Abstract
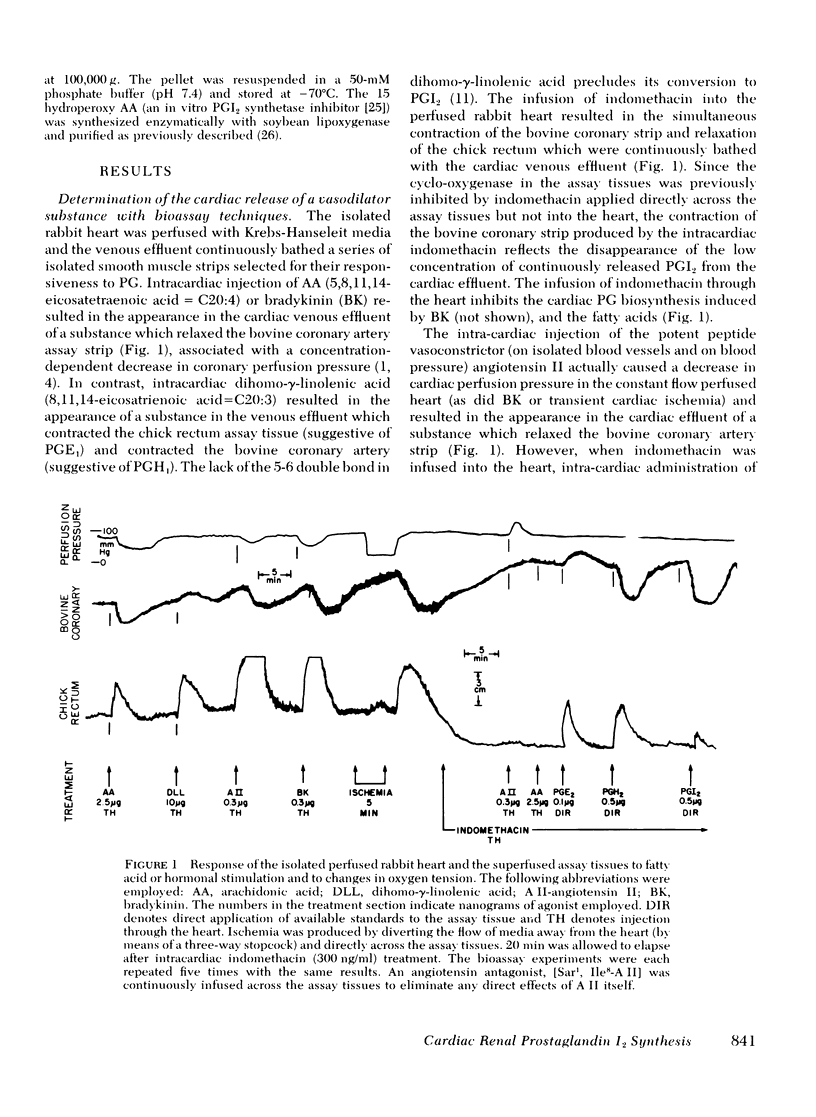
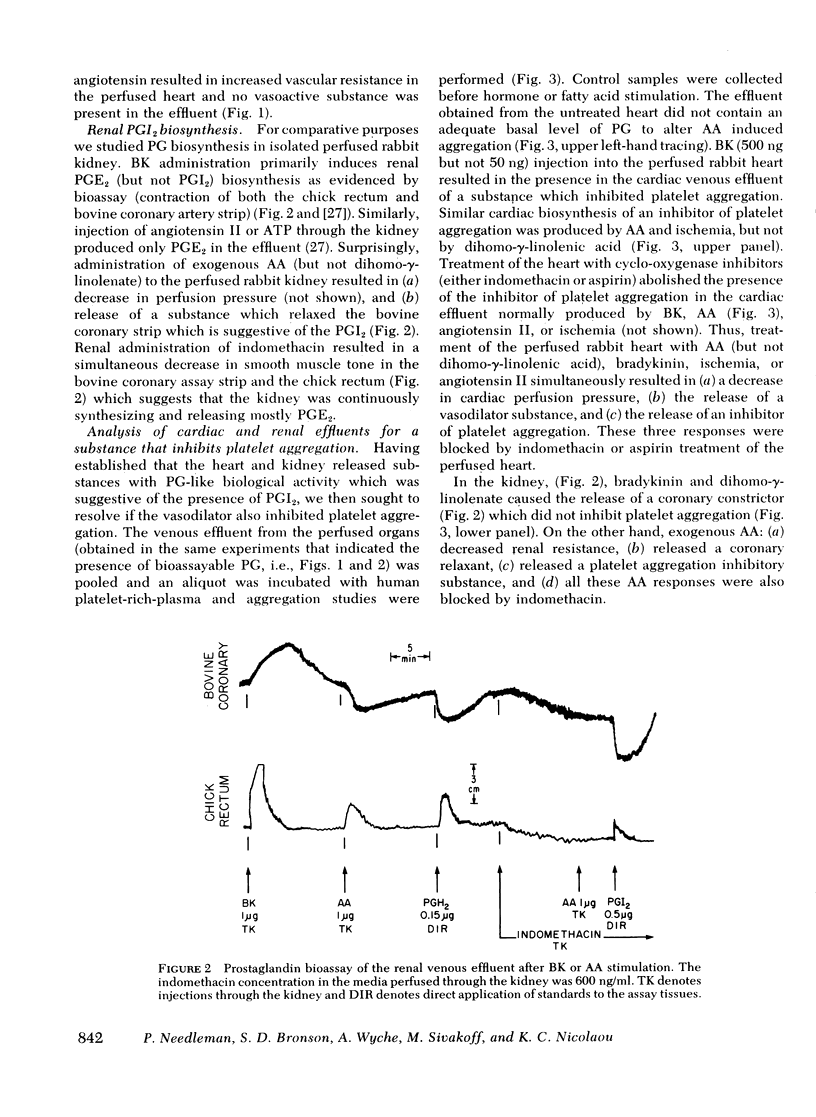
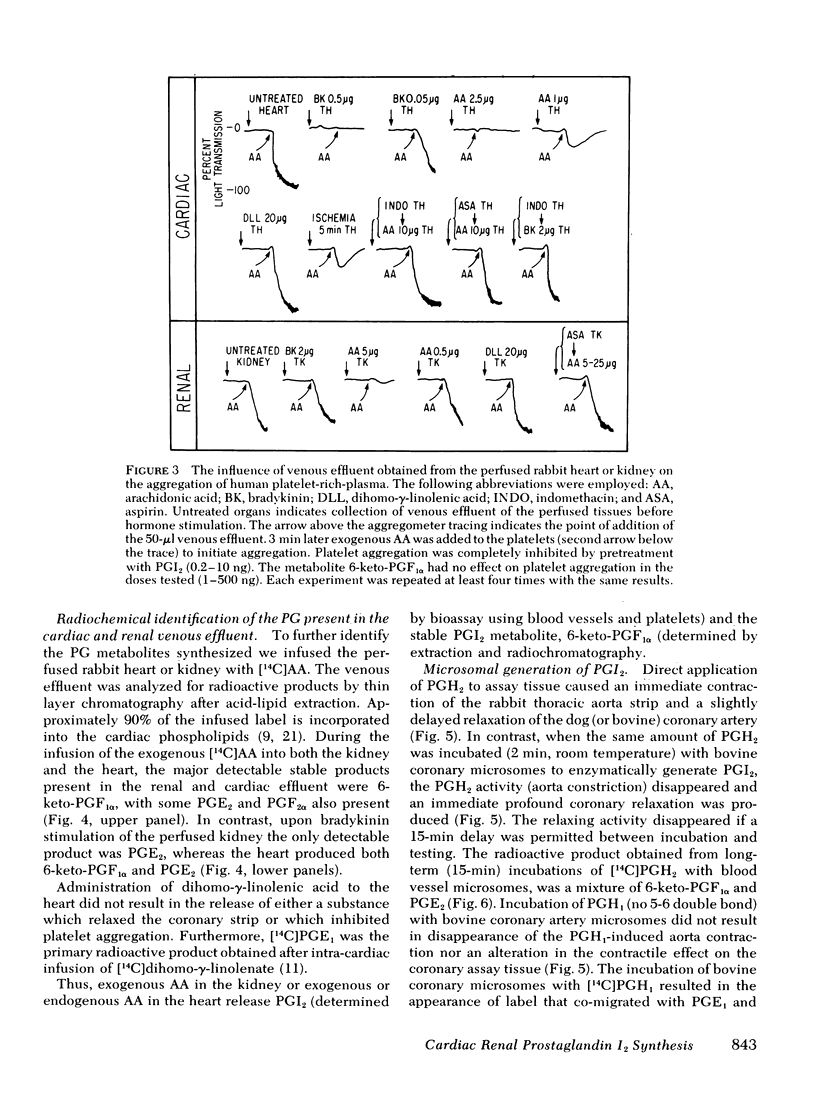
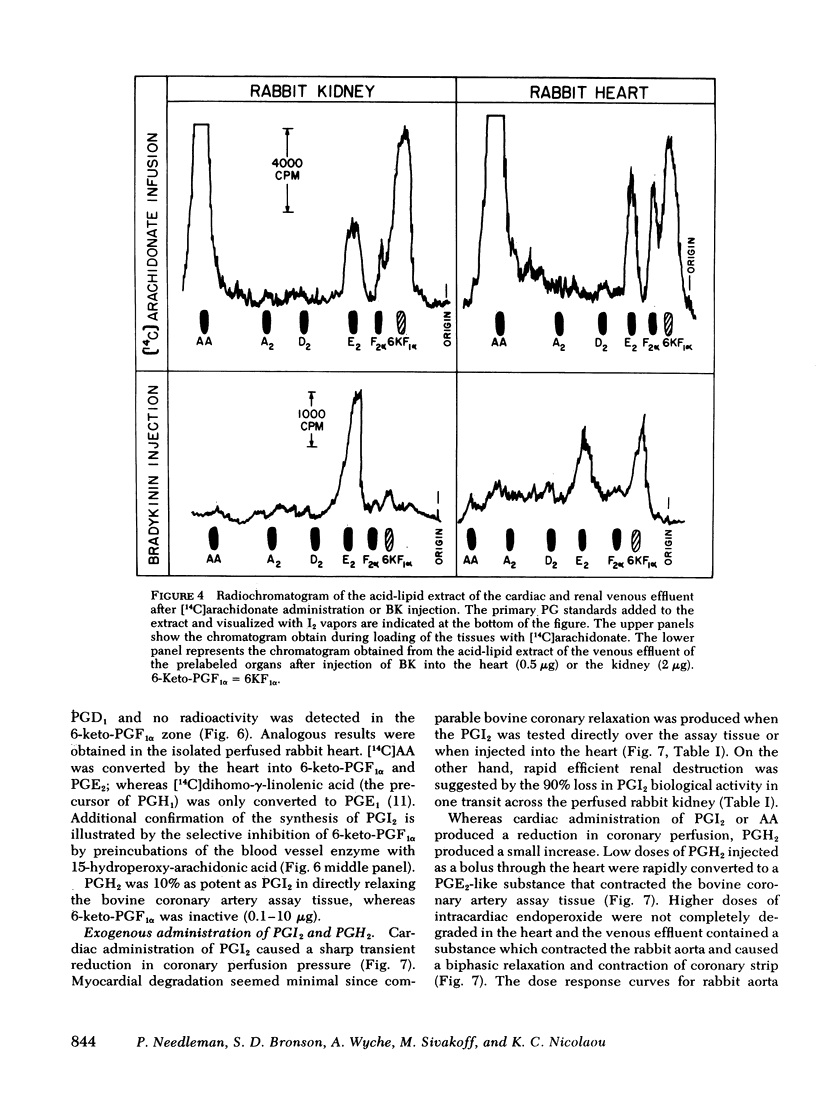
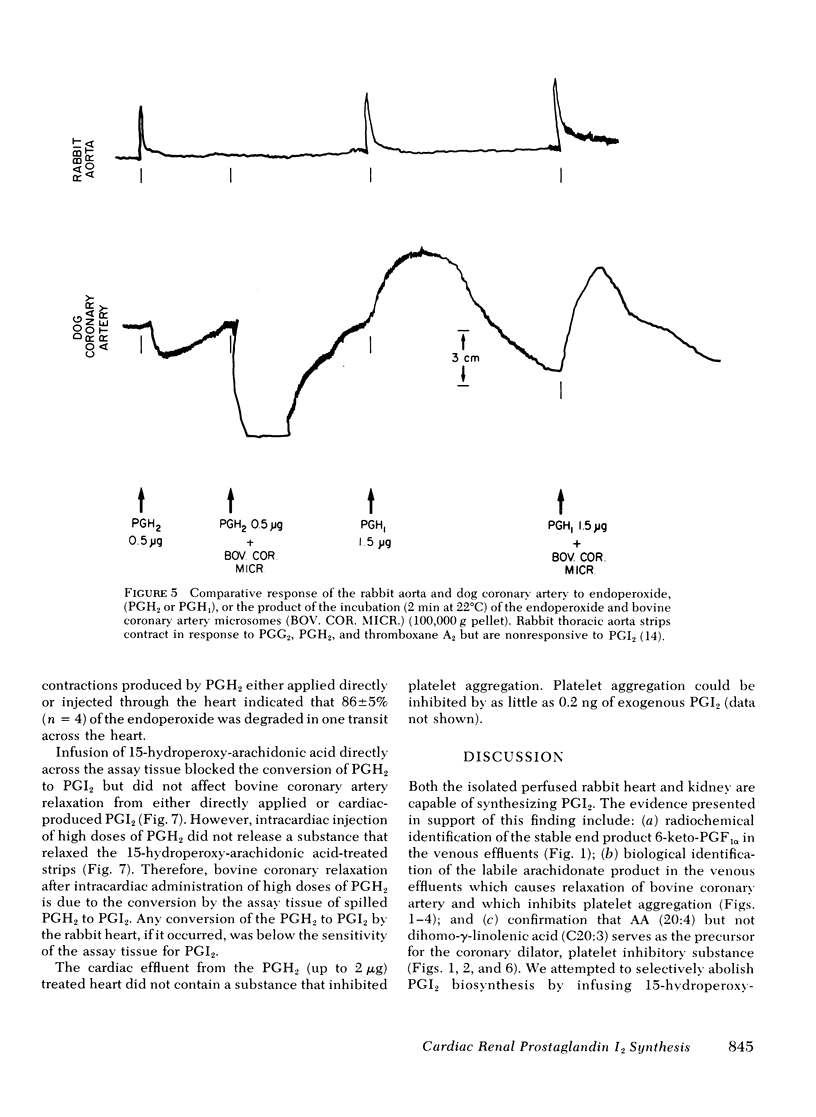
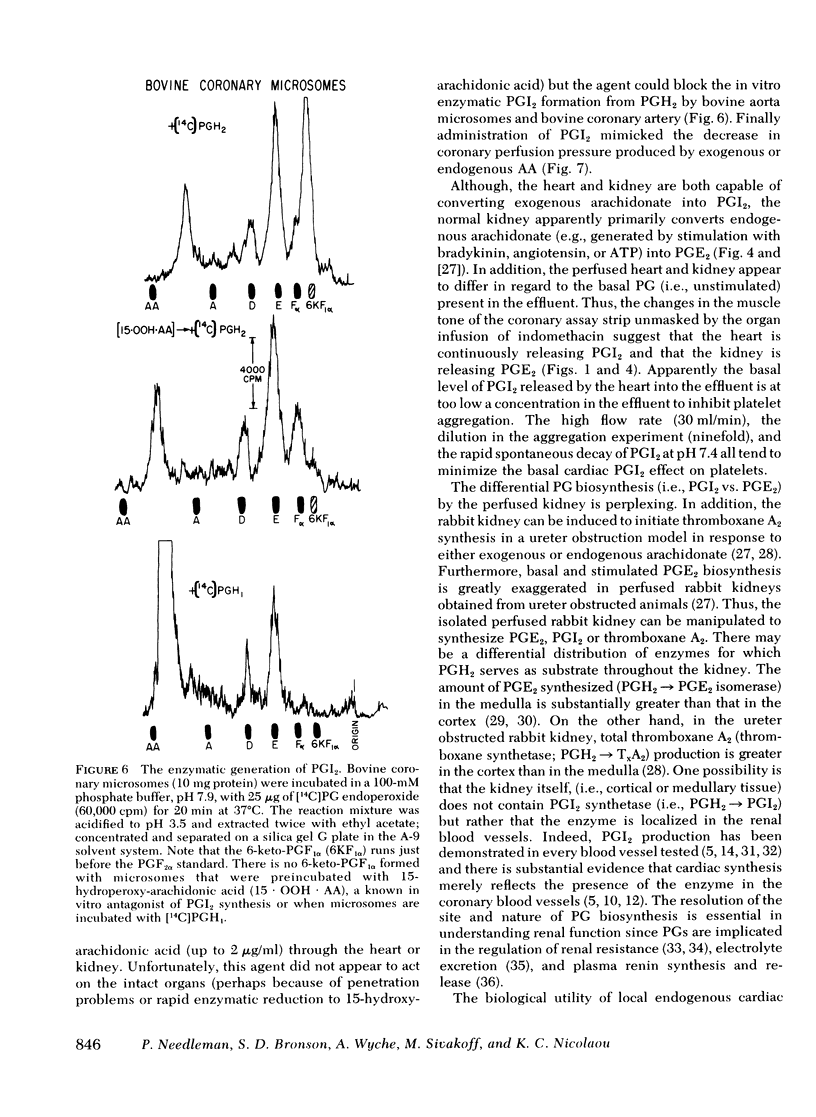
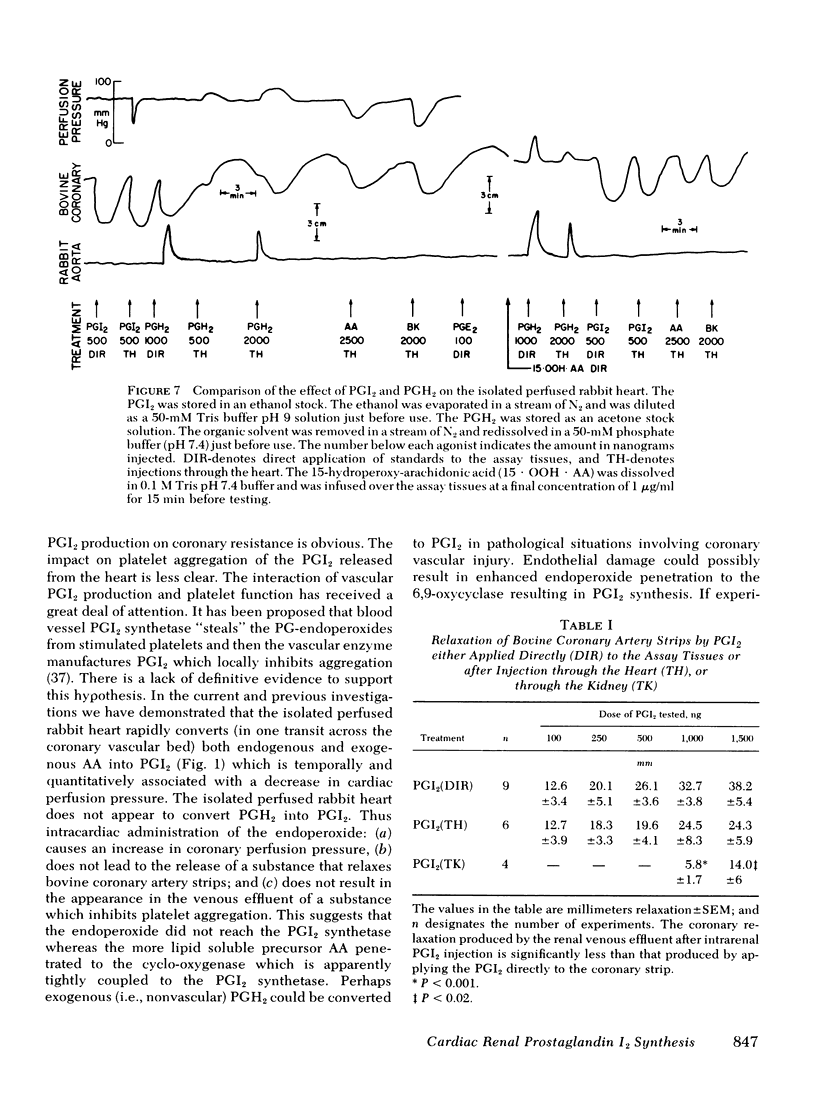
Both the isolated perfused rabbit heart and kidney are capable of synthesizing prostaglandin (PG) I2. The evidence that supports this finding includes: (a) radiochemical identification of the stable end-product of PGI2, 6-keto-PGF1α, in the venous effluent after arachidonic acid administration; (b) biological identification of the labile product in the venous effluents which causes relaxation of the bovine coronary artery assay tissue and inhibition of platelet aggregation; and (c) confirmation that arachidonic acid and its endoperoxide PGH2, but not dihomo-γ-linolenic acid and its endoperoxide PGH1, serve as the precursor for the coronary vasodilator and the inhibitor of platelet aggregation. The rabbit heart and kidney are both capable of converting exogenous arachidonate into PGI2 but the normal perfused rabbit kidney apparently primarily converts endogenous arachidonate (e.g., generated by stimulation with bradykinin, angiotensin, ATP, or ischemia) into PGE2; while the heart converts endogenous arachidonate primarily into PGI2. Indomethacin inhibition of the cyclo-oxygenase unmasks the continuous basal synthesis of PGI2 by the heart, and of PGE2 by the kidney. Cardiac PGI2 administration causes a sharp transient reduction in coronary perfusion pressure, whereas the intracardiac injection of the PGH2 causes an increase in coronary resistance without apparent cardiac conversion to PGI2. The perfused heart rapidly degrades most of the exogenous endoperoxide probably into PGE2, while exogenous PGI2 traverses the heart without being metabolized. The coronary vasoconstriction produced by PGH2 in the normal perfused rabbit heart suggests that the endoperoxide did not reach the PGI2 synthetase, whereas the more lipid soluble precursor arachidonic acid (exogenous or endogenous) penetrated to the cyclooxygenase, which apparently is tightly coupled to the PGI2 synthetase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W., Vane J. R. Intrarenal prostaglandin release attenuates the renal vasoconstrictor activity of angiotensin. J Pharmacol Exp Ther. 1973 Mar;184(3):678–687. [PubMed] [Google Scholar]
- Block A. J., Poole S., Vane J. R. Modification of basal release of prostaglandins from rabbit isolated hearts. Prostaglandins. 1974 Sep 25;7(6):473–486. doi: 10.1016/0090-6980(74)90092-6. [DOI] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Crowshaw K., Szlyk J. Z. Distribution of prostaglandins in rabbit kidney. Biochem J. 1970 Feb;116(3):421–424. doi: 10.1042/bj1160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusting G. J., Moncada S., Vane J. R. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins. 1977 Jan;13(1):3–15. doi: 10.1016/0090-6980(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Funk M. O., Isacc R., Porter N. A. Preparation and purification of lipid hydroperoxides from arachidonic and gamma-linolenic acids. Lipids. 1976 Feb;11(2):113–117. doi: 10.1007/BF02532660. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Sun F. F., Miller O. V., Johnson R. A. Prostaglandins H1 and H2. Convenient biochemical synthesis and isolation. Further biological and spectroscopic characterization. Prostaglandins. 1977 Jun;13(6):1043–1053. doi: 10.1016/0090-6980(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Isakson P. C., Needleman P. Hormone selective lipase activation in the isolated rabbit heart. Prostaglandins. 1977 Jun;13(6):1073–1091. doi: 10.1016/0090-6980(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Denny S. E., Pure E., Needleman P. A novel prostaglandin is the major product of arachidonic acid metabolism in rabbit heart. Proc Natl Acad Sci U S A. 1977 Jan;74(1):101–105. doi: 10.1073/pnas.74.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Needleman P. Selective incorporation of 14C-arachidonic acid into the phospholipids of intact tissues and subsequent metabolism to 14C-prostaglandins. Prostaglandins. 1976 Nov;12(5):739–748. doi: 10.1016/0090-6980(76)90049-6. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Endogenous prostaglandin release contributes directly to coronary artery tone. Can J Physiol Pharmacol. 1975 Aug;53(4):560–565. doi: 10.1139/y75-079. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Intrinsic prostaglandin release. A mediator of anoxia-induced relaxation in an isolated coronary artery preparation. Blood Vessels. 1976;13(3):155–166. [PubMed] [Google Scholar]
- Kulkarni P. S., Roberts R., Needleman P. Paradoxical endogenous synthesis of a coronary dilating substance from arachidonate. Prostaglandins. 1976 Sep;12(3):337–353. doi: 10.1016/0090-6980(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Larsson C., Anggård E. Regional differences in the formation and metabolism of prostaglandins in the rabbit kidney. Eur J Pharmacol. 1973 Jan;21(1):30–36. doi: 10.1016/0014-2999(73)90202-1. [DOI] [PubMed] [Google Scholar]
- Larsson C., Weber P., Anggård E. Arachidonic acid increases and indomethacin decreases plasma renin activity in the rabbit. Eur J Pharmacol. 1974 Oct;28(2):391–394. doi: 10.1016/0014-2999(74)90296-9. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R. J., Bunting S., Vane J. R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976 Nov;12(5):715–737. doi: 10.1016/0090-6980(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature. 1977 May 19;267(5608):259–260. doi: 10.1038/267259a0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Key S. L., Denny S. E., Isakson P. C., Marshall G. R. Mechanism and modification of bradykinin-induced coronary vasodilation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2060–2063. doi: 10.1073/pnas.72.6.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Kulkarni P. S., Raz A. Coronary tone modulation: formation and actions of prostaglandins, endoperoxides, and thromboxanes. Science. 1977 Jan 28;195(4276):409–412. doi: 10.1126/science.831285. [DOI] [PubMed] [Google Scholar]
- Needleman P., Marshall G. R., Sobel B. E. Hormone interactions in the isolated rabbit heart. Synthesis and coronary vasomotor effects of prostaglandins, angiotensin, and bradykinin. Circ Res. 1975 Dec;37(6):802–808. doi: 10.1161/01.res.37.6.802. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Needleman P. The synthesis and function of prostaglandins in the heart. Fed Proc. 1976 Oct;35(12):2376–2381. [PubMed] [Google Scholar]
- Nishikawa K., Morrison A., Needleman P. Exaggerated prostaglandin biosynthesis and its influence on renal resistance in the isolated hydronephrotic rabbit kidney. J Clin Invest. 1977 Jun;59(6):1143–1150. doi: 10.1172/JCI108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. A novel prostaglandin derivative formed from arachidonic acid by rat stomach homogenates. Biochemistry. 1971 Sep 28;10(20):3657–3664. doi: 10.1021/bi00796a004. [DOI] [PubMed] [Google Scholar]
- Raz A., Isakson P. C., Minkes M. S., Needleman P. Characterization of a novel metabolic pathway of arachidonate in coronary arteries which generates a potent endogenous coronary vasodilator. J Biol Chem. 1977 Feb 10;252(3):1123–1126. [PubMed] [Google Scholar]
- Strong C. G., Bohr D. F. Effects of prostaglandins E1, E2, A1, and F1-alpha on isolated vascular smooth muscle. Am J Physiol. 1967 Sep;213(3):725–733. doi: 10.1152/ajplegacy.1967.213.3.725. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Whittaker N., Bunting S., Salmon J., Moncada S., Vane J. R., Johnson R. A., Morton D. R., Kinner J. H., Gorman R. R., McGuire J. C. The chemical structure of prostaglandin X (prostacyclin). Prostaglandins. 1976 Dec;12(6):915–928. doi: 10.1016/0090-6980(76)90126-x. [DOI] [PubMed] [Google Scholar]


