Abstract
Breastfeeding protects the neonate against pathogen infection. Major mechanisms of protection include human milk glycoconjugates functioning as soluble receptor mimetics that inhibit pathogen binding to the mucosal cell surface, prebiotic stimulation of gut colonization by favorable microbiota, immunomodulation, and as a substrate for bacterial fermentation products in the gut. Human milk proteins are predominantly glycosylated, and some biological functions of these human milk glycoproteins (HMGPs) have been reported. HMGPs range in size from 14 kDa to 2,000 kDa and include mucins, secretory immunoglobulin A, bile salt-stimulated lipase, lactoferrin, butyrophilin, lactadherin, leptin, and adiponectin. This review summarizes known biological roles of HMGPs that may contribute to the ability of human milk to protect neonates from disease.
Introduction
Human milk is widely accepted as containing an ideal mixture of nutrients for infants, while also conveying immunologic and other health benefits.1 Glycans in human milk contain oligosaccharide moieties in their free and conjugated form, and many function as competitive inhibitors of pathogen binding, thereby protecting infants against infection.2 The most plentiful and well-defined inhibitors of pathogen binding are the human milk oligosaccharides, but human milk glycoproteins (HMGPs) are also principal components of human milk that, in aggregate, display inhibitory activity against a broad spectrum of pathogens.
HMGPs vary in size, structure, and abundance. More than 400 proteins, most of which are glycosylated, have been identified in human milk by mass spectrometry.3 Some of these HMGPs have shown activity that might protect infants against pathogens. In many cases, glycoproteins with reported activities were isolated from milk of other species, especially cows. Glycosylation of human milk proteins differs from that of glycoproteins from other milks. Therefore, only published data regarding HMGPs were selected in this review. Much of the published evidence for biological activities is for those molecules present in milk at relatively high concentrations. Of these, the HMGPs whose activities are most widely recognized in the literature include mucins, secretory immunoglobulin A (sIgA), xanthine dehydrogenase/oxidase, bile salt-stimulated lipase (BSSL), lactoferrin, lactoperoxidase, butyrophilin, lactadherin, adiponectin, β-casein, κ-casein, leptin, lysozyme, and α-lactalbumin, and these are included in this review. The molecular sizes and concentrations of these HMGPs are presented in Table 1. Major HMGPs protect against microbial infection4 and excessive inflammatory responses in vitro.5 This suggests that HMGPs may be important for the nursing mother to protect her immature infant against pathogen infection and other pathologies. HMGPs that are known to modulate human pathophysiology are described herein.
Table 1.
Molecular Size and Concentration of Major Human Milk Glycoproteins
| Glycoprotein | Molecular size (kDa) | Concentration (mg/L) |
|---|---|---|
| Mucins | 200–2,000 | 729±756 |
| sIgA | 160 | 200–6,2007 |
| Xanthine dehydrogenase/oxidase | 146 | Not reported |
| BSSL | 120–140 | 100–2008 |
| Lactoferrin | 80 | 1,000–7,0009 |
| Lactoperoxidase | 77.5 | 0.77±0.3810 |
| Butyrophilin | 66 | 41±36 |
| Lactadherin | 46 | 93±106 |
| Adiponectin | 30 | 4–8811 |
| β-Casein | 24 | 4,670±89012 |
| κ-Casein | 19 | 100–4,6007 |
| Leptin | 16 | 0.00313 |
| Lysozyme | 14.4 | 21±1314 |
| α-Lactalbumin | 14.2 | 2,440±64015 |
BSSL, bile salt-stimulated lipase; sIgA, secretory immunoglobulin A.
Mucins
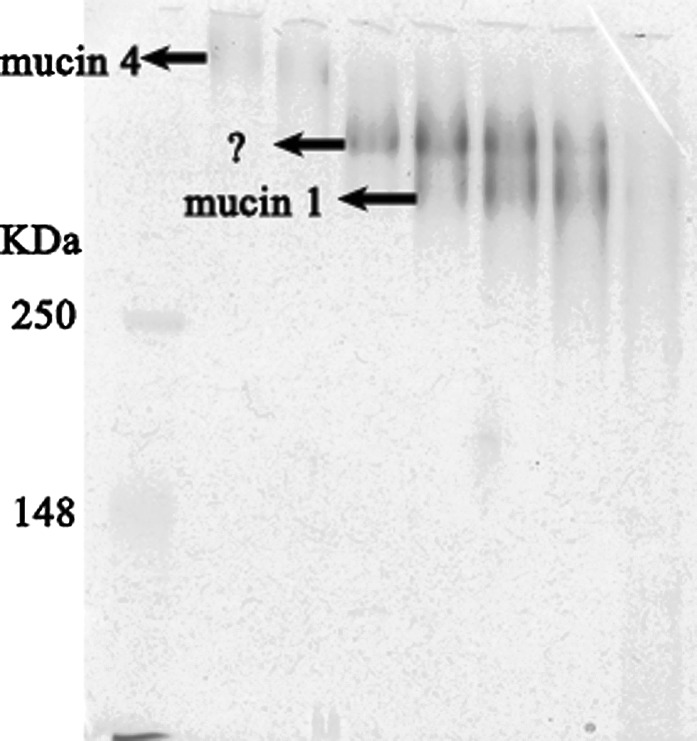
Mucins are high-molecular-mass glycoproteins ranging from about 200 kDa to 2,000 kDa in size. Mucins are major components of the extracellular matrix and are involved in diverse functions, including shielding the epithelium against pathogenic infection, regulating cellular signaling, and transcription.16 The mucin family of large, heavily glycosylated proteins are characterized by a variable number of tandem repeats termed the mucin domain, which makes up much of the protein component of mucus. At least 16 mucins have been identified in humans, and the expression profile of the mucins varies among tissues, with the gastrointestinal tract showing the highest and most diverse expression. The mucin family can be divided into three subfamilies according to their location relative to the cell surface: (a) gel-forming (secreted) mucins, such as mucin 1, mucin 4, and mucin 16; (b) cell surface (transmembrane, membrane-tethered) mucins, such as mucin 2, mucin 5, and mucin 6; and (c) secreted non–gel-forming mucins, such as mucin 7.17 The physical characteristics of the mucins (i.e., their large size and hydrophobicity) can make them difficult to isolate and purify, especially from the complex matrix of milk. That notwithstanding, the major human milk mucins have been identified, initially as mucin 1 and a higher-molecular-weight electrophoresis band,18,19 designated mucin X.20,21 Recently our laboratory purified mucin 4 from human milk and identified it (Fig. 1)22; mucin 4 seems to be the band previously designated mucin X. We observe another band that runs between mucin 1 and mucin 4 (Fig. 1), which is currently under investigation. Other types of mucins have not been isolated from human milk to date, but our research indicates that some others may be present in minor amounts.
FIG. 1.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of human milk mucins from a pool of 20 human milk donors. The 4–12% gradient sodium dodecyl sulfate–polyacrylamide gel–electrophoresed gel was stained with periodic acid–Schiff reagent. Mucin 1 and mucin 4 were identified by western blot. The arrows indicate mucin 1, mucin 4, and an unknown band.
Mucin 1 and mucin 4 are dimers; each dimer is formed by cleavage of an intact single peptide product of a single gene (Fig. 2). The larger subunit is wholly extracellular, heavily glycosylated, and almost entirely composed of a variable number of tandem repeats.16 Mucin 1 and mucin 4 can interact with microorganisms (Table 2). The most commonly studied mechanism is a sialic acid moiety of mucin 1 interacting with the pathogen, thereby inhibiting the ability of the pathogen to bind to its infant host cell surface glycan receptor. Thus, mucin 1 plays a role in innate immune defense of the infant against invading microorganisms. However, other human milk mucins, like mucin 4, have only begun to be investigated for their role in interaction with microorganisms. These data would help understand the full biological role of human milk mucins in protecting infants.
FIG. 2.
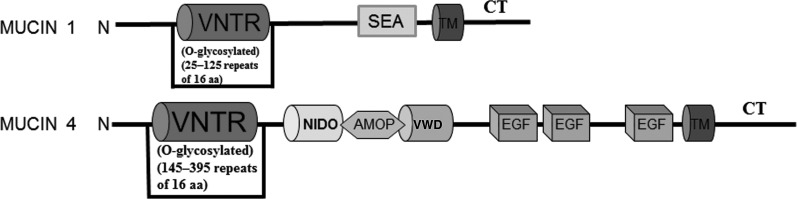
Structural motifs of mucin 1 and mucin 4. Key domains include the following: a variable number of tandem repeats (VNTR); sperm protein, exterokinase, and agrin (SEA) modules; transmembrane (TM) domains; cytoplasmic tail (CT); nidogen homology sequence (NIDO); adhesion-associated domain in mucin 4 and other proteins (AMOP); von Willebrand factor D sequence (VWD); and epidermal growth factor (EGF)-like regions. aa, amino acids.
Table 2.
Known Pathogens That Interact with Human Milk Mucin 1 and Mucin 4
| Mucin | Molecular size | Microorganism |
|---|---|---|
| Mucin 1 | ∼400 kDa | HIV23 |
| Rotavirus24 | ||
| Escherichia coli25 | ||
| Salmonella22 | ||
| Mucin 4 | ∼900 kDa16 | Salmonella22 |
HIV, human immunodeficiency virus.
sIgA
sIgA is the principal immunoglobulin in human milk. Typical sIgA consists of two monomeric IgA units and two additional polypeptide chains: the J chain and the secretory component (SC). The heavy and light chains in plasma cells assemble into IgA, which on association with J chain become polymerized; subsequently, SC is added during transport across the epithelium.26 sIgA is present at quite high concentrations in colostrum and is consistently present at substantial concentrations throughout lactation. However, only 72% of sIgA activity survives pasteurization at 62.5°C for 30 minutes.27 When first detected in human milk, sIgA was considered the first protective line of defense against pathogens because of its involvement in extracellular neutralization of pathogen infectivity and its intracellular neutralization of bacterial lipopolysaccharide and viruses within epithelial cells. Human milk sIgA protects infants against human pathogens (Table 3); when sIgA specifically binds to a pathogen antigen, it renders the pathogen less infective. In contrast, the sugar on sIgA plays more general structural and functional roles.28 sIgAs are resistant to proteolytic digestion in the gut, and this resistance is most often attributed to the glycan sugar moieties attached to secretory antibodies. These glycans also participate in intracellular trafficking of the antibodies in the cell. As with some other glycoproteins, the glycans of sIgA containing galactose, sialic acid, mannose, or fucose can act as decoys to prevent binding by pathogenic bacteria to their glycosylated targets on mucosal surfaces in the gut.28 For example, the mannose-containing oligosaccharides of sIgA can inhibit Vibrio cholerae biofilm formation.29 The glycosylation of sIgAs in general (irrespective of the antigen specificity of the antibody) may provide a broad-spectrum antipathogen activity that complements the very specific antigen binding inhibition by the protein portion of specific antibodies.
Table 3.
Antipathogen Activities of Human Milk Secretory Immunoglobulin A
| Pathogen | |
|---|---|
| Bacteria | Clostridium botulinum30 |
| Clostridium difficile33 | |
| Escherichia coli36 | |
| Haemophilus influenzae38 | |
| Mycobacterium tuberculosis40 | |
| Salmonella typhimurium42 | |
| Shigellae44 | |
| Staphylococcus aureus46 | |
| Helicobacter pylori47 | |
| Vibrio cholerae29 | |
| Viruses | Coxsackie B4 virus31 |
| Norovirus34 | |
| Rotavirus37 | |
| Poliovirus39 | |
| Rubella41 | |
| Measles43 | |
| HIV45 | |
| Fungi/protozoa | Candida albicans32 |
| Entamoeba histolytica35 |
HIV, human immunodeficiency virus.
BSSL
BSSL, a major glycoprotein in human milk, functions in milk lipid digestion.48 BSSL is present in human milk at a concentration of between 100 and 200 μg/mL.49 Human milk BSSL migrates as a heterogeneous protein with an apparent molecular size of 120–140 kDa on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (conditions that disaggregate proteins).50 BSSL contains 722 amino acids and an N-glycosylation site at asparagine-187. The catalytically active site of BSSL is located at serine-194.51 BSSL exhibits a triglyceridase activity that may aid in the fat digestion in newborns, particularly in preterm infants who have low lipase activity and poor lipid utilization.52 Heating human milk to 40–55°C for 39 minutes (typical pasteurization conditions for donor human milk is 65°C for 30 minutes) destroys the activity of BSSL and results in decreased lipid absorption in premature infants.53 BSSL may have essential functions in lipid digestion in term infants, as this enzyme has uniquely wide substrate specificity. It hydrolyzes mono-, di-, and triacylglycerols, cholesterol esters, and diacylphosphatidylglycerols and can hydrolyze these lipids in both micellar and water-soluble forms.52 Human milk BSSL has other nonenzymatic functions: it binds dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells.54 Human milk BSSL inhibits the binding of Norwalk virus capsids to their carbohydrate ligands; the tandem repeat O-glycosylated sequences of BSSL may act as decoy receptors for the Norwalk virus.55 Thus, human milk BSSL is an example of an HMGP with multiple functions: it degrades a large spectrum of lipids, which is an essential role in nutrition, and also inhibits virus invasion.
Lactoferrin
Lactoferrin, an 80-kDa iron-binding glycoprotein, was first isolated from bovine milk and subsequently from human milk.56,57 It is abundant in colostrum at up to 7 g/L, and its concentration declines sevenfold as lactation progresses.58–60 After pasteurization at 62.5°C for 30 minutes, only 39% of the original lactoferrin remains in the milk.27 This major protein of human milk chelates free iron, which may assist in iron absorption by the infant, and iron chelation also inhibits bacterial growth. Thus, its biological functions range from antimicrobial activities against a large panel of microorganisms, including bacteria, viruses, fungi, and parasites, to regulation of cellular proliferation and differentiation, as well as anti-inflammatory and anticancer activities.61
Lactoferrin is a polypeptide chain of about 700 amino acids and forms two homologous globular domains: the N-and C-lobes. The N-lobe corresponds to amino acid residues 1–333, and the C-lobe corresponds to positions 345–692; the ends of those domains are connected by a short α-helix.62 Each lobe can reversibly bind one ferric ion. Lactoferrin exhibits bacteriostatic activity against a wide range of bacteria because of its ability to chelate iron, which is essential for microbial growth. Lactoferrin also displays innate antibacterial, antivirus, antifungal, and antiprotozoan activity that may be independent of iron chelation, for example, through disruption of the bacterial cell membranes or blocking of cell–virus interactions63,64 (Table 4).
Table 4.
Antipathogen Activities of Human Milk Lactoferrin
| Pathogen | |
|---|---|
| Bacteria | Escherichia coli65 |
| Salmonella typhimurium66 | |
| Shigella dysenteriae67 | |
| Listeria monocytogenes68 | |
| Streptococcus spp.69 | |
| Vibrio cholerae70 | |
| Legionella pneumophila71 | |
| Bacillus stearothermophilus72 | |
| Bacillus subtilis73 | |
| Viruses | Rotavirus74,75 |
| HIV76 | |
| Herpes simplex virus77,78 | |
| Cytomegalovirus79 | |
| Hepatitis virus80,81 | |
| Human papillomavirus82 | |
| Adenovirus83 | |
| Fungi/protozoa | Candida spp.84 |
| Entamoeba histolytica85 | |
| Tritrichomonas foetus86 | |
| Eimeria stiedai87 |
HIV, human immunodeficiency virus.
Moreover, lactoferrin is a key modulator of inflammatory and immune responses,88 revealing host-protective effects not only against microbial infections but also in inflammatory disorders such as cancer, allergies, and arthritis.89 These activities may be mediated through modulation of the immune system, such as binding to lipopolysaccharide, inhibition of several cytokines (tumor necrosis factor-α and interleukin-1β), or binding to bacterial nonmethylated cytosine–guanosine motifs.64 Lactoferrin elevates the number and the activity of T and B lymphocytes and natural killer cells, stimulates the release of several cytokines, accelerates the maturation of T and B cells, and elevates the expression of several types of cellular receptors.80 Moreover, lactoferrin protects against chemically induced carcinogenesis, tumor growth, and/or metastasis in several animal model experiments. It targets tumors of specific organs, such as the esophagus, tongue, lung, liver, colon, and bladder.64
Butyrophilin
A major feature of milk is the specialized structure that allows the stable dispersion of fat droplets, denoted the milk fat globule. It is surrounded by the milk fat globule membrane, which is derived from the maternal mammary epithelium, and contains a large and complex glycocalyx (i.e., its extracellular matrix). When isolated, this membrane exhibits four major protein bands on gel electrophoresis: butyrophilin, mucins, lactadherin, and xanthene oxidase. Butyrophilin is the prominent band of apparent 66 kDa in Coomassie Brilliant Blue–stained gels.90–92 It is a type I transmembrane glycoprotein with a cytoplasmic C-terminal tail.93 Butyrophilin is expressed only during lactation and appears to be essential for milk fat globule production. Butyrophilin may function as an integral receptor for cytoplasmic fat droplets; budding of the droplets at the cell surface is initiated by interactions between the cytoplasmic tail of butyrophilin and other proteins, notably the redox enzyme xanthine oxidase.93 Butyrophilin functions in vivo to stabilize the association of xanthine oxidase with the milk fat globule membrane by direct interactions through the PRY/SPRY/B30.2 domain.94 The autoimmune encephalomyelitis that follows immunization with myelin/oligodentrocyte glycoprotein is prevented by butyrophilin treatment, which also improves the clinical manifestations of preexisting disease.95
Lactadherin
Lactadherin is the 46-kDa glycoprotein of the human milk fat globule membrane, which is also known as PAS-6/7, indicating the two glycosylation variants on sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis.96 Human milk lactadherin binds specifically to rotavirus and inhibits its replication, thereby protecting human milk-fed infants against symptomatic rotavirus infection.97 Human lactadherin inhibits rotavirus infection of MA 104 and Caco-2 cells by direct interaction between rotavirus and the oligosaccharides of the lactadherin molecule.98 Removal of sialic acid from lactadherin results in loss of this inhibitory activity.99 The protein core also exhibits specific biological activities: lactadherin exhibits a vascular endothelial growth factor pro-angiogenic effect in adult neovascularization,100 suggesting a use in modulating blood vessel growth in a pathological setting. Lactadherin has EGF1–EGF2 domains (epidermal growth factor homology) at the amino-terminus and C1 and C2 domains that share homology to the phosphatidylserine-binding domains of blood coagulation factors V and VIII96,101; lactadherin can mediate clearance of phosphatidylserine-expressing procoagulant platelet-derived microvesicles.102 Milk lactadherin is present in the intestines of breastfed infants before the tight junctions of the intestinal epithelium close and when fat complexes can cross the mucosa by bulk transport. Thus, human milk lactadherin could gain access to the circulation of the neonate, where its strong anticoagulant effects (half-maximal concentration is 1–4 nM) would be mediated through modulating factor V and VIII activities and through microvesicle clearance. Although the potential function of lactadherin is not understood, it may participate in early homeostasis of circulating cells; also, many diseases induce strong procoagulation processes, including sepsis, suggesting other possible domains of protective activity. Indeed, a recent report indicates that recombinant lactadherin may attenuate sepsis-induced apoptosis.103 Lactadherin interacts with damaged intestinal epithelium in vivo and plays an important role in stimulating growth of intestinal epithelial cells in vitro.104 Thus, orally ingested lactadherin could have potential in the prevention and treatment of intestinal injury in infants.
Leptin and Adiponectin
Leptin and adiponectin are members of adipose-secreted glycoprotein metabolic regulators known as adipokines105 that are present in human milk.11,106,107 Various adipokines have pro-inflammatory or anti-inflammatory activities and have potential as regulators of metabolic function.108 Leptin is a 16-kDa glycoprotein hormone that regulates energy intake and energy expenditure, including appetite and metabolism.109 In experimental studies on animals, leptin is transferred from the maternal circulation to breastmilk and then passes to neonatal blood, suggesting that maternal leptin may exert biological effects on the infant.110 Adiponectin is a 30-kDa glycoprotein produced primarily in adipose tissue and participates in several physiologic processes that may affect human development.11 Human milk adiponectin was first reported in 2006, and it seems to play a role in the early growth and development of breastfed infants.11 Furthermore, adiponectin inhibits the proliferation of myelomonocytic progenitor cells and induces apoptosis, and this may contribute to the anti-inflammatory effects of this adiponectin.111 Immunoreactive adiponectin was detected in skim milk at concentrations significantly higher than those of milk leptin.110 The leptin/adiponectin ratio in mid-infancy correlates with weight gain in healthy term infants.112 Thus, leptin and adiponectin in human milk may play a role in growth and development of infants.
Other Glycoproteins in Human Milk
For those with sufficient data, a section is dedicated to reviewing their activities (above); those with limited published data on antipathogen activity are summarized in Table 5.
Table 5.
Antipathogen Activities of Other Human Milk Glycoproteins
| HMGP | Pathogen |
|---|---|
| β-Casein | Haemophilus influenzae,113 streptococci114 |
| κ-Casein | Helicobacter pylori115 |
| α-Lactalbumin | Reovirus,116 streptococci117 |
| Lysozyme | Escherichia coli118 |
| Lactoperoxidase | Helicobacter pylori,119 HIV119 |
| Xanthine dehydrogenase/oxidase | Burkholderia cepacia120 |
HIV, human immunodeficiency virus; HMGP, human milk glycoprotein.
Future Directions and Implications
Described above are many examples of HMGPs that inhibit the pathobiology of human diseases. Prevalent among these inhibitory processes is the ability to competitively bind to the pathogen or pathogen receptor, thereby interfering with the essential first step of pathogenesis, the binding of the pathogen to its host cell surface receptor. Even this most widely recognized bioactive mechanism requires elucidation. For example, glycan moieties of different HMGPs can show similar protection against the same pathogens. Mucin 1 and lactoferrin inhibit Escherichia coli and Salmonella infection in vitro. However, it is not known if only the carbohydrate moiety is responsible for the inhibition, which carbohydrate moiety inhibits each pathogen, or whether two or more specific carbohydrate moieties act together to inhibit pathogen infection. With the involvement of multiple glycan moieties, would inhibition be additive or synergistic? These questions beg for research on the relationship between structural features of a biologically active molecule and its activity. Such structure–function research requires pure compound and robust, sensitive bioassays.
The relationship between structure and functional glycobiology of HMGPs is now ripe for fruitful human milk research. The difficulties of glycan analysis are now yielding to sophisticated new separation and analytic technologies, obviating preparation of pure compounds. Progress had been hampered by the enormous complexity of glycoprotein–ligand interactions, which can now be measured by nano-surface plasmon resonance (SPR) and other emerging techniques121 and efficient high-throughput screening methods, now possible with shotgun glycan microarray122 and robotic technology.
Recent developments in SPR are quite promising. Nano-SPR is a surface-sensitive optical method to study molecular binding events on a functionalized biosensor. SPR can be used to study both protein–carbohydrate interactions and carbohydrate-mediated inhibition of protein–protein interactions. SPR represents a powerful, high-throughput approach to defining the relevant carbohydrate moiety of an HMGP that is responsible for binding inhibition.123
In addition to the aforementioned inhibition of binding by pathogen adhesins or host cell receptors, other mechanisms for protection of the infant by HMGPs are possible, including a prebiotic affect. In 1905, Tissier124 described the distinctive microflora (now microbiota) of breastfed infants as containing more Lactobacillus bifidus (now classified as Bifidobacterium bifidum). A molecule in human milk was hypothesized to be responsible for this bifidus growth activity and designated as the “bifidus factor.” After approximately 70 years of research, the “bifidus factor” was isolated and identified as a glycan moiety of an HMGP. This should be considered the prototype of a family of glycans now known as prebiotics, which are indigestible dietary glycans that promote proper colonization of the gut. After birth, the vacant infant gut undergoes colonization by a succession of microbes, resulting in the complex stable microbiota of the more mature child.125 Human milk glycans that stimulate growth by mutualist bacteria126 are now defined as prebiotics: indigestible dietary glycans that stimulate colonization by beneficial bacteria and provide a health benefit. Typical prebiotics enhance growth of bifidobacteria and lactobacilli, are fermented to produce organic acids, lower intestinal pH,127 suppress potentially harmful bacteria in the microbiota, and confer other health benefits to the host. The human milk oligosaccharides have already been demonstrated to be prebiotic. However, the HMGPs, which are also indigestible by the human gut enzymes and therefore move into the colon during intestinal transit and provide bacteria access to a potential carbon source, are essentially untested. Prebiotic HMGPs could also contribute toward the lower risk of morbidity and mortality in breastfed infants.128,129 We hypothesize that the glycans of milk could work in concert with glycoconjugates expressed on the surface of the intestinal mucosa to direct initial colonization leading toward a normal, beneficial gut microbiota.
Another function of prebiotics is their use as substrates for bacterial fermentation that results in small organic acid metabolites, such as short-chain fatty acids and other small acids, like acetate, lactate, butyrate, succinate, valerate, propionate, etc. Some of these acids have strong metagenomic effects in intestinal epithelial cells at biologically relevant concentrations. Different microbes of the microbiota produce different complements of organic acids.130 It follows that a prebiotic effect by HMGPs could have profound influences on some aspects of cell signaling and control and therefore contribute to modulation of intestinal response to injury and other types of intestinal inflammation conditions. This could contribute to the known reduction of risk of necrotizing enterocolitis and other inflammatory conditions in premature infants fed human milk.
HMGPs could also directly affect inflammation of the intestinal mucosa. This is discussed above for lactoferrin but is largely unexplored for other of these human milk molecules.
The importance of the human milk oligosaccharides, another major family of complex glycans in human milk, is increasingly recognized as clinically relevant to neonates and term infants alike. The higher-molecular-weight glycoproteins are more difficult to isolate and test, which accounts for the relative lack of definition of their structures and of their biological functions and clinical relevance. The confluence of newly emerging technologies for the isolation, purification, identification, and biological testing of these molecules creates the promise of newly recognized glycans becoming sources of novel prophylactic and therapeutic agents that inhibit diseases caused by a variety of pathogens.
Acknowledgments
This study was supported by grants HD013021, HD059140, HD061930, and AI075563 from the National Institutes of Health.
Disclosure Statement
D.S.N. owns stock in Glycosyn, LLC, whose purpose is synthesis of bioactive human milk glycans. B.L. declares that no competing financial interests exist.
References
- 1.Newburg DS. Human milk glycoconjugates that inhibit pathogens. Curr Med Chem. 1999;6:117–127. [PubMed] [Google Scholar]
- 2.Newburg DS. Ruiz-Palacios GM. Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 3.Molinari CE. Casadio YS. Hartmann BT, et al. Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res. 2012;11:1696–1714. doi: 10.1021/pr2008797. [DOI] [PubMed] [Google Scholar]
- 4.Florisa R. Recio I. Berkhout B, et al. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des. 2003;9:1257–1275. doi: 10.2174/1381612033454810. [DOI] [PubMed] [Google Scholar]
- 5.Brissette MJ. Lepage S. Lamonde AS, et al. MFG-E8 released by apoptotic endothelial cells triggers anti-inflammatory macrophage reprogramming. PLoS One. 2012;7:e36368. doi: 10.1371/journal.pone.0036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson JA. Hamosh M. Scallan CD, et al. Milk fat globule glycoproteins in human milk and in gastric aspirates of mother's milk-fed preterm infants. Pediatr Res. 1998;44:499–506. doi: 10.1203/00006450-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Montagne PM. Tregoat VS. Cuilliere ML, et al. Measurement of nine human milk proteins by nephelometric immunoassays: Application to the determination of mature milk protein profile. Clin Biochem. 2000;33:181–186. doi: 10.1016/s0009-9120(00)00059-x. [DOI] [PubMed] [Google Scholar]
- 8.Stromqvist M. Lindgren K. Hansson L, et al. Differences in the glycosylation of recombinant and native human milk bile salt-stimulated lipase revealed by peptide mapping. J Chromatogr A. 1995;718:53–58. doi: 10.1016/0021-9673(95)00632-x. [DOI] [PubMed] [Google Scholar]
- 9.Masson PL. Heremans JF. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971;39:119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 10.Shin K. Hayasawa H. Lonnerdal B. Purification and quantification of lactoperoxidase in human milk with use of immunoadsorbents with antibodies against recombinant human lactoperoxidase. Am J Clin Nutr. 2001;73:984–989. doi: 10.1093/ajcn/73.5.984. [DOI] [PubMed] [Google Scholar]
- 11.Martin LJ. Woo JG. Geraghty SR, et al. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83:1106–1111. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 12.Chtourou A. Brignon G. Ribadeau-Dumas B. Quantification of beta-casein in human milk. J Dairy Res. 1985;52:239–247. doi: 10.1017/s0022029900024109. [DOI] [PubMed] [Google Scholar]
- 13.Ilcol YO. Hizli ZB. Ozkan T. Leptin concentration in breast milk and its relationship to duration of lactation and hormonal status. Int Breastfeed J. 2006;1:21. doi: 10.1186/1746-4358-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun OH. Sandkuhler H. Relationships between lysozyme concentration of human milk, bacteriologic content, and weight gain of premature infants. J Pediatr Gastroenterol Nutr. 1985;4:583–586. doi: 10.1097/00005176-198508000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Jackson JG. Janszen DB. Lonnerdal B, et al. A multinational study of alpha-lactalbumin concentrations in human milk. J Nutr Biochem. 2004;15:517–521. doi: 10.1016/j.jnutbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Hattrup CL. Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 17.Linden SK. Sutton P. Karlsson NG, et al. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patton S. Huston GE. Jenness R, et al. Differences between individuals in high-molecular weight glycoproteins from mammary epithelia of several species. Biochim Biophys Acta. 1989;980:333–338. doi: 10.1016/0005-2736(89)90321-0. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu M. Yamauchi K. Miyauchi Y, et al. High-Mr glycoprotein profiles in human milk serum and fat-globule membrane. Biochem J. 1986;233:725–730. doi: 10.1042/bj2330725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J. Perez A. Yasin M, et al. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol. 2005;204:166–177. doi: 10.1002/jcp.20277. [DOI] [PubMed] [Google Scholar]
- 21.Patton S. Gendler SJ. Spicer AP. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta. 1995;1241:407–423. doi: 10.1016/0304-4157(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu B. Yu Z. Chen C, et al. Human milk mucin 1 and mucin 4 inhibit Salmonella enterica serovar Typhimurium invasion of human intestinal epithelial cells in vitro. J Nutr. 2012;142:1504–1509. doi: 10.3945/jn.111.155614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeland E. de Jong MA. Nabatov AA, et al. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol. 2009;46:2309–2316. doi: 10.1016/j.molimm.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Yolken RH. Peterson JA. Vonderfecht SL, et al. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroten H. Hanisch FG. Plogmann R, et al. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: A novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect Immun. 1992;60:2893–2899. doi: 10.1128/iai.60.7.2893-2899.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phalipon A. Cardona A. Kraehenbuhl JP, et al. Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 27.Czank C. Prime DK. Hartmann B, et al. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr Res. 2009;66:374–379. doi: 10.1203/PDR.0b013e3181b4554a. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JN. Wormald MR. Sim RB, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 29.Murthy AK. Chaganty BK. Troutman T, et al. Mannose-containing oligosaccharides of non-specific human secretory immunoglobulin A mediate inhibition of Vibrio cholerae biofilm formation. PLoS One. 2011;6:e16847. doi: 10.1371/journal.pone.0016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura T. Fujinaga Y. Jin Y, et al. Human milk SIgA binds to botulinum type B 16S toxin and limits toxin adherence on T84 cells. Biochem Biophys Res Commun. 2007;352:867–872. doi: 10.1016/j.bbrc.2006.11.095. [DOI] [PubMed] [Google Scholar]
- 31.Sane F. Alidjinou EK. Kacet N, et al. Human milk can neutralize Coxsackievirus B4 in vitro. J Med Virol. 2013;85:880–887. doi: 10.1002/jmv.23518. [DOI] [PubMed] [Google Scholar]
- 32.Vudhichamnong K. Walker DM. Ryley HC. The effect of secretory immunoglobulin A on the in-vitro adherence of the yeast Candida albicans to human oral epithelial cells. Arch Oral Biol. 1982;27:617–621. doi: 10.1016/0003-9969(82)90184-4. [DOI] [PubMed] [Google Scholar]
- 33.Rolfe RD. Song W. Immunoglobulin and non-immunoglobulin components of human milk inhibit Clostridium difficile toxin A-receptor binding. J Med Microbiol. 1995;42:10–19. doi: 10.1099/00222615-42-1-10. [DOI] [PubMed] [Google Scholar]
- 34.Makita K. Hayakawa Y. Okame M, et al. First detection of IgA against norovirus in breast milk. Clin Lab. 2007;53:125–128. [PubMed] [Google Scholar]
- 35.Acosta-Altamirano G. Torres-Sanchez E. Meraz E, et al. Detection of class IgA antibodies directed against a lipopeptidophosphoglycan of E. histolytica in samples of human colostrum. Arch Invest Med (Mex) 1986;17(Suppl 1):291–295. [PubMed] [Google Scholar]
- 36.Ciardelli L. Garofoli F. Avanzini MA, et al. Escherichia coli specific secretory IgA and cytokines in human milk from mothers of different ethnic groups resident in northern Italy. Int J Immunopathol Pharmacol. 2007;20:335–340. doi: 10.1177/039463200702000213. [DOI] [PubMed] [Google Scholar]
- 37.Asensi MT. Martinez-Costa C. Buesa J. Anti-rotavirus antibodies in human milk: Quantification and neutralizing activity. J Pediatr Gastroenterol Nutr. 2006;42:560–567. doi: 10.1097/01.mpg.0000221892.59371.b3. [DOI] [PubMed] [Google Scholar]
- 38.Harabuchi Y. Faden H. Yamanaka N, et al. Human milk secretory IgA antibody to nontypeable Haemophilus influenzae: Possible protective effects against nasopharyngeal colonization. J Pediatr. 1994;124:193–198. doi: 10.1016/s0022-3476(94)70302-7. [DOI] [PubMed] [Google Scholar]
- 39.Zaman S. Carlsson B. Jalil F, et al. Specific antibodies to poliovirus type I in breastmilk of unvaccinated mothers before and seven years after start of community-wide vaccination of their infants with live, oral poliovirus vaccine. Acta Paediatr Scand. 1991;80:1174–1182. doi: 10.1111/j.1651-2227.1991.tb11806.x. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez N. Otero O. Camacho F, et al. Passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection [abstract] BMC Immunol. 2013;14(Suppl 1):S3. doi: 10.1186/1471-2172-14-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Losonsky GA. Fishaut JM. Strussenberg J, et al. Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk. J Infect Dis. 1982;145:654–660. doi: 10.1093/infdis/145.2.654. [DOI] [PubMed] [Google Scholar]
- 42.Bessler HC. de Oliveira IR. Giugliano LG. Human milk glycoproteins inhibit the adherence of Salmonella typhimurium to HeLa cells. Microbiol Immunol. 2006;50:877–882. doi: 10.1111/j.1348-0421.2006.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 43.Mandomando IM. Naniche D. Pasetti MF, et al. Measles-specific neutralizing antibodies in rural Mozambique: Seroprevalence and presence in breast milk. Am J Trop Med Hyg. 2008;79:787–792. [PubMed] [Google Scholar]
- 44.Cam PD. Achi R. Lindberg AA, et al. Antibodies against invasion plasmid coded antigens of shigellae in human colostrum and milk. Acta Microbiol Hung. 1992;39:263–270. [PubMed] [Google Scholar]
- 45.Mabuka J. Nduati R. Odem-Davis K, et al. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saadi AT. Gordon AE. MacKenzie DA, et al. The protective effect of breast feeding in relation to sudden infant death syndrome (SIDS): I. The effect of human milk and infant formula preparations on binding of toxigenic Staphylococcus aureus to epithelial cells. FEMS Immunol Med Microbiol. 1999;25:155–165. doi: 10.1111/j.1574-695X.1999.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 47.Thomas JE. Austin S. Dale A, et al. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet. 1993;342:121. doi: 10.1016/0140-6736(93)91327-i. [DOI] [PubMed] [Google Scholar]
- 48.Blackberg L. Hernell O. Bile salt-stimulated lipase in human milk. Evidence that bile salt induces lipid binding and activation via binding to different sites. FEBS Lett. 1993;323:207–210. doi: 10.1016/0014-5793(93)81340-6. [DOI] [PubMed] [Google Scholar]
- 49.Blackberg L. Angquist KA. Hernell O. Bile-salt-stimulated lipase in human milk: Evidence for its synthesis in the lactating mammary gland. FEBS Lett. 1987;217:37–41. doi: 10.1016/0014-5793(87)81237-1. [DOI] [PubMed] [Google Scholar]
- 50.Stromqvist M. Hernell O. Hansson L, et al. Naturally occurring variants of human milk bile salt-stimulated lipase. Arch Biochem Biophys. 1997;347:30–36. doi: 10.1006/abbi.1997.0307. [DOI] [PubMed] [Google Scholar]
- 51.Baba T. Downs D. Jackson KW, et al. Structure of human milk bile salt activated lipase. Biochemistry. 1991;30:500–510. doi: 10.1021/bi00216a028. [DOI] [PubMed] [Google Scholar]
- 52.Hernell O. Blackberg L. Digestion of human milk lipids: Physiologic significance of sn-2 monoacylglycerol hydrolysis by bile salt-stimulated lipase. Pediatr Res. 1982;16:882–885. doi: 10.1203/00006450-198210000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Fredrikzon B. Hernell O. Blackberg L, et al. Bile salt-stimulated lipase in human milk: Evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr Res. 1978;12:1048–1052. doi: 10.1203/00006450-197811000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Naarding MA. Dirac AM. Ludwig IS, et al. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells. Antimicrob Agents Chemother. 2006;50:3367–3374. doi: 10.1128/AAC.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruvoen-Clouet N. Mas E. Marionneau S, et al. Bile-salt-stimulated lipase and mucins from milk of 'secretor' mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006;393:627–634. doi: 10.1042/BJ20050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montreuil J. Tonnelat J. Mullet S. Preparation and properties of lactosiderophilin (lactotransferrin) of human milk. Biochim Biophys Acta. 1960;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- 57.Blanc B. Isliker H. Isolation and characterization of the red siderophilic protein from maternal milk: Lactotransferrin. Bull Soc Chim Biol (Paris) 1961;43:929–943. [PubMed] [Google Scholar]
- 58.Hirai Y. Kawakata N. Satoh K, et al. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J Nutr Sci Vitaminol (Tokyo) 1990;36:531–544. doi: 10.3177/jnsv.36.531. [DOI] [PubMed] [Google Scholar]
- 59.Lonnerdal B. Iyer S. Lactoferrin: Molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 60.Hennart PF. Brasseur DJ. Delogne-Desnoeck JB, et al. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: Influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991;53:32–39. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- 61.Kanwar JR. Kanwar RK. Sun X, et al. Molecular and biotechnological advances in milk proteins in relation to human health. Curr Protein Pept Sci. 2009;10:308–338. doi: 10.2174/138920309788922234. [DOI] [PubMed] [Google Scholar]
- 62.Baker EN. Baker HM. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci. 2005;62:2531–2539. doi: 10.1007/s00018-005-5368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward PP. Conneely OM. Lactoferrin: Role in iron homeostasis and host defense against microbial infection. Biometals. 2004;17:203–208. doi: 10.1023/b:biom.0000027693.60932.26. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues L. Teixeira J. Schmitt F, et al. Lactoferrin and cancer disease prevention. Crit Rev Food Sci Nutr. 2009;49:203–217. doi: 10.1080/10408390701856157. [DOI] [PubMed] [Google Scholar]
- 65.Bullen JJ. Rogers HJ. Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. BMJ. 1972;1:69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naidu SS. Svensson U. Kishore AR, et al. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1993;37:240–245. doi: 10.1128/aac.37.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willer Eda M. Lima Rde L. Giugliano LG. In vitro adhesion and invasion inhibition of Shigella dysenteriae, Shigella flexneri and Shigella sonnei clinical strains by human milk proteins. BMC Microbiol. 2004;4:18. doi: 10.1186/1471-2180-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murdock CA. Cleveland J. Matthews KR, et al. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett Appl Microbiol. 2007;44:255–261. doi: 10.1111/j.1472-765X.2006.02076.x. [DOI] [PubMed] [Google Scholar]
- 69.von Hunolstein C. Ricci ML. Valenti P, et al. Lack of activity of transferrins towards Streptococcus spp. Med Microbiol Immunol. 1992;181:351–357. doi: 10.1007/BF00191547. [DOI] [PubMed] [Google Scholar]
- 70.Ascencio F. Ljungh A. Wadstrom T. Lactoferrin binding properties of Vibrio cholerae. Microbios. 1992;70:103–117. [PubMed] [Google Scholar]
- 71.Bortner CA. Miller RD. Arnold RR. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986;51:373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlsson A. Bjorck L. Persson K. Lactoferrin and lysozyme in milk during acute mastitis and their inhibitory effect in Delvotest P. J Dairy Sci. 1989;72:3166–3175. doi: 10.3168/jds.S0022-0302(89)79475-3. [DOI] [PubMed] [Google Scholar]
- 73.Bellamy WR. Wakabayashi H. Takase M, et al. Role of cell-binding in the antibacterial mechanism of lactoferricin B. J Appl Bacteriol. 1993;75:478–484. [PubMed] [Google Scholar]
- 74.Superti F. Ammendolia MG. Valenti P, et al. Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med Microbiol Immunol. 1997;186:83–91. doi: 10.1007/s004300050049. [DOI] [PubMed] [Google Scholar]
- 75.Superti F. Siciliano R. Rega B, et al. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim Biophys Acta. 2001;1528:107–115. doi: 10.1016/s0304-4165(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 76.Groot F. Geijtenbeek TB. Sanders RW, et al. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J Virol. 2005;79:3009–3015. doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valimaa H. Tenovuo J. Waris M, et al. Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol J. 2009;6:53. doi: 10.1186/1743-422X-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jenssen H. Sandvik K. Andersen JH, et al. Inhibition of HSV cell-to-cell spread by lactoferrin and lactoferricin. Antiviral Res. 2008;79:192–198. doi: 10.1016/j.antiviral.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Beljaars L. van der Strate BW. Bakker HI, et al. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antiviral Res. 2004;63:197–208. doi: 10.1016/j.antiviral.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Actor JK. Hwang SA. Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikeda M. Sugiyama K. Tanaka T, et al. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem Biophys Res Commun. 1998;245:549–553. doi: 10.1006/bbrc.1998.8481. [DOI] [PubMed] [Google Scholar]
- 82.Drobni P. Naslund J. Evander M. Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antiviral Res. 2004;64:63–68. doi: 10.1016/j.antiviral.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Pietrantoni A. Di Biase AM. Tinari A, et al. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob Agents Chemother. 2003;47:2688–2691. doi: 10.1128/AAC.47.8.2688-2691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakabayashi H. Okutomi T. Abe S, et al. Enhanced anti-Candida activity of neutrophils and azole antifungal agents in the presence of lactoferrin-related compounds. Adv Exp Med Biol. 1998;443:229–237. doi: 10.1007/978-1-4757-9068-9_27. [DOI] [PubMed] [Google Scholar]
- 85.Lopez-Soto F. Leon-Sicairos N. Nazmi K, et al. Microbicidal effect of the lactoferrin peptides lactoferricin17–30, lactoferrampin265–284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals. 2010;23:563–568. doi: 10.1007/s10534-010-9295-3. [DOI] [PubMed] [Google Scholar]
- 86.Grab DJ. Lonsdale-Eccles JD. Oli MW, et al. Lactoferrin-binding proteins of Tritrichomonas foetus. J Parasitol. 2001;87:1064–1070. doi: 10.1645/0022-3395(2001)087[1064:LBPOTF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 87.Omata Y. Satake M. Maeda R, et al. Reduction of the infectivity of Toxoplasma gondii and Eimeria stiedai sporozoites by treatment with bovine lactoferricin. J Vet Med Sci. 2001;63:187–190. doi: 10.1292/jvms.63.187. [DOI] [PubMed] [Google Scholar]
- 88.Ward PP. Paz E. Conneely OM. Multifunctional roles of lactoferrin: A critical overview. Cell Mol Life Sci. 2005;62:2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Legrand D. Pierce A. Elass E, et al. Lactoferrin structure and functions. Adv Exp Med Biol. 2008;606:163–194. doi: 10.1007/978-0-387-74087-4_6. [DOI] [PubMed] [Google Scholar]
- 90.Kobylka D. Carraway KL. Proteins and glycoproteins of the milk fat globule membrane. Biochim Biophys Acta. 1972;288:282–295. doi: 10.1016/0005-2736(72)90249-0. [DOI] [PubMed] [Google Scholar]
- 91.Mather IH. Tamplin CB. Irving MG. Separation of the proteins of bovine milk-fat-globule membrane by electrofocusing with retention of enzymatic and immunological activity. Eur J Biochem. 1980;110:327–336. doi: 10.1111/j.1432-1033.1980.tb04871.x. [DOI] [PubMed] [Google Scholar]
- 92.Mather IH. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J Dairy Sci. 2000;83:203–247. doi: 10.3168/jds.S0022-0302(00)74870-3. [DOI] [PubMed] [Google Scholar]
- 93.Banghart LR. Chamberlain CW. Velarde J, et al. Butyrophilin is expressed in mammary epithelial cells from a single-sized messenger RNA as a type I membrane glycoprotein. J Biol Chem. 1998;273:4171–4179. doi: 10.1074/jbc.273.7.4171. [DOI] [PubMed] [Google Scholar]
- 94.Jeong J. Rao AU. Xu J, et al. The PRY/SPRY/B30.2 domain of butyrophilin 1A1 (BTN1A1) binds to xanthine oxidoreductase: Implications for the function of BTN1A1 in the mammary gland and other tissues. J Biol Chem. 2009;284:22444–22456. doi: 10.1074/jbc.M109.020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mana P. Goodyear M. Bernard C, et al. Tolerance induction by molecular mimicry: Prevention and suppression of experimental autoimmune encephalomyelitis with the milk protein butyrophilin. Int Immunol. 2004;16:489–499. doi: 10.1093/intimm/dxh049. [DOI] [PubMed] [Google Scholar]
- 96.Hvarregaard J. Andersen MH. Berglund L, et al. Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. Eur J Biochem. 1996;240:628–636. doi: 10.1111/j.1432-1033.1996.0628h.x. [DOI] [PubMed] [Google Scholar]
- 97.Newburg DS. Peterson JA. Ruiz-Palacios GM, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet. 1998;351:1160–1164. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- 98.Kvistgaard AS. Pallesen LT. Arias CF, et al. Inhibitory effects of human and bovine milk constituents on rotavirus infections. J Dairy Sci. 2004;87:4088–4096. doi: 10.3168/jds.S0022-0302(04)73551-1. [DOI] [PubMed] [Google Scholar]
- 99.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87(13 Suppl):26–34. doi: 10.2527/jas.2008-1347. [DOI] [PubMed] [Google Scholar]
- 100.Silvestre JS. Thery C. Hamard G, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 101.Andersen MH. Graversen H. Fedosov SN, et al. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 102.Dasgupta SK. Abdel-Monem H. Niravath P, et al. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheyuo C. Jacob A. Wu R, et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology. 2012;62:890–900. doi: 10.1016/j.neuropharm.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bu HF. Zuo XL. Wang X, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673–3683. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weyermann M. Brenner H. Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology. 2007;18:722–729. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 106.Bielicki J. Huch R. von Mandach U. Time-course of leptin levels in term and preterm human milk. Eur J Endocrinol. 2004;151:271–276. doi: 10.1530/eje.0.1510271. [DOI] [PubMed] [Google Scholar]
- 107.Resto M. O'Connor D. Leef K, et al. Leptin levels in preterm human breast milk and infant formula. Pediatrics. 2001;108:E15. doi: 10.1542/peds.108.1.e15. [DOI] [PubMed] [Google Scholar]
- 108.Ouchi N. Parker JL. Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brennan AM. Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 110.Casabiell X. Pineiro V. Tome MA, et al. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- 111.Yokota T. Oritani K. Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 112.Diamon F. Dharamraj C. Luther S, et al. The leptin/adiponectin ratio in mid-infancy correlates with weight gain in healthy term infants, but is unrelated to serum insulin concentrations, body mass index, or skin fold thickness. J Pediatr Endocrinol Metab. 2008;21:1133–1138. doi: 10.1515/JPEM.2008.21.12.1133. [DOI] [PubMed] [Google Scholar]
- 113.Kroening TA. Baxter JH. Anderson SA, et al. Concentrations and anti-Haemophilus influenzae activities of beta-casein phosphoforms in human milk. J Pediatr Gastroenterol Nutr. 1999;28:486–491. doi: 10.1097/00005176-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Danielsson Niemi L. Hernell O. Johansson I. Human milk compounds inhibiting adhesion of mutans streptococci to host ligand-coated hydroxyapatite in vitro. Caries Res. 2009;43:171–178. doi: 10.1159/000213888. [DOI] [PubMed] [Google Scholar]
- 115.Stromqvist M. Falk P. Bergstrom S, et al. Human milk kappa-casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. J Pediatr Gastroenterol Nutr. 1995;21:288–296. doi: 10.1097/00005176-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Iskarpatyoti JA. Morse EA. Paul McClung R, et al. Serotype-specific differences in inhibition of reovirus infectivity by human-milk glycans are determined by viral attachment protein sigma1. Virology. 2012;433:489–497. doi: 10.1016/j.virol.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 117.Hakansson A. Svensson M. Mossberg AK, et al. A folding variant of alpha-lactalbumin with bactericidal activity against Streptococcus pneumoniae. Mol Microbiol. 2000;35:589–600. doi: 10.1046/j.1365-2958.2000.01728.x. [DOI] [PubMed] [Google Scholar]
- 118.Duhaiman AS. Purification of camel milk lysozyme and its lytic effect on Escherichia coli and Micrococcus lysodeikticus. Comp Biochem Physiol B. 1988;91:793–796. doi: 10.1016/0305-0491(88)90210-6. [DOI] [PubMed] [Google Scholar]
- 119.Shin K. Yamauchi K. Teraguchi S, et al. Susceptibility of Helicobacter pylori and its urease activity to the peroxidase-hydrogen peroxide-thiocyanate antimicrobial system. J Med Microbiol. 2002;51:231–237. doi: 10.1099/0022-1317-51-3-231. [DOI] [PubMed] [Google Scholar]
- 120.Segal BH. Sakamoto N. Patel M, et al. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47(phox–/–) mouse model of chronic granulomatous disease. Infect Immun. 2000;68:2374–2378. doi: 10.1128/iai.68.4.2374-2378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reuel NF. Mu B. Zhang J, et al. Nanoengineered glycan sensors enabling native glycoprofiling for medicinal applications: Towards profiling glycoproteins without labeling or liberation steps. Chem Soc Rev. 2012;41:5744–5779. doi: 10.1039/c2cs35142k. [DOI] [PubMed] [Google Scholar]
- 122.Yu Y. Mishra S. Song X, et al. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J Biol Chem. 2012;287:44784–44799. doi: 10.1074/jbc.M112.425819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shang J. Cheng F. Dubey M, et al. An organophosphonate strategy for functionalizing silicon photonic biosensors. Langmuir. 2012;28:3338–3344. doi: 10.1021/la2043153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tissier H. Repartition des microbes dans l'intenstin du nourrinson. Ann Inst Pasteur. 1905;19:109–123. [Google Scholar]
- 125.Mackie RI. Sghir A. Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 126.Gyorgy P. Norris RF. Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48:193–201. doi: 10.1016/0003-9861(54)90323-9. [DOI] [PubMed] [Google Scholar]
- 127.Gibson GR. Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 128.Cunningham AS. Morbidity in breast-fed and artificially fed infants. II. J Pediatr. 1979;95:685–689. doi: 10.1016/s0022-3476(79)80711-8. [DOI] [PubMed] [Google Scholar]
- 129.Newburg DS. Bioactive components of human milk: Evolution, efficiency, and protection. Adv Exp Med Biol. 2001;501:3–10. doi: 10.1007/978-1-4615-1371-1_1. [DOI] [PubMed] [Google Scholar]
- 130.Yu ZT. Chen C. Kling DE, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;2:169–177. doi: 10.1093/glycob/cws138. [DOI] [PMC free article] [PubMed] [Google Scholar]