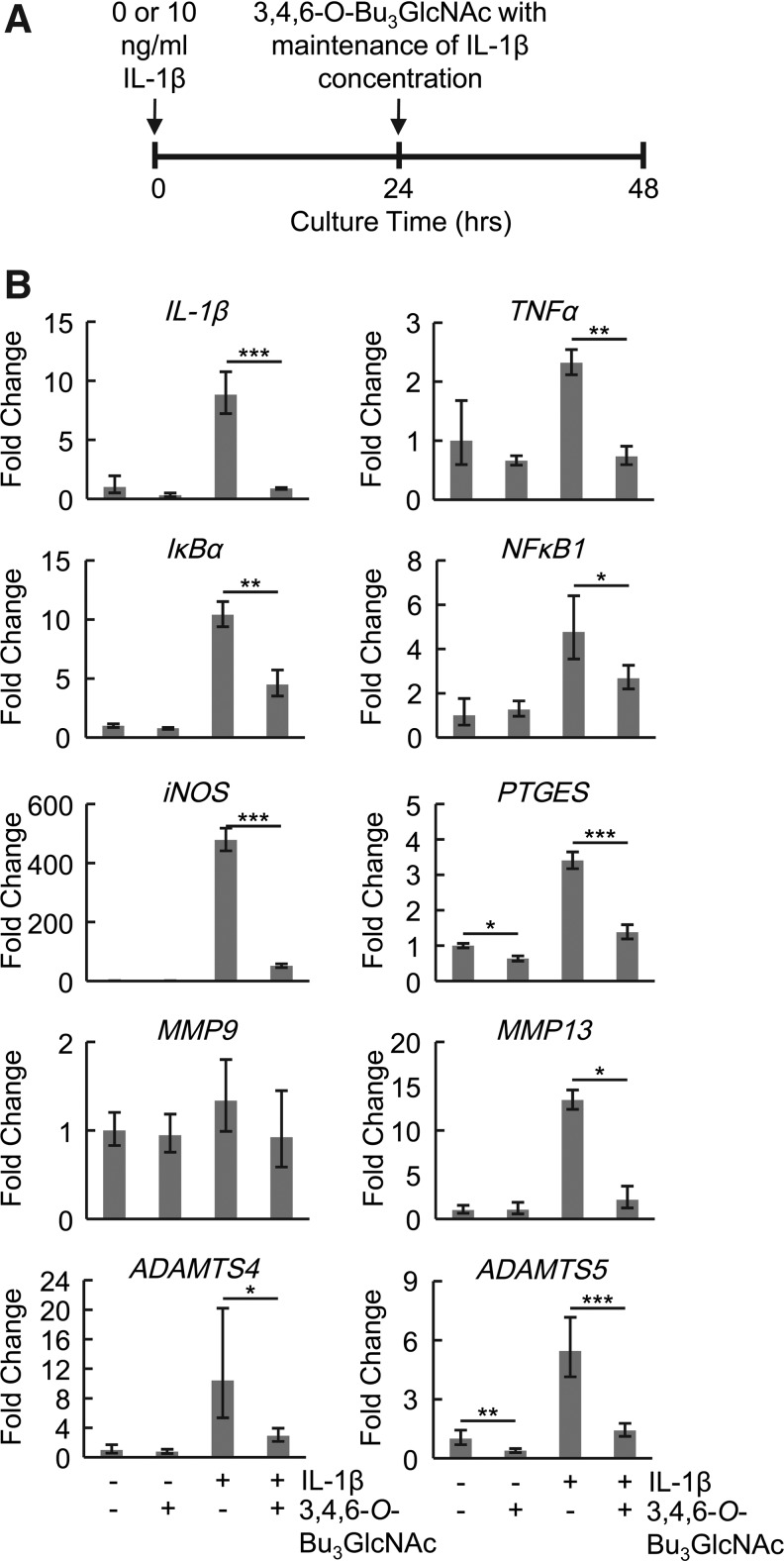
The biological impact of 3,4,6-O-Bu3GlcNAc was initially evaluated in monolayer culture over 24 h (Fig. 2). IL-1β stimulation was used to activate the NFκB activity (Fig. 1A). Genes that are transcriptional targets of NFκB and are of relevance to OA were evaluated using real-time PCR, including IL-1β, MMP13, ADAMTS4, ADAMTS5, iNOS, PTGES, and the transcription factor, NFκB1, and its inhibitor IκBα. All were upregulated after 48 h of IL-1β exposure with the exception of MMP9. Exposure of the IL-1β-stimulated chondrocytes to 3,4,6-O-Bu3GlcNAc decreased the expression levels of all genes evaluated after a 24-h exposure again with the exception of MMP9 (Fig. 2). Additionally, 3,4,6-O-Bu3GlcNAc in combination with IL-1β stimulation was able to alter expression levels of some of the genes after only 4 h of exposure, specifically IL-1β, IκBα, and iNOS (Supplementary Fig. S1).
FIG. 2.
Anti-inflammatory effects of 3,4,6-O-Bu3GlcNAc exposure on chondrocytes in monolayer culture. (A) Time line of monolayer gene expression study. (B) Expression levels of genes related to osteoarthritis and NFκB signaling in the presence of 50 μM of 3,4,6-O-Bu3GlcNAc with or without interleukin-1β (IL-1β). Data are presented as averages and SEM of 3 independent samples (*p<0.05, **p<0.01, ***p<0.001 vs. the negative control with respective IL-1β concentrations).
3,4,6-O-Bu3GlcNAc influence on ECM deposition by IL-1β-stimulated chondrocytes
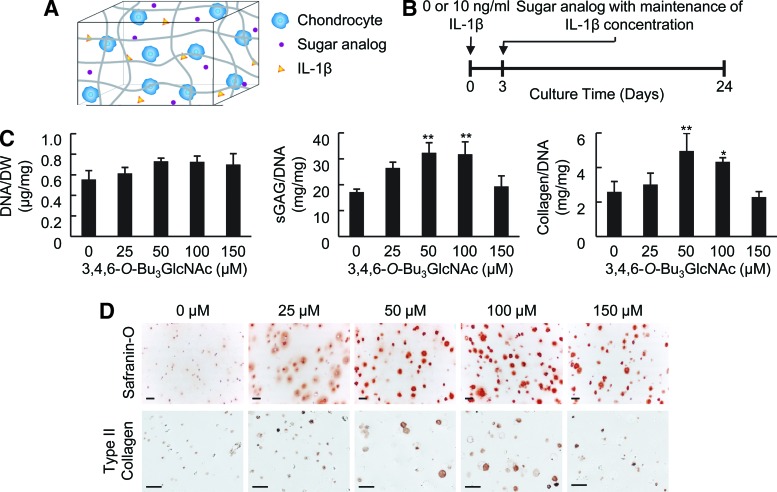
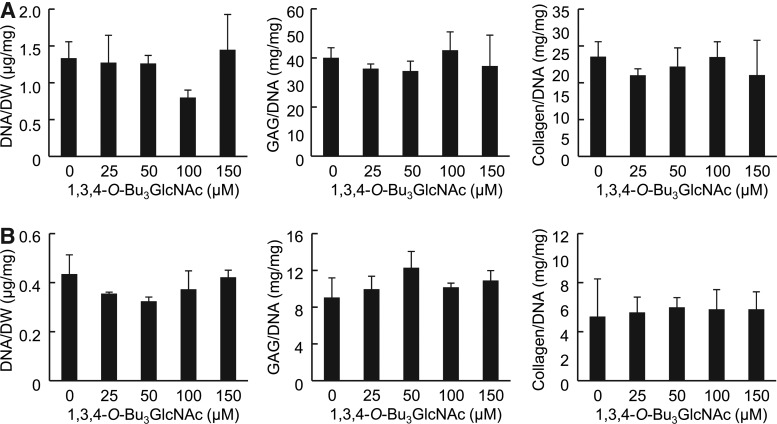
To elucidate the potential of 3,4,6-O-Bu3GlcNAc to affect ECM accumulation, we evaluated the response of chondrocytes to this molecule when cultured in 3D with or without IL-1β. Chondrocytes were encapsulated in PEGDA hydrogels to mimic a 3D tissue environment and help to maintain a chondrocytic phenotype as compared to monolayer cultures (Fig. 3A, B).19 This hydrogel system has previously been shown to support chondrocyte viability and matrix production.14,20–25 The exposure of chondrocytes to 3,4,6-O-Bu3GlcNAc did not elicit a statistically significant difference in cell viability, as evident by the DNA content of the cell-laden hydrogels, after 21 days of exposure (Figs. 3C and 4A).
FIG. 3.
Effect of 3,4,6-O-Bu3GlcNAc exposure on the biochemical content of IL-1β-stimulated chondrocytes in three-dimensional (3D) hydrogels after 21 days of exposure. (A) Schematic of a 3D culture system and (B) experimental time line (also applies to Figs. 4–6). (C) DNA normalized to dry weight, sulfated glycosaminoglycan (sGAG) normalized to the DNA content and total collagen normalized to the DNA content of the cell-laden hydrogels after 24 days of culture (n=3; * p<0.05, ** p<0.01 vs. 0 μM 3,4,6-O-Bu3GlcNAc exposure). (D) Histological staining for Safranin O and immunostaining for type II collagen of the cell-laden hydrogels (scale bars: 50 μm). An increase in both stains can be observed at as low as 25 μM 3,4,6-O-Bu3GlcNAc exposure, but is more evident at 50 μM and 100 μM 3,4,6-O-Bu3GlcNAc exposure. Color images available online at www.liebertpub.com/tea
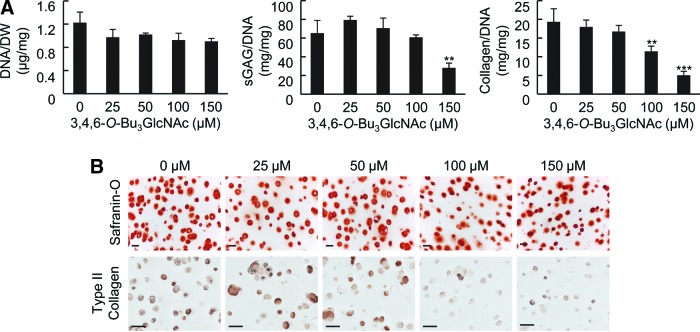
FIG. 4.
Effect of 3,4,6-O-Bu3GlcNAc exposure on the biochemical content of unstimulated (no IL-1β exposure) chondrocytes in 3D hydrogel cultures. (A) DNA normalized to dry weight, sGAG normalized to the DNA content and total collagen normalized to the DNA content of the cell-laden hydrogels after 21 days of exposure (n=3, **p<0.01, ***p<0.001). (B) Histological staining for Safranin O and immunostaining for type II collagen of the cell-laden hydrogels (scale bars: 50 μm). Color images available online at www.liebertpub.com/tea
3,4,6-O-Bu3GlcNAc stimulated sGAG accumulation. Specifically, 3,4,6-O-Bu3GlcNAc induced an increase in sGAG accumulation starting at 25 μM and continued through 100 μM 3,4,6-O-Bu3GlcNAc exposure in IL-1β-stimulated chondrocytes (Fig. 3C). This change in sGAG corresponded to an almost doubling in sGAG deposition with 50 μM 3,4,6-O-Bu3GlcNAc exposure as compared to no 3,4,6-O-Bu3GlcNAc exposure. However, the sGAG content decreased at 150 μM 3,4,6-O-Bu3GlcNAc exposure to levels similar to no analog controls. The decrease is plausibly attributed to the onset of cell stress and toxicity, which was manifested more overtly in the form of reduced cell viability in monolayer culture.26 In unstimulated chondrocytes, 3,4,6-O-Bu3GlcNAc did not have an effect on sGAG deposition until 150 μM 3,4,6-O-Bu3GlcNAc exposure (Fig. 4A), where a decrease was observed. Histological staining for proteoglycans using Safranin-O morphologically confirmed the changes in sGAG accumulations (Figs. 3D and 4B).
To further investigate the impact of 3,4,6-O-Bu3GlcNAc on ECM accumulation, the total collagen content of the cell-laden hydrogels was quantified. 3,4,6-O-Bu3GlcNAc induced a significant increase in total collagen accumulation at 50 and 100 μM 3,4,6-O-Bu3GlcNAc exposure in IL-1β-stimulated chondrocytes (Fig. 3C). This increase corresponded to an almost doubling in collagen accumulation compared to no 3,4,6-O-Bu3GlcNAc exposure. Unstimulated chondrocytes maintained collagen accumulation through 50 μM 3,4,6-O-Bu3GlcNAc exposure with a concentration-dependent decrease thereafter (Fig. 4A). As total collagen deposition captures information on the collagen content regardless of the type being produced, we sought to determine the impact 3,4,6-O-Bu3GlcNAc had on type II collagen, the most abundant type of collagen in articular cartilage, using immunohistochemistry. Similar to total collagen accumulation, 3,4,6-O-Bu3GlcNAc induced an increase in type II collagen immunostaining at 50 and 100 μM 3,4,6-O-Bu3GlcNAc exposure (Fig. 3D) in IL-1β stimulated chondrocytes. A slight increase in stain intensity was also observed at 25 and 150 μM 3,4,6-O-Bu3GlcNAc exposure as compared to no analog exposure. Unstimulated chondrocytes exhibited no differences in type II collagen immunostaining through 50 μM 3,4,6-O-Bu3GlcNAc exposure after which, a dose-dependent decrease was observed thereafter (Fig. 4B).
Unique chemical functionality required for ECM accumulation changes
After confirming the anti-inflammatory and chondroprotective potential of 3,4,6-O-Bu3GlcNAc, we next sought to ensure that the observed results were not a result of the liberated GlcNAc or butyrate generated upon hydrolysis of the ester linkage in the analog (Fig. 1C). Therefore, we evaluated the ECM accumulation by chondrocytes exposed to 1,3,4-O-Bu3GlcNAc, a molecule that has identical hydrolysis byproducts as 3,4,6-O-Bu3GlcNAc. 1,3,4-O-Bu3GlcNAc exposure produced no statistical differences in the DNA content of the cell-laden hydrogels by unstimulated (Fig. 5A) or IL-1β-stimulated chondrocytes (Fig. 5B). Additionally, 1,3,4-O-Bu3GlcNAc did not alter the sGAG and total collagen deposition at the concentrations evaluated regardless of the inflammatory state (Fig. 5A, B). Therefore, the contribution of the core GlcNAc to GAG production is expected to be occurring in our system based on literature precedent,27 the lack of activity of 1,3,4-O-Bu3GlcNAc clearly demonstrates that metabolic flux considerations have a negligible impact on increased ECM deposition. Instead, the beneficial effects of 3,4,6-O-Bu3GlcNAc emanate from the intact pharmacophore rather than from any latent effects of liberated hydrolysis products.
FIG. 5.
Effect of 1,3,4-O-Bu3GlcNAc exposure on the biochemical content on chondrocytes in 3D hydrogel cultures. (A) Chondrocytes without IL-1 β stimulation exhibited no statistical differences in DNA normalized to dry weight, sGAG normalized to the DNA content or total collagen normalized to the DNA content. (B) IL-1β-stimulated chondrocytes exhibited no statistical differences in DNA normalized to dry weight, sGAG normalized to the DNA content or total collagen normalized to the DNA content.
Altered expression of ECM and inflammatory markers in response to 3,4,6-O-Bu3GlcNAc exposure in 3D hydrogel cultures
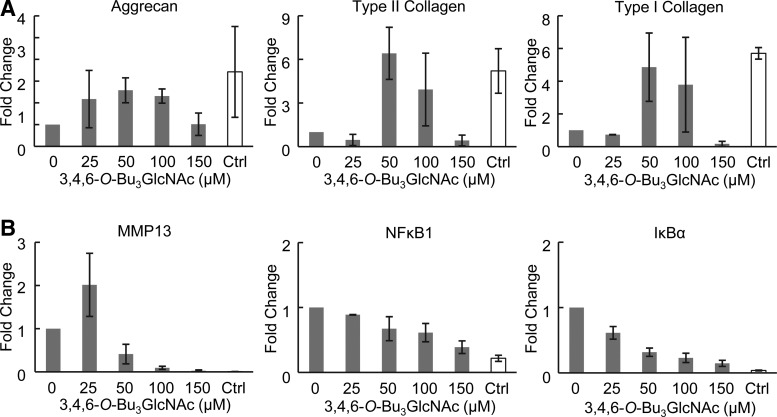
We next evaluated the gene expression changes in chondrocytes exposed to 3,4,6-O-Bu3GlcNAc for 21 days with and without IL-1β stimulation. 3,4,6-O-Bu3GlcNAc exposure increased aggrecan and type II collagen gene expression at concentrations similar to the increased ECM accumulation in IL-1β-stimulated chondryoctes (Fig. 6A). Additionally, 3,4,6-O-Bu3GlcNAc exposure increased the expression of type I collagen at intermediate concentrations in IL-1β-stimulated chondrocytes; whereas, a concentration-dependent decrease in expression was observed in unstimulated chondrocytes (Fig. 6A and Supplementary Fig. S2A). The increases observed in IL-1β-stimulated chondrocytes for aggrecan, type II collagen and type I collagen, though highly variable, approached expression levels found in unstimulated chondrocytes (Fig. 6A, gray bars compared to white bar).
FIG. 6.
Effect of 3,4,6-O-Bu3GlcNAc exposure on gene expression of IL-1β-stimulated chondrocytes in 3D hydrogel cultures after 21 days of exposure. (A) Gene expression for extracellular matrix markers, aggrecan, type II collagen and type I collagen. (B) Gene expression for inflammatory markers, MMP13, NFκB1, and IκBα. Ctrl denotes control chondrocytes without IL-1β stimulation. Data are presented as averages and SEM of RQ values (n=2 for 25 and 150 μM, n=3 for 0, 50, and 100 μM.).
To evaluate the effect of the 3,4,6-O-Bu3GlcNAc on NFκB activity after 21 days of exposure, we investigated genes transcriptionally regulated by NFκB; specifically, MMP13 for matrix degradation, and NFκB1 and IκBα for the autoregulatory behavior of NFκB. An initial increase in MMP13 expression at the lowest dose of 3,4,6-O-Bu3GlcNAc was observed followed by a decrease through 100 μM of analog exposure regardless of the inflammatory state (Fig. 6B and Supplementary Fig. S2B). With IL-1β-stimulated chondrocytes, a further decrease in MMP13 gene expression was observed at 150 μM 3,4,6-O-Bu3GlcNAc exposure (Fig. 6B). The initial increase in MMP13 gene expression observed may reflect ECM-mediated signaling as the cells begin to accumulate the ECM. 3,4,6-O-Bu3GlcNAc exposure decreased both IκBα and NFκB1 gene expression in IL-1β-stimulated chondrocytes in a concentration-dependent manner (Fig. 6B). Neither of these genes changed in unstimulated cells except at the highest concentrations studied, where an increase was observed likely due to toxic effects at high concentrations. Critical from the therapeutic perspective, exposure of the IL-1β-stimulated chondrocytes to the analog decreased MMP13, NFκB1, and IκBα expression levels near to that of unstimulated (normal, healthy) chondrocytes (Fig. 6B, gray bars compared to white bar).
Discussion
OA is characterized by changes in tissues associated with the joint, including the cartilage, underlying bone and synovium. Damage to the articular cartilage stems from ECM degradation initiated, in part, by inflammation. Proinflammatory cytokines in the osteoarthritic joint space, including IL1β, result in the activation of NFκB.28 This activation in turn increases transcription of numerous inflammatory mediators, including enzymes and cytokines that result in ECM degradation and further NFκB activation. Therefore, targeting NFκB signaling before symptomatic OA and major pathological changes may have significant therapeutic benefits in preventing OA progression28 and restoring tissue homeostasis. The findings of this study indicate that a small molecule with the potential to decrease the NFκB activity, 3,4,6-O-Bu3GlcNAc, induces ECM synthesis by IL-1β-stimulated chondrocytes, suggesting a potential role for this molecule to be included in tissue-engineering strategies for building cartilage in a pathological (OA) environment.
Investigating small molecules as disease modifying agents have become an attractive area of research and clinical investigation.29–32 Libraries of small molecules can be readily synthesized, which allows for evaluation of numerous small molecules with minor structural differences cocurrently.28,33 In this work, we utilize a SCFA-modified hexosamine, 3,4,6-O-Bu3GlcNAc, as a small molecule drug candidate for reducing osteoarthritic changes that represents an emerging paradigm wherein monosaccharides present an attractive 3D scaffold for drug discovery efforts.9 Furthermore, although sugar-based drugs have been regarded as nondruggable, hexosamines with ester-linked substituents have recently shown efficacy in an animal model of hereditary inclusion body myopathy3 and have better than expected serum stability.2 This and a similar molecule, 3,4,6-O-Bu3ManNAc, have been previously reported to reduce the NFκB activity in human embryonic kidney (HEK) AD293 cells, in contrast to the 1,3,4-O-Bu3ManNAc isomer that did not affect the NFκB activity.8 Further protein docking modeling experiments showed that 3,4,6-O-Bu3ManNAc has binding affinity for NFκB1, one of the NFκB proteins. The binding energy was lowest and therefore the strongest with 3,4,6-O-Bu3ManNAc compared to 1,3,4-O-Bu3ManNAc and Bu4ManNAc.10
Initial stages of OA onset are difficult to identify. The initiating events in OA remain unclear, but may result from biomechanical forces that activate localized inflammatory processes originating at the level of both the chondrocytes and synovial lining cells.5 Utilizing IL-1β stimulation of chondrocytes as a model for an OA phenotype, we examined the effects of 3,4,6-O-Bu3GlcNAc on early gene expression changes related to NFκB activation that are implicated in OA. 3,4,6-O-Bu3GlcNAc decreased the expression of all NFκB target genes that we studied (with the exception of MMP9 as it was not found to be changed upon IL-1β stimulation). The gene expression changes we observed cannot be unambiguously attributed to reduced NFkB activity because hexosamine analogs may also affect other signaling pathways. However, the altered expression of NFκB target genes we observed is consistent with the hypothesis that a significant portion of the beneficial effects of 3,4,6-O-Bu3GlcNAc is realized through decreased NFκB activity. These results, as well as the previous findings of reduced NFκB activity in cancer cells, indicate that this molecule may have chondroprotective effects in preventing inflammation-related tissue damage.
As early stages of OA are marked by an increase in catabolic enzymes resulting in ECM loss and concomitant tissue degeneration, therapeutics that increase ECM synthesis and accumulation are of importance to maintain proper tissue mechanics. After establishing that 3,4,6-O-Bu3GlcNAc exposure decreases the expression levels of MMP13, ADAMTS4 and ADAMTS5, degradation enzymes regulated by NFκB and increased in OA tissue, we demonstrated that prolonged 3,4,6-O-Bu3GlcNAc exposure resulted in an increased ECM accumulation by IL-1β-stimulated chondrocytes. Specifically, sGAG and type II collagen, both of which are abundant in articular cartilage and necessary for proper mechanical loading, increased with analog exposure.34 Both decreased ECM degradation and increased ECM synthesis play a role in the observed tissue accumulation as evident by the spatial distribution of proteoglycans observed histologically. Anabolic effects of the analog were supported by increases in both aggrecan and type II collagen gene expression at similar 3,4,6-O-Bu3GlcNAc concentrations as the increased ECM accumulation. Additionally, histological changes in the localization of proteoglycans were observed with different concentrations of 3,4,6-O-Bu3GlcNAc exposure. Specifically, at 25 μM 3,4,6-O-Bu3GlcNAc exposure, the proteoglycans were diffuse and radiated beyond the pericellular matrix of the chondrocytes, indicative of smaller degradation fragments. At 50 and 100 μM 3,4,6-O-Bu3GlcNAc exposure, the proteoglycans were retained in the pericellular space, supporting the presence of larger matrix molecules that were unable to diffuse through the hydrogel. This difference in the GAG structure was even more evident when comparing the 0 and 150 μM 3,4,6-O-Bu3GlcNAc treatment, where quantification of sGAG was equivalent, but proteoglycan staining was spatially different (the sample without analog exhibited very diffuse staining with no pericellular matrix, while 150 μM 3,4,6-O-Bu3GlcNAc exposure exhibited pericellular matrix accumulation). Combined, these results support the hypothesis that both decreased ECM degradation and increased ECM synthesis play a pivotal role in altering the disease state of the cells with analog treatment.
The increased ECM deposition observed with 3,4,6-O-Bu3GlcNAc exposure is unlikely due to the hydrolysis byproducts of the molecule, n-butyrate and GlcNAc, as the isomer 1,3,4-O-Bu3GlcNAc did not have an effect on matrix deposition. Although the intact molecule is required for the primary biological effect, the hydrolysis products are unlikely to have negative side effects especially at these low concentrations. N-Acetylglucosamine has been shown to have anti-inflammatory effects on chondrocytes by reducing nitric oxide and IL-6 levels via inhibiting cytokine-induce gene expression.35 Hwang et al. reported that GlcNAc reduced reactive oxygen species formation, and MMP1 and MMP13 activity in UV-B irritated human dermal fibroblasts.36 Furthermore, Shikhman et al. showed that GlcNAc has chondroprotective and chondroinductive effects in an in vivo model of OA.37 However, these previous studies were performed at concentrations significantly higher than the target concentrations observed in the current studies (millimolar vs. micromolar range). The other degradation byproduct, sodium butyrate, has been shown to decrease levels of nitric oxide and PGE2 in IL-1β-stimulated chondrocytes in the culture medium at concentrations of 5 mM and 10 mM.38 Sodium butyrate was also shown to decrease proteoglycans loss into the culture medium at those concentrations.38
The target concentration for the observed effects is in the micromolar range. This concentration is significantly lower than the target concentration observed for glucosamine and GlcNAc alone. However, this concentration is generally considered a nonstarter for drug discovery research. However, intra-articular injections would circumvent this concern. Intra-articular delivery of viscosupplements and corticosteroids is already a common clinical intervention to relieve OA symptoms.11,39 Additionally, controlled-release systems for intra-articular injections would allow for sustained, target concentrations over days, weeks, or even months. From a biomaterial perspective, incorporation of 3,4,6-O-Bu3GlcNAc into biomaterial scaffolds would allow for tissue-engineering discrete cartilage defects and the adjacent tissues.
In conclusion, we demonstrated that 3,4,6-O-Bu3GlcNAc, and not its hydrolysis byproducts, increases cartilage-specific ECM accumulation by IL-1β-stimulated chondrocytes, but not unstimulated chondrocytes. The increased matrix accumulation is likely due to the molecule's ability to decrease the effect of IL-1β stimulation on the NFκB activity. 3,4,6-O-Bu3GlcNAc may have therapeutic potential as a disease modifying drug candidate for reducing progression or prevention of OA.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (R01AR054005). J.M.C. was supported, in part, by a Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship (F31AG033999).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence R.C. Felson D.T. Helmick C.G. Arnold L.M. Choi H. Deyo R.A. Gabriel S. Hirsch R. Hochberg M.C. Hunder G.G. Jordan J.M. Katz J.N. Kremers H.M. Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew M.P. Tan E. Shah S. Bhattacharya R. Adam Meledeo M. Huang J. Espinoza F.A. Yarema K.J. Extracellular and intracellular esterase processing of SCFA-hexosamine analogs: implications for metabolic glycoengineering and drug delivery. Bioorg Med Chem Lett. 2012;22:6929. doi: 10.1016/j.bmcl.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malicdan M.C. Noguchi S. Tokutomi T. Goto Y. Nonaka I. Hayashi Y.K. Nishino I. Peracetylated N-acetylmannosamine, a synthetic sugar molecule, efficiently rescues muscle phenotype and biochemical defects in mouse model of sialic acid-deficient myopathy. J Biol Chem. 2012;287:2689. doi: 10.1074/jbc.M111.297051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedbom E. Häuselmann H.J. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci. 2002;59:45. doi: 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito M.J. Veale D.J. FitzGerald O. van den Berg W.B. Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahl H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 7.Moos V. Fickert S. Muller B. Weber U. Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999;26:870. [PubMed] [Google Scholar]
- 8.Campbell C.T. Aich U. Weier C.A. Wang J.J. Choi S.S. Wen M.M. Maisel K. Sampathkumar S.-G. Yarema K.J. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogues inhibits the mobility of metastatic MDA-MB-231 breast cancer cells. J Med Chem. 2008;51:8135. doi: 10.1021/jm800873k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meutermans W. Le G.T. Becker B. Carbohydrates as scaffolds in drug discovery. ChemMedChem. 2006;1:1164. doi: 10.1002/cmdc.200600150. [DOI] [PubMed] [Google Scholar]
- 10.Elmouelhi N. Aich U. Paruchuri V.D. Meledeo M.A. Campbell C.T. Wang J.J. Srinivas R. Khanna H.S. Yarema K.J. Hexosamine template. A platform for modulating gene expression and for sugar-based drug discovery. J Med Chem. 2009;52:2515. doi: 10.1021/jm801661m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutjes A.W. Juni P. da Costa B.R. Trelle S. Nuesch E. Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 12.Aich U. Campbell C.T. Elmouelhi N. Weier C.A. Sampathkumar S.G. Choi S.S. Yarema K.J. Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxicity and MUC1 suppression. ACS Chem Biol. 2008;3:230. doi: 10.1021/cb7002708. [DOI] [PubMed] [Google Scholar]
- 13.Kim E.J. Sampathkumar S.G. Jones M.B. Rhee J.K. Baskaran G. Goon S. Yarema K.J. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acyl-modified N-acetylmannosamine analogs in Jurkat cells. J Biol Chem. 2004;279:18342. doi: 10.1074/jbc.M400205200. [DOI] [PubMed] [Google Scholar]
- 14.Kim T.K. Sharma B. Williams C.G. Ruffner M.A. Malik A. McFarland E.G. Elisseeff J.H. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y.-J. Sah R.L.Y. Doong J.-Y.H. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 16.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Woessner J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 18.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Stokes D.G. Liu G. Coimbra I.B. Piera-Velazquez S. Crowl R.M. Jiménez S.A. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002;46:404. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- 20.Sharma B. Williams C.G. Kim T.K. Sun D. Malik A. Khan M. Leong K. Elisseeff J.H. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 21.Bryant S.J. Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 22.Lee W.K. Ichi T. Ooya T. Yamamoto T. Katoh M. Yui N. Novel poly(ethylene glycol) scaffolds crosslinked by hydrolyzable polyrotaxane for cartilage tissue engineering. J Biomed Mater Res A. 2003;67:1087. doi: 10.1002/jbm.a.10570. [DOI] [PubMed] [Google Scholar]
- 23.Lin-Gibson S. Bencherif S. Cooper J.A. Wetzel S.J. Antonucci J.M. Vogel B.M. Horkay F. Washburn N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 24.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Sims C.D. Butler P.E. Casanova R. Lee B.T. Randolph M.A. Lee W.P. Vacanti C.A. Yaremchuk M.J. Injectable cartilage using polyethylene oxide polymer substrates. Plast Reconstr Surg. 1996;98:843. doi: 10.1097/00006534-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Coburn J.M. Bernstein N. Bhattacharya R. Aich U. Yarema K.J. Elisseeff J.H. Differential response of chondrocytes and chondrogenic-induced mesenchymal stem cells to C1-OH tributanoylated N-acetylhexosamines. PLoS One. 2013;8:e58899. doi: 10.1371/journal.pone.0058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhakar V. Sasisekharan R. The biosynthesis and catabolism of galactosaminoglycans. In: Nicola V., editor. Advances in Pharmacology. Vol. 53. Academic Press; 2006. p. 69. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X. Smith D.L. Meriin A.B. Engemann S. Russel D.E. Roark M. Washington S.L. Maxwell M.M. Marsh J.L. Thompson L.M. Wanker E.E. Young A.B. Housman D.E. Bates G.P. Sherman M.Y. Kazantsev A.G. A potent small molecule inhibits polyglutamine aggregation in Huntington's disease neurons and suppresses neurodegeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:892. doi: 10.1073/pnas.0408936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friman S. Arns W. Nashan B. Vincenti F. Banas B. Budde K. Cibrik D. Chan L. Klempnauer J. Mulgaonkar S. Nicholson M. Wahlberg J. Wissing K.M. Abrams K. Witte S. Woodle E.S. Sotrastaurin, a novel small molecule inhibiting protein-kinase C: randomized phase II study in renal transplant recipients. Am J Translant. 2011;11:1444. doi: 10.1111/j.1600-6143.2011.03538.x. [DOI] [PubMed] [Google Scholar]
- 30.MacMillan K.S. Naidoo J. Liang J. Melito L. Williams N.S. Morlock L. Huntington P.J. Estill S.J. Longgood J. Becker G.L. McKnight S.L. Pieper A.A. De Brabander J.K. Ready J.M. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc. 2011;133:1428. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong C.C. Yu P.B. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne J.C. Lambert P.D. Schenk S. Carney D.P. Smith J.J. Gagne D.J. Jin L. Boss O. Perni R.B. Vu C.B. Bemis J.E. Xie R. Disch J.S. Ng P.Y. Nunes J.J. Lynch A.V. Yang H. Galonek H. Israelian K. Choy W. Iffland A. Lavu S. Medvedik O. Sinclair D.A. Olefsky J.M. Jirousek M.R. Elliott P.J. Westphal C.H. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calamini B. Silva M.C. Madoux F. Hutt D.M. Khanna S. Chalfant M.A. Saldanha S.A. Hodder P. Tait B.D. Garza D. Balch W.E. Morimoto R.I. Small-molecule proteostasis regulators for protein conformational diseases. Nat Chem Biol. 2012;8:185. doi: 10.1038/nchembio.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mow V.C. Ratcliffe A. Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 35.Shikhman A.R. Kuhn K. Alaaeddine N. Lotz M. N-Acetylglucosamine prevents IL-1β-mediated activation of human chondrocytes. J Immunol. 2001;166:5155. doi: 10.4049/jimmunol.166.8.5155. [DOI] [PubMed] [Google Scholar]
- 36.Hwang Y.P. Kim H.G. Han E.H. Choi J.H. Park B.H. Jung K.H. Shin Y.C. Jeong H.G. N-Acetylglucosamine suppress collagenases activation in ultraviolet B-irradiated human dermal fibroblasts: involvement of calcium ions and mitogen-activated protein kinases. J Dermatol Sci. 2011;63:93. doi: 10.1016/j.jdermsci.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Shikhman A.R. Amiel D. D'Lima D. Hwang S.B. Hu C. Xu A. Hashimoto S. Kobayashi K. Sasho T. Lotz M.K. Chondroprotective activity of N-acetylglucosamine in rabbits with experimental osteoarthritis. Ann Rheum Dis. 2005;64:89. doi: 10.1136/ard.2003.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chabane N. Zayed N. Afif H. Mfuna-Endam L. Benderdour M. Boileau C. Martel-Pelletier J. Pelletier J.P. Duval N. Fahmi H. Histone deacetylase inhibitors suppress interleukin-1β-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis Cartilage. 2008;16:1267. doi: 10.1016/j.joca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Stephens M.B. Beutler A.I. O'Connor F.G. Musculoskeletal injections: a review of the evidence. Am Fam Physician. 2008;78:971. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.