Abstract
The mixed lymphocyte reaction (MLR) is the proliferative response of one individual's lymphocytes cultured in the presence of another individual's lymphocytes. In man, the MLR is elicited by cell surface antigens coded for by the HLA-D gene locus. This locus is among a cluster of genes which are located on the sixth chromosome and which include genes coding for the major histocompatibility antigens HLA-A, B, and C as well as HLA-D. If the stimulator cell possesses D locus antigens not present in the responder, the lymphocytes of the latter will undergo blast transformation resulting in DNA synthesis which can be measured. A vigorous response in the MLR to allogeneic cells is the rule among healthy individuals.
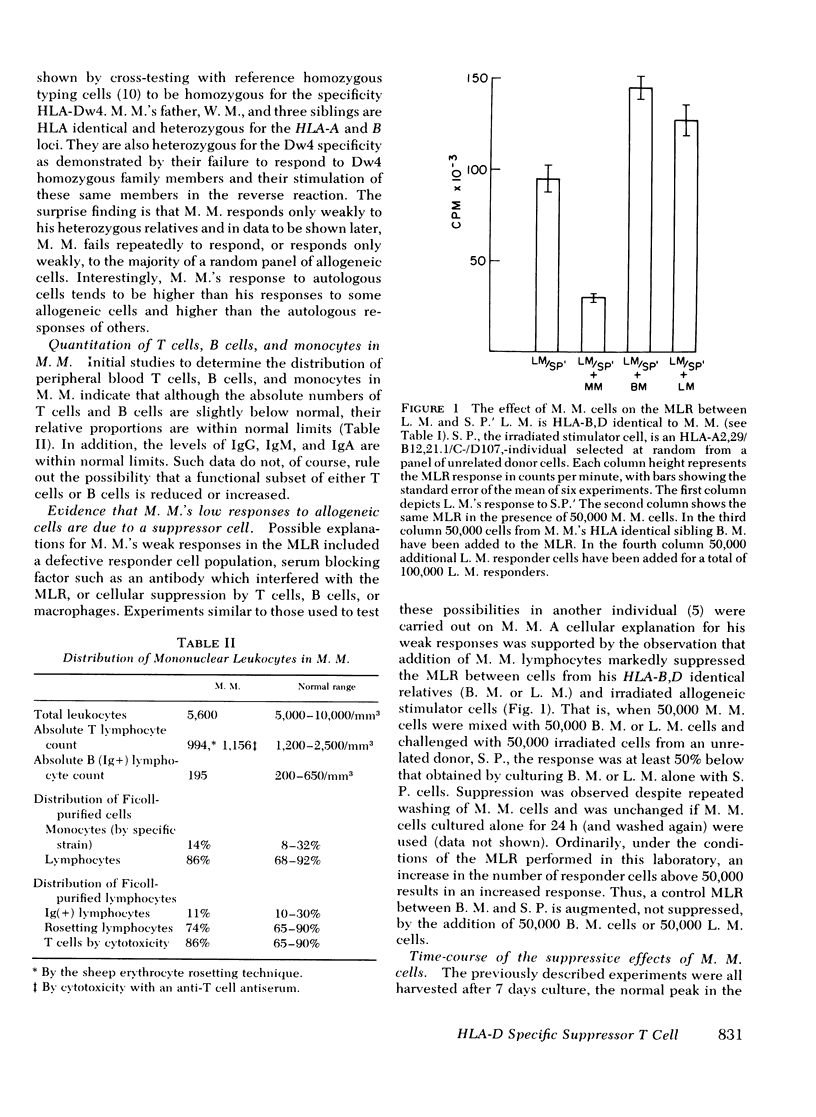
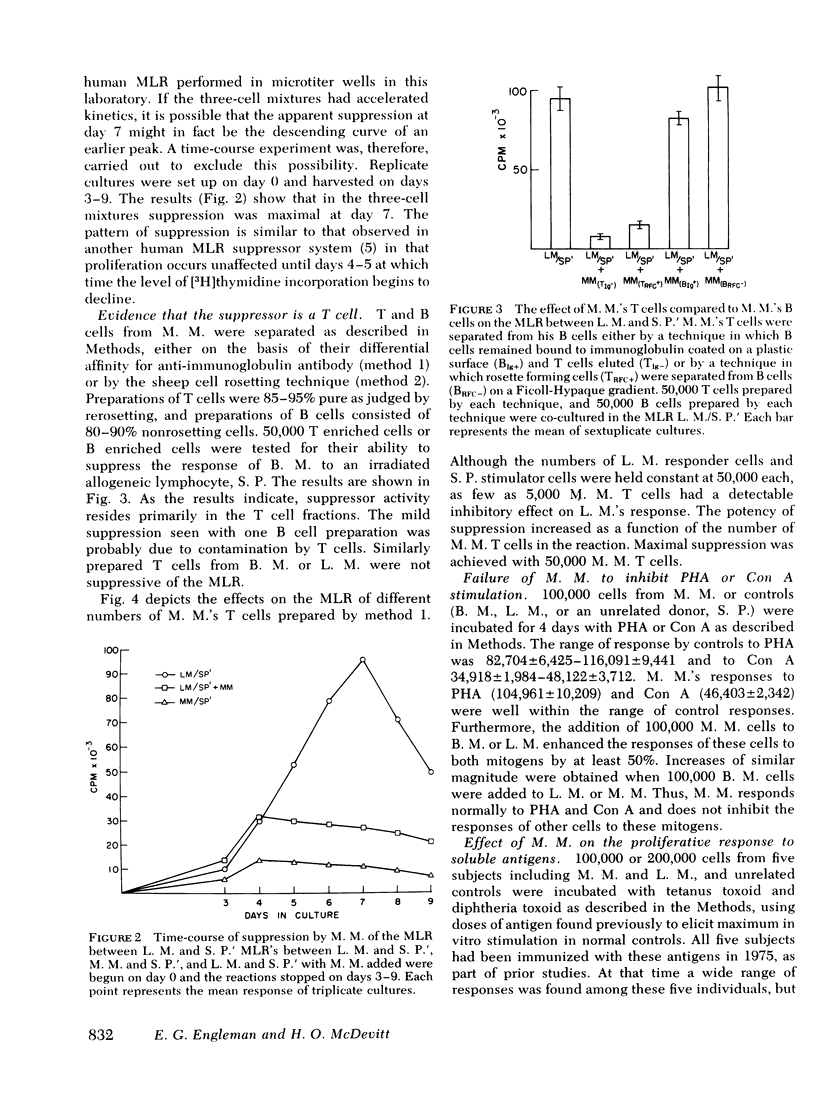
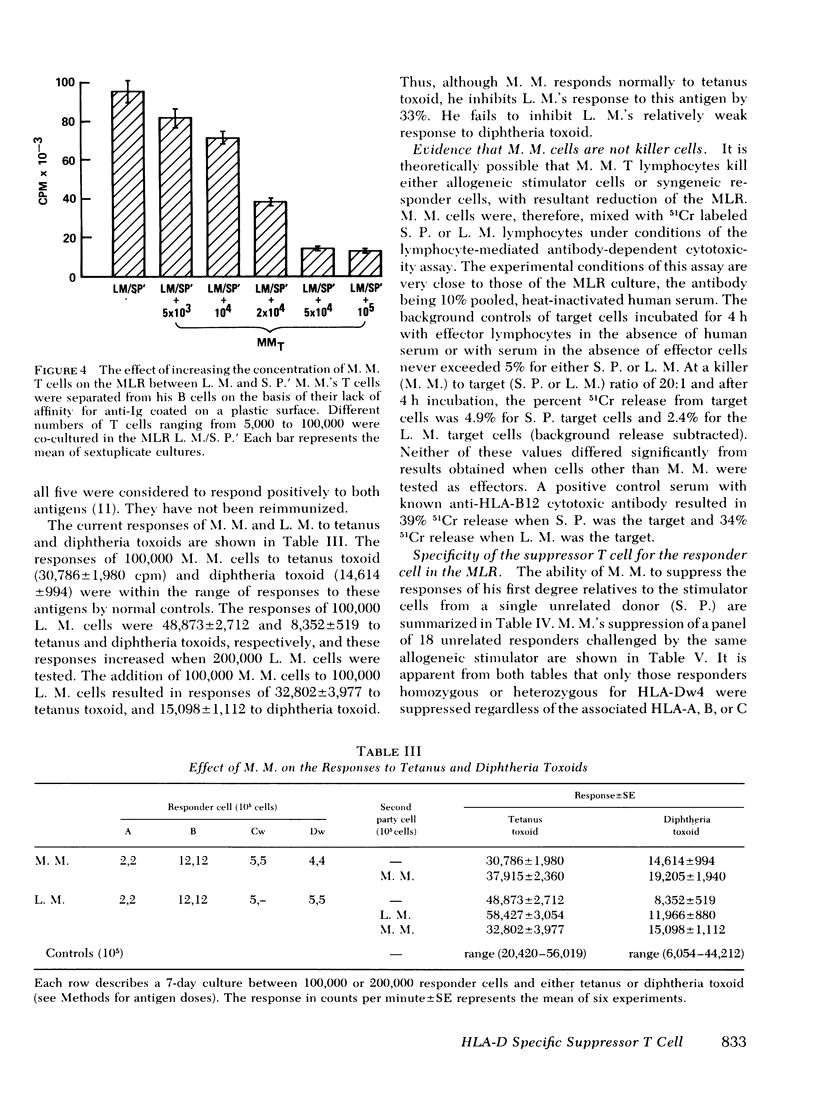
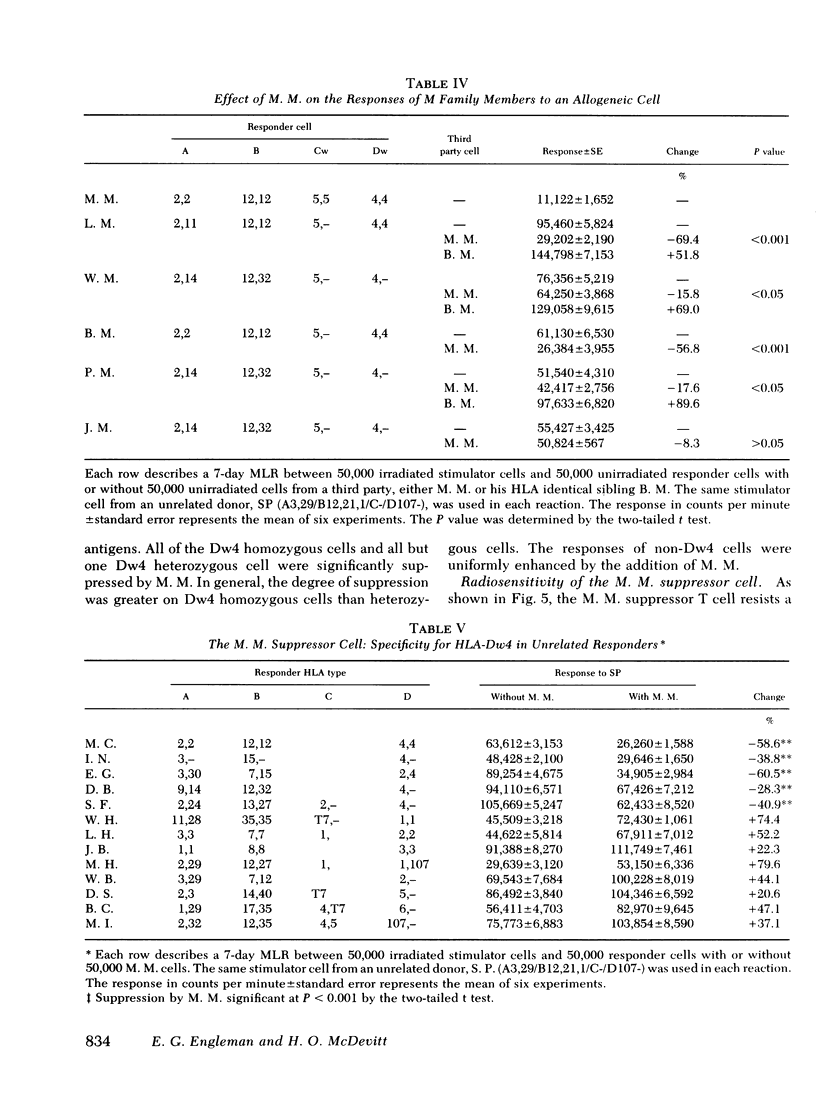
We describe studies of a 23-yr-old man whose lymphocytes respond normally to mitogens and soluble antigens but fail to respond to allogeneic cells in the MLR. His medical history is unremarkable except that he received thymic irradiation as an infant. HLA typing revealed that he is homozygous for HLA-A2, B12, and Cw5 as well as for the D locus antigen Dw4. When his lymphocytes were added to the responder lymphocytes of other persons homozygous for the same HLA antigens, their responses to allogeneic cells but not mitogens were suppressed by 50-95%. Their responses to a soluble antigen, tetanus toxoid, were suppressed to a lesser degree. These inhibitory effects were mediated by a relatively radioresistant thymus-derived (T) lymphocyte.
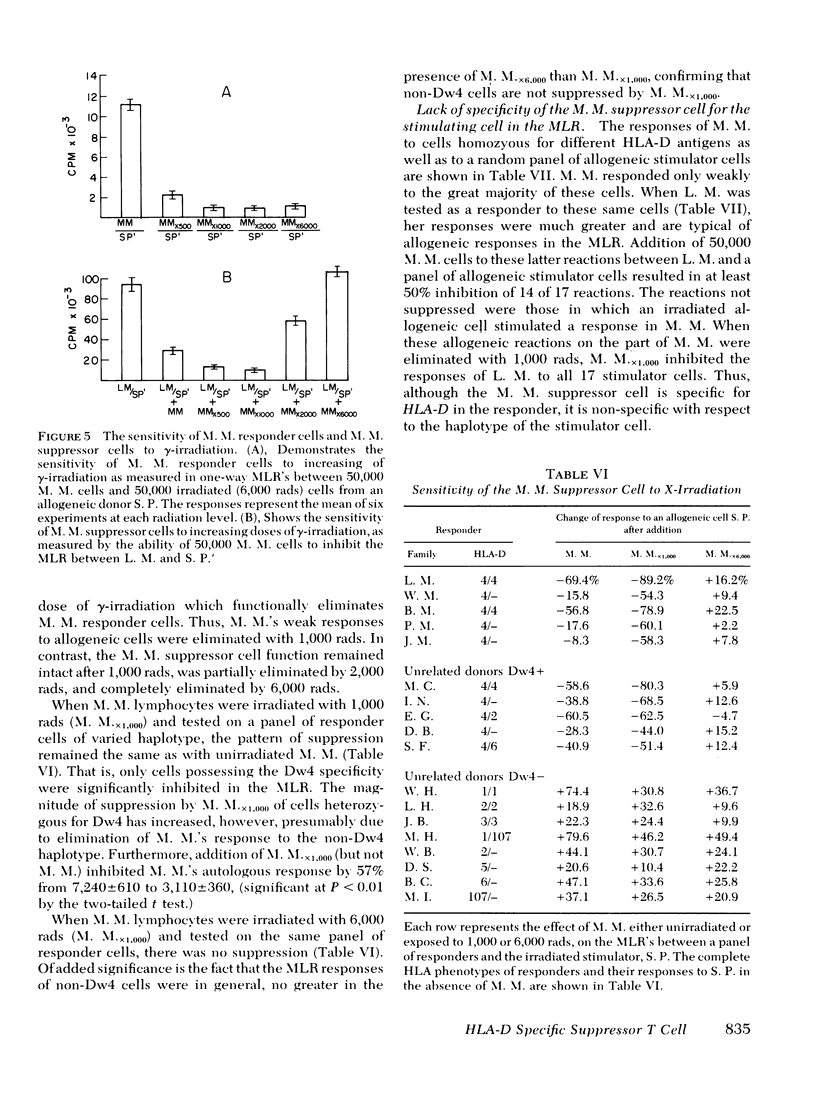
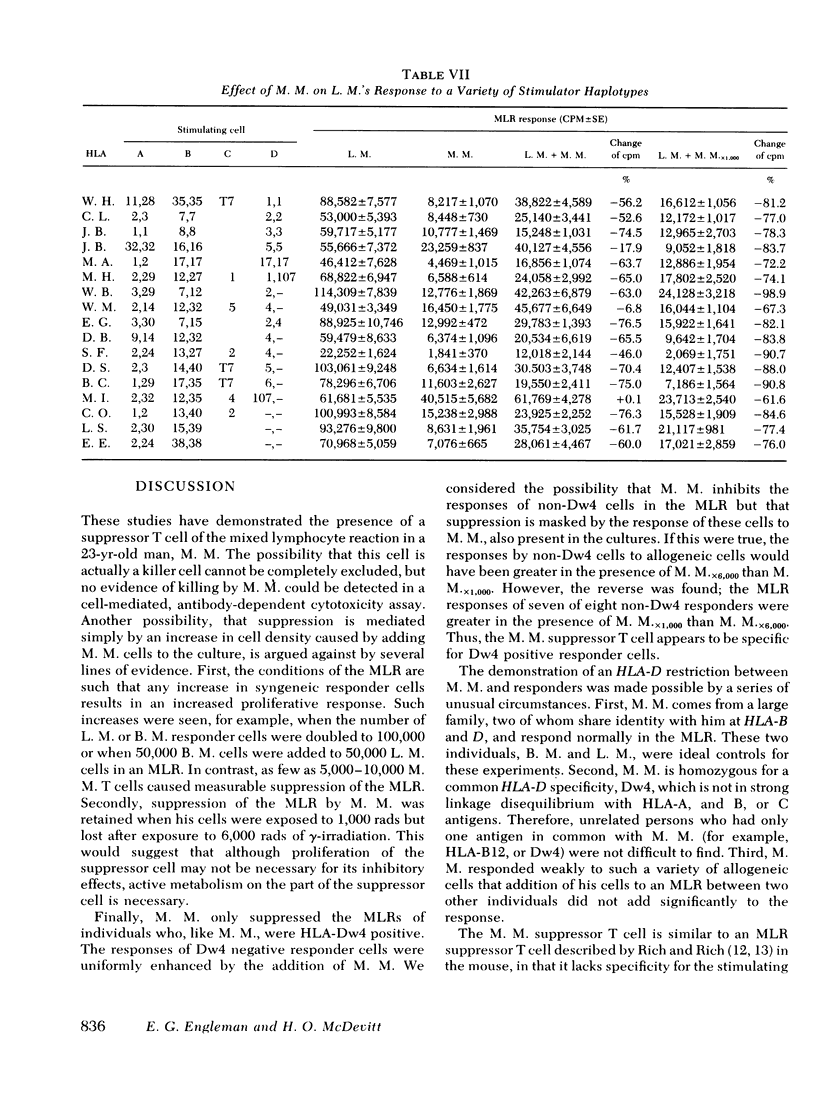
Further studies of the requirements for MLR suppression revealed that only persons heterozygous or homozygous for the Dw4 antigen were inhibited by the suppressor T cell. This effect was not altered by differences in the HLA-A, B, or C antigens between the suppressor and responder. It is concluded that genes in or near the HLA-D locus code not only for antigens (primarily on bone marrow-derived (B) cells), that elicit the MLR, but also for structures on T cells, or possibly macrophages, which are recognized by MLR suppressor T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Blumenthal M. N., Amos D. B., Noreen H. Genetic mapping of Ir locus in man: linkage to second locus of HL-A. Science. 1974 Jun 21;184(4143):1301–1303. doi: 10.1126/science.184.4143.1301. [DOI] [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- DUFFY B. J., Jr, FITZGERALD P. J. Thyroid cancer in childhood and adolescence; a report on 28 cases. Cancer. 1950 Nov;3(6):1018–1032. doi: 10.1002/1097-0142(1950)3:6<1018::aid-cncr2820030611>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goh K., Reddy M. M., Hempelmann L. H. Chromosomal abberations in lymphocytes of normal adults long after thymus irradiation. Radiat Res. 1976 Jul;67(1):82–85. [PubMed] [Google Scholar]
- Levine B. B., Stember R. H., Fotino M. Ragweed hay fever: genetic control and linkage to HL-A haplotypes. Science. 1972 Dec 15;178(4066):1201–1203. doi: 10.1126/science.178.4066.1201. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T. A suppressor T cell in the human mixed lymphocyte reaction. J Exp Med. 1977 Aug 1;146(2):368–380. doi: 10.1084/jem.146.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T., McDevitt H. O. The immune response to diphtheria toxoid in humans. Transplant Proc. 1977 Mar;9(1 Suppl 1):191–194. [PubMed] [Google Scholar]
- McMichael A., McDevitt H. The association between the HLA system and disease. Prog Med Genet. 1977;2:39–100. [PubMed] [Google Scholar]
- Miller J. F., De Burgh P. M., Dukor P., Grant G., Allman V., House W. Regeneration of thymus grafts. II. Effects on immunological capacity. Clin Exp Immunol. 1966 Jan;1(1):61–76. [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. II. A genetically restricted suppressor of mixed lymphocyte reactions released by alloantigen-activated spleen cells. J Exp Med. 1975 Dec 1;142(6):1391–1402. doi: 10.1084/jem.142.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. III. I-region control of suppressor cell interaction with responder cells in mixed lymphocyte reactions. J Exp Med. 1976 Mar 1;143(3):672–677. doi: 10.1084/jem.143.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger C. H., Kraft S. C., Rothberg R. M. Alteration of T cell function in healthy persons with a history of thymic x-irradiation. J Allergy Clin Immunol. 1975 Oct;56(4):273–281. doi: 10.1016/0091-6749(75)90101-3. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swasdikul D., Block M. Effect of radiation upon the "embryonic" thymus. Radiat Res. 1972 Apr;50(1):73–84. [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Widmer M. B., Omodei-Zorini C., Bach M. L., Bach F. H., Klein J. Importance of different regions of H-2 for MLC stimulation. Tissue Antigens. 1973;3(4):309–315. doi: 10.1111/j.1399-0039.1973.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Haegert D. G., Coombs R. R. Increased sensitivity of the rosette-forming reaction of human T lymphocytes with sheep erythrocytes afforded by papain treatment of the sheep cells. Clin Exp Immunol. 1975 Oct;22(1):177–182. [PMC free article] [PubMed] [Google Scholar]
- Women I. P. Immunity to tissue sensitisation, HL-A and non-HL-A, as detected by the ABCIL system. Transplantation. 1974 May;17(5):453–461. doi: 10.1097/00007890-197405000-00003. [DOI] [PubMed] [Google Scholar]
- Yunis E. J., Amos D. B. Three closely linked genetic systems relevant to transplantation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3031–3035. doi: 10.1073/pnas.68.12.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]


