Abstract
After ionising radiation double-strand breaks (dsb) are lethal if not repaired or misrepaired. Cell killing is greatly enhanced by hyperthermia and it is questioned here whether heat not only affects dsb repair capacity but also fidelity in a chromosomal context. dsb repair experiments were designed so as to mainly score non-homologous end joining, while homologous recombination was largely precluded. Human male G0 fibroblasts were either preheated (45°C, 20 min) or not before X-irradiation. dsb induction and repair were measured by conventional gel electrophoresis and an assay combining restriction digestion using a rare cutting enzyme (NotI) and Southern hybridisation, which detects large chromosomal rearrangements (>100 kb). dsb induction rate in an X-chromosomal NotI fragment was 4.8 × 10–3 dsb/Gy/Mb. Similar values were found for the genome overall and also when cells were preheated. After 50 Gy, fibroblasts were competent to largely restore the original restriction fragment size. Five per cent of dsb remained non-rejoined and 14% were misrejoined. Correct restitution of restriction fragments occurred preferably during the first hour but continued at a slow rate for 12–16 h. In addition, dsb appeared to misrejoin throughout the entire repair period. After hyperthermia the fractions of non-rejoined and misrejoined dsb were similarly increased to 13 and 51%, respectively. It is suggested that heat increases the probability of dsb being incorrectly rejoined but it is not likely to interfere with one dsb repair pathway in particular.
INTRODUCTION
DNA double-strand breaks (dsb) are considered the most critical lesions after ionising radiation (IR). Cell fate is in particular determined by the result of dsb repair. Non-rejoined as well as misrejoined dsb possibly give rise to chromosomal breaks, exchanges, translocations and loss of heterozygocity, which are all potentially lethal. Numerous efforts have thus been made by us and others to understand the kinetics, outcome and consequences of dsb repair. The kinetics can uniformly be described by three components, a fast and a slow repair phase and a constant component of non-repairable dsb (1–3). We sought to identify the nature of these repair components and found that neither of them depend on the size of DNA fragments involved (1). Using repair-deficient mutants we found that the slow repair kinetics are due to high numbers of non-repaired dsb rather than to differences in repair velocity (4,5). The different capacities to rejoin dsb were shown to closely correlate with cell survival. This was not only true for repair-deficient mutants (2,5,6), but also for normal fibroblasts (3), tumour cells (7,8) and cells treated with different types of radiation (9,10)
The number of non-repairable dsb was also shown to be considerably enhanced when cells were heated prior to irradiation (11–15). Moreover, the amount of residual damage was found to correlate well with the pronounced cell inactivation achieved by hyperthermia combined with irradiation (13,14). We suggested that this mechanism is relevant and may largely contribute to heat effects observed in clinical settings, as hyperthermia significantly improved the tumour response and cure rates of radiotherapy (16,17). The few chromosomal studies conducted hitherto showed that not only the number of frank breaks were increased but also exchange aberrations (18), suggesting that hyperthermia might promote misrepair, resulting in alterations to the gross chromosomal structure.
Recently Löbrich and co-workers introduced a method to detect misrepair at the DNA level by scoring the joining of correct and incorrect DNA ends which flank dsb introduced by IR. The method combines restriction digestion of genomic DNA followed by gel separation with Southern hybridization (19,20). Protection of intact DNA in agarose allowed restriction with rare cutting enzymes, such as NotI, which leads to large fragments of up to 3.5 Mb. Fragments of this size need only moderate radiation doses (i.e. 50–80 Gy) to be hit and to enable repair to be followed. It is currently a unique method to quantify correct repair in a natural chromosomal context. In contrast, cytogenetic methods only score erroneous repair. It was shown that high LET radiation not only prevents overall dsb rejoining, but also increases misrepair (19,21). Misrejoining was further shown to depend on the X-ray dose (22), but not on chromosomal location of the repair event (20,22). No differences were observed for misrejoining in human fibroblast, tumour or rodent cells (22,23), which all had a repair-proficient phenotype. In repair-deficient cells (e.g. XRCC5), however, correct repair appeared drastically reduced and misrejoining was also noted (M.Löbrich, personal communication).
Here we investigate the effect of heat on correct and incorrect dsb repair using the above method. Correct rejoining is considered a repair event, which joins proper dsb ends and thus retains the gross chromosomal structure. The underlying repair process is likely to be non-homologous end joining (NHEJ), as this is the dominant pathway in mammalian cells (24,25). NHEJ, however, usually leaves small deletions or insertions of several bases (for a review see 26). Mammalian chromatin tolerates these minor lesions and even loss of up to 1000 kb as long as non-essential sequences are involved (27,28). In contrast, conservative error-free repair can only be achieved by gene conversion through homologous recombination. Other homology-based pathways, such as single-strand annealing (SSA) (29) and break-induced replication (BIR) (30), as well as non-homologous recombination (31–33), can result in junctions between distant DNA ends and sequences. All these products produce altered restriction fragment sizes, in the present study designated non-correct repair.
The experimental system was designed so as to measure the dominant NHEJ pathway and to largely exclude homologous recombination. Non-transformed human fibroblasts were strictly kept in plateau phase (95% in G0) to eliminate any replication–repair connection (30,34). An X-chromosomal fragment was studied in male cells, hence providing no obvious homology.
MATERIALS AND METHODS
Cell culture
Primary human fibroblasts were obtained by skin biopsy and propagated as described elsewhere (J.Dahm-Daphi, manuscript submitted). Cells were maintained in Dulbecco’s modified Eagle’s medium containing 15% foetal calf serum under an incubation atmosphere of 95% air, 5% CO2. Experiments were done between passages 4 and 10 with 4 day confluent cultures with 95% of cells in G0 phase.
Hyperthermia and irradiation
Cells were heated for 20 min in a 45°C water bath and then immediately cooled to 4°C for irradiation. Irradiation was performed on ice using a Philips X-ray machine (220 kVp, 15 mA) at a dose rate of 4 Gy/min.
Cell survival
Plateau phase cells were trypsinised immediately after irradiation with or without preheating and plated at appropriate dilutions. Cells were grown for 2 weeks for colony formation. Those having >50 cells were counted. Results were normalised to the plating efficiency of unirradiated cells.
dsb in the overall genome: constant field gel electrophoresis (CFGE)
Experiments were performed as described previously (1). Briefly, cells were washed and suspended in prewarmed 0.8% low melting point agarose (Bio-Rad, Munich, Germany), resulting in a concentration of 3 × 106 cells/ml. The liquid suspension was immediately pipetted into plug-forming moulds and was allowed to solidify at 4°C. The agarose plugs were irradiated on ice in Eppendorf vials. Immediately after irradiation cell lysis (0.4 mol/dm3 EDTA, 2% sodium N-laurylsarcosine, 1 mg/ml proteinase K, pH 8.0; all Sigma-Aldrich, Deisenhofen, Germany) was started for 30 min on ice and continued overnight at 37°C. Subsequently the plugs were washed three times in TE buffer (10 mmol/dm3 Tris, 1 mmol/ dm3 EDTA, pH 8.0; Sigma) and sliced into pieces containing ∼1 × 105 cells. These pieces were inserted into an agarose gel and electrophoresis was performed (30 h at 0.6 V/cm) in 0.5× TBE buffer. The gel was then stained overnight (0.5 µg/ml ethidium bromide), destained and the UV fluorescence recorded using a CCD video camera. The fraction of DNA released into the gel (FDR), corresponding to fragmented DNA, was calculated as
FDR = FIrel/(FIplug + FIrel)
where FIrel and FIplug correspond to the fluorescence intensity of DNA released and retained in the well, respectively.
dsb in a restriction fragment: pulsed field gel electrophoresis (PFGE)/Southern hybridisation
Cells were embedded in agarose as described above but at a higher cell density of 106 cells/100 µl plug. Cell lysis was continued for 36 h at 50°C with one buffer exchange. Thereafter plugs were washed six times for 1 h at room temperature and once overnight at 4°C in large volumes of TE buffer to remove all traces of EDTA and sarcosine. Plugs were equilibrated in 250 µl of restriction buffer, which was then replaced by buffer plus 20 U NotI enzyme (Oncor, Heidelberg, Germany), followed by overnight incubation. After insertion of plugs the agarose gel was covered by a second thin layer of agarose, enabling DNA transfer to a membrane not only from the gel but also from the plugs. PFGE (CHEF-mapper system; Bio-Rad) was performed at 14°C in 0.5× TBE buffer for 120 h at 1.5 V/cm with increasing switch times ranging from 1 to 60 min with an included angle of 106°. The gel was stained and photographed. DNA was vacuum transferred (7.5 cm H2O, 2.5 h) to a nylon membrane (Hybond N; Amersham, Braunschweig, Germany) under alkaline conditions (0.4 mol/dm3 NaOH, 1 mol/dm3 NaCl). The membrane was then briefly washed in 2× SSC.
A 5.9 kb EcoRI fragment embracing the X-chromosomal marker DXS53 (35) was used as probe. Aliquots of 0.3 ng/cm2 denatured DNA were labelled overnight using a Rediprime kit (Amersham) and [32P]dCTP (sp. act. 222 TBq/mol). Non-incorporated nucleotides were removed on 200-S spin columns (MoBiTech, Göttingen, Germany). Membranes were prehybridised overnight at 65°C in 0.2 ml/cm2 of 5× SSPE, 0.5% SDS, 5× Denhardt’s solution plus 100 µg/ml denatured salmon sperm DNA. Hybridisation (0.075 ml/cm2 + probe) was continued overnight at 65°C. The membranes were then washed twice in 2× SSPE, 0.1% SDS (30 min at room temperature) and twice in 1× SSPE, 0.1% SDS (65°C). The membranes were rinsed in 2× SSC and exposed to a phosphorimager screen (Fujix BAS 2000; Fuji, Straubenhardt, Germany). Signal intensities (minus background) were quantified by defining rectangles around each band and the entire lane, respectively, using the manufacturer’s software.
Statistical analysis
Data are given as a means (± SEM) of three independent experiments. Statistical analyses, data fitting and graphics were performed using the GraphPad Prism program (San Diego, CA).
RESULTS
Hyperthermia enhances cellular radiosensitivity
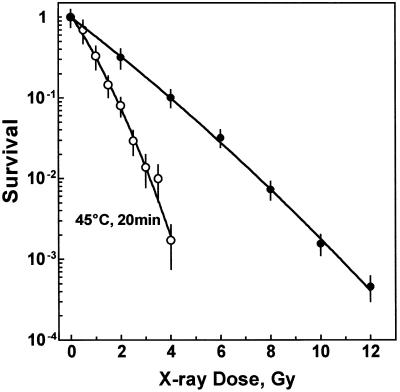
Figure 1 shows cell survival after X-irradiation alone or combined with a preceding heat treatment of 45°C for 20 min. Hyperthermia alone decreased colony formation to 29 ± 7% of that of non-heated cells. In addition, it strongly increased the radiosensitivity of primary fibroblasts. Comparing equitoxic doses, a thermal enhancement ratio (TER10%) of 2.2 was calculated.
Figure 1.
Cell survival. Cells were either preheated at 45°C for 20 min (open circles) or mock treated (closed circles). Cells were then irradiated on ice and immediately plated for colony formation. Survival of irradiated cells was normalized to survival of their non-irradiated counterparts.
Induction of DNA dsb in chromosomal NotI restriction fragments
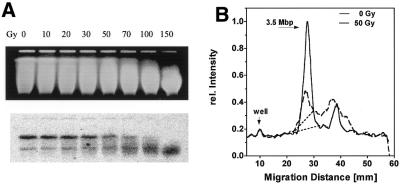
Figure 2 shows the induction of DNA dsb by various X-ray doses (Fig. 2A, top, EB gel; bottom, Southern blot). The radiation effect was reflected in an increasing shift of the DNA smear towards smaller molecular weights (Fig. 2A, top). Southern blots revealed two specific bands of varying intensity (compare experiments depicted in Figs 2, 4 and 5) the sizes of which were determined to be 3.5 and 1.8 Mb by comparing their migration distances with those of yeast chromosomes (Fig. 4A and 5A, top). The exact nature of the smaller band is not clear. A GenBank search for homologous sequences at other chromosomal locations was non-productive. An alternative explanation might be an additional NotI site within the 3.5 Mb fragment which eventually escaped from restriction, i.e. by methylation. The intensity of the smaller band varied between, but importantly not within, experiments (see Figs 2–5). The 3.5 Mb band is likely to be equivalent to the X-chromosomal NotI fragment sized by Lippert et al. at 3 Mb (36) and further characterized by Rothkamm and Löbrich (22). These papers did not mention an optional NotI site.
Figure 2.
Induction of dsb by X-rays. Cells were embedded in agarose, irradiated on ice and lysed. DNA was then digested with NotI and resolved in a pulsed field gel. (A) The gel stained with ethidium bromide (top) was then blotted and hybridized against the DXS53 probe (bottom). Signals were detected using a phosphorimager. (B) Intensity profiles for the entire lanes 1 and 5.
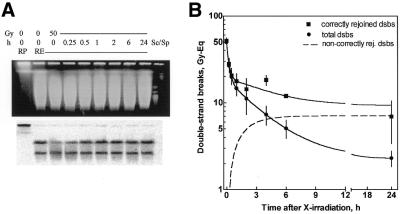
Figure 4.
Repair of dsb. Fibroblasts were irradiated, incubated for repair and prepared for two assays. (A) Restitution of NotI fragments as a measure of correct rejoining. Lane 1, 0 Gy, restriction buffer; lane 2, 0 Gy, NotI; lanes 3–9, 50 Gy, repair as indicated, NotI; lanes 10 and 11, S.pombe and S.cerevisiae chromosomes as size markers. (B) Band destruction and restitution were quantified using a phosphorimager and expressed as remaining damage (squares). The overall rejoining was measured using CFGE (circles). Both data sets, as well as their differences (dashed line, see text), were fitted by non-linear regression.
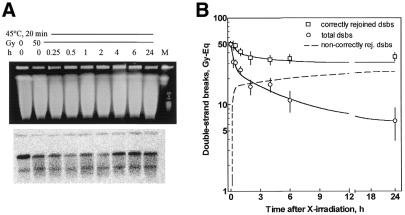
Figure 5.
Repair of dsb after heat treatment (45°C, 20 min). Experiments, analysis and blots are as described in the legend to Figure 4. (A) Lane 1, 0 Gy, NotI; lanes 2–9, 50 Gy, repair, NotI; lane 10, marker.
For quantification of radiation and repair effects, in the following only the intensity of the larger band was quantified and related to the overall radioactivity of each lane individually (Irel). This approach measures destruction and restoration of the specific NotI fragment independent of the variation in cell load. With increasing dose the intensity of the 3.5 Mb band decreased and it was completely extinguished beyond 100 Gy. A smear of smaller fragments became prominent instead. It should be noted that the intensity of the smaller band apparently increased with dose. This effect is due to the superimposed smear from the larger band. dsb induction was also measured in cells which were heated to 45°C for 20 min and irradiated immediately thereafter at 4°C without allowing for repair. These experiments gave identical pictures to those in Figure 2A (data not shown). The number of dsb induced within the 3.5 Mb fragment was equivalent to
Ndsb = –ln(Irel/Icon)
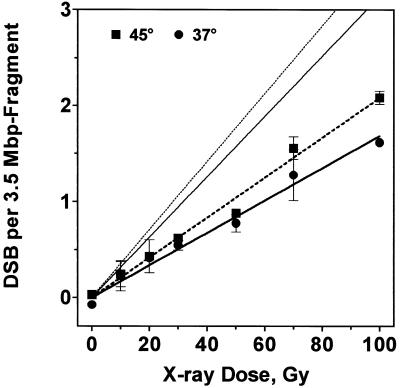
where Irel and Icon are the relative intensities of the respective band and of the unirradiated control. Figure 3 shows the number of radiation-induced dsb for heated and non-heated cells increasing linearly with dose. The induction rate was 4.8 ± 0.2 × 10–3 dsb/Gy/Mb for non-heated and 5.9 ± 0.2 × 10–3 dsb/Gy/Mb for preheated cells (not significantly different, Student’s t-test, P = 0.12). Recently Rothkamm and Löbrich (22) favoured an alternative approach for dsb quantification which takes into account that the band signal represents not only intact fragments but also those containing a dsb in close proximity to either NotI site. If the fraction of these slightly smaller fragments (assumed to correspond to the area below the straight lines in Fig. 2B) is considered as non-intact DNA then dsb induction would nearly double to 9.2 and 10.1 × 10–3 dsb/Gy/Mb for non-heated and preheated cells, respectively (steeper lines without symbols in Fig. 3B).
Figure 3.
From relative band intensities dsb induction rate was calculated for non-heated and preheated cells (circles and squares) and fitted by linear regression. Alternatively, intensity profiles of lanes of the Southern blot (as shown in Fig. 2B) were used to calculate dsb rate (see text), giving 2-fold higher rates (straight lines without symbols).
Overall dsb rejoining in the whole genome and correct rejoining in a NotI fragment
Rejoining of dsb in the genome overall was recorded by means of CFGE for heated and non-heated cells. The FDR measured after 50 Gy and repair incubation was calibrated from induction curves (data not shown) as described previously (1) and the remaining damage was expressed as dose equivalents (Fig. 4B, circles). Initially the repair proceeded quickly (half-time τ = 9 min), followed by a slower component (τ = 2 h), finally reaching a constant plateau, analogous to our previous observations (1–3). In parallel, repair was also recorded as restitution of the NotI fragment using the combined PFGE/Southern assay (Fig. 4A). Irradiation with 50 Gy clearly reduced the signal of intact NotI fragment (Fig. 4A, bottom, lane 3), which was restored upon repair incubation (Fig.4A, bottom, lanes 4–9). The band intensity appeared to reach the control level within 1–2 h. Quantitative determination also revealed a fast and a slow phase for this correct rejoining process (Fig. 4B, squares). During the first hour both assays (overall and correct repair) showed nearly identical kinetics, which suggests that all fast rejoined dsb are correctly rejoined. During the slow phase, however, the kinetics of overall and correct rejoining diverged, indicating that not all dsb are correctly rejoined. The difference between correct and overall repair corresponds to misrejoining (20,23), for which the kinetics were calculated (Fig. 4B, dashed line, a single exponential function). This analysis revealed that misrejoining might start virtually ab initio. However, due to small numbers it was not obvious before 1 h after irradiation. Lastly, the fraction of misrejoined dsb amounted to 14%, which was about three times more than the fraction of non-rejoined dsb (5%) (Table 1).
Table 1. Parameters of overall, correct and incorrect dsb repair.
| Fraction of total damage after 50 Gy (%) | ||||
| Non-heated | Preheated (45°C, 20 min) | |||
| |
Gy equiv |
(%)a |
Gy equiv |
(%)a |
| Nfast | 31.3 | (62) | 23.1 | (49) |
| Nslow | 16.2 | (32) | 17.5 | (37) |
| Nnon-rep | 2.3 | (5) | 6.3 | (13) |
| Ncorrect | 40.5 | (82) | 16.5 | (35) |
| Nnon-correct | 7.0 | (14) | 24.1 | (51) |
Nfast, Nslow and Nnon-rep are derived from the fit to the overall genome data (FDR, Figs 3B and 4B, circles), it should be noted that for heated cells best fits converged to an initial damage of 46.9 Gy equiv instead of the actual 50 Gy.
Ncorrect are derived from the fit to the data on restitution of the restriction fragment (NotI assay, Figs 4B and 5B, squares).
Nnon-correct are derived from the fit to the difference between Ncorrect and Nnon-rep (Figs 4B and 5B, dashed lines).
aFractions as related to the initial damage without repair.
Hyperthermia inhibits both overall rejoining and rejoining of correct ends
It is known from several cell systems (11,12,14,15) that heat hinders the repair machinery leading to increased numbers of non-repairable dsb. This was confirmed here for primary human fibroblasts after combined preheating and IR (Fig. 4B, circles, compared to 5B, circles, and Table 1). The overall kinetics after hyperthermia also showed a fast and a slow repair phase and a final plateau. The fraction of fast rejoined dsb was reduced while the fraction of slowly and non-rejoined dsb was enhanced, which apparently slowed down the overall kinetics. Analogous repair experiments were conducted for restitution of NotI restriction fragments (Fig. 5A) to elucidate whether hyperthermia has an impact on repair fidelity. It can be seen from Figure 5A (bottom) that band restitution proceeded much slower compared to non-heated cells and never reached the level of controls. Most importantly, repair curves (Fig. 5B) showed an immediate divergence of overall and correct rejoining indicative of a large number of misrejoined dsb arising early during the repair process. Moreover, the kinetics of misrejoining (Fig. 5B, dashed line) and overall rejoining (Fig. 5B, circles) proceeded with fairly similar kinetics (τ = 9 versus 10 min), confirming that initial repair was predominantly erroneous, which is the opposite of non-heated cells. After repair had been completed many more dsb remained either non-rejoined (13%) or misrejoined (51%) as compared to non-heated cells (5 and 14%, respectively) (Table 1). However, the ratio between both types of residual damage remained similar for the normal and heated conditions.
DISCUSSION
The present study shows that human fibroblasts largely repair radiation-induced dsb. Even after high doses of 50 Gy cells are competent to efficiently restore DNA continuity (rejoining of fragmented DNA) and to mostly correctly rejoin DNA ends, as original NotI restriction fragment sizes were recovered (only 14% misrejoining). We found correct rejoining to proceed mainly during the fast but also during the slow repair phase. The high fidelity recorded here contrasts with previous observations of 30–50% misrejoining after comparable X-ray doses (20–23,37,38). The observation of a long-lasting correct repair process was also not shared by those studies. Löbrich and colleagues found no correct NotI fragment restitution beyond 1–2 h using the same method and suggested a separation between a fast correct and a slow error-prone repair process. The present analysis further shows that incorrect rejoining might start immediately after irradiation and continue for the entire repair period, as described by Fouladi et al. (23). Our results can be summarised as follows. One fraction of dsb is mainly quickly and correctly rejoined. To some extent this correct rejoining continued later with much slower kinetics. Another fraction of dsb is subjected to incorrect repair exclusively with slower kinetics that is, hence, only perceptible during the slow phase. A third fraction of dsb remains non-repairable. Either repair outcome is probably determined by the process which keeps DNA ends together (see below). The discrepancies in kinetics and extent of repair compared to the above-mentioned studies cannot be sufficiently explained, neither by log versus linear plots nor by the slightly lower doses applied here (see 38).
Preceding hyperthermia considerably modified dsb repair in human fibroblast, as was similarly reported for other cell systems (11,12,14,15,39–41). However, this process appeared not simply retarded but to be affected in a complex manner with an impact on completeness and fidelity. Correct repair was reduced by heat while the fractions of non-repairable and misrejoined dsb were both enhanced (Table 1). Kinetic analysis showed that misrejoined dsb arose extremely early at the same rate as overall rejoining took place (Fig. 5B), which can be explained in several ways. (i) After preheating the dsb repair complex may simply fail to bring together the correct DNA ends and rejoining is predominantly erroneous. The way promiscuous joining is performed should be different for hyperthermic and normal conditions, as judged from the kinetics (τ of 10 versus 70 min). It is, however, unlikely that this misrepair process operates faster in heated than in non-heated cells. (ii) High numbers of misrejoined dsb can also be explained by additional dsb, which we have previously shown arise during repair in heated CHO (14) and HeLa cells (15). We have suggested clustered base lesions as critical precursors. Hyperthermia permits DNA to be incised at these loci but prevents or delays their complete repair, leaving new open dsb. These additional dsb could accumulate quickly due to the high level of clustered damage present after IR and can easily explain the early detection of misrejoined dsb. If heat only increases the number of candidates for repair failure but has no impact on the repair process per se, both non-repaired and misrepaired dsb should be similarly enhanced. This was in fact found for radiation-induced chromosome aberrations after prior heating. The numbers of both deletions and exchanges were increased, but their ratio remained constant (18). We consider these types of aberrations equivalent to non-rejoined and misrejoined dsb as recorded here. Our data likewise suggest (within experimental limits) that each dsb which cannot be repaired correctly has the same probability of remaining non-rejoined or of finding an incorrect partner independent of hyperthermic or normal conditions. However, it cannot be excluded that heat additionally favours the misrejoining process, as the increase in misrejoined dsb was slightly higher (3.6-fold) than that of non-rejoined dsb (2.6-fold).
The Ku–DNA-PK complex is considered the most important dsb repair protein in mammalian cells, in particular for the dominant NHEJ pathway. Our experiments were designed so as to measure non-homologous repair and to largely exclude homologous recombination (we made use of an X-chromosomal probe in male G0 fibroblasts). Ku protein is likely to permit correct dsb rejoining due to its alignment function (42,43) and it was suggested to be thermally inactivated (44). It was shown, however, not to be a critical heat target as equal thermal radiosensitisation could be achieved in Ku–DNA-PK-proficient and -deficient cells (45). Heat inactivation of both Ku and DNA-PK (44) appear not to be responsible for the above heat effects. These results support our view that an increase in critical lesions during repair in heated cells rather than an altered dsb rejoining process leads to more non-rejoined dsb and also to more incorrect joining. However, it should be noted that a defective repair pathway alone can produce a similar outcome. Chromosomal breaks and exchanges were found to be enhanced in ATM- (46), NBS- (47), Ku- and DNA-PK-deficient cells (48,49). In yeast, gross chromosomal rearrangements particularly were observed when cells lacked components of the mre11/rad50/xrs2 complex (and RFA1 and rad27) (50). From these and from our results it can be speculated as to whether these phenotypes have a common basic mechanism, i.e. a failure to bring the correct ends together. After hyperthermia incomplete processing of base damage might hinder other enzymes (such as Ku) from bridging the open dsb, which then favours promiscuous end joining or complete repair failure. The same outcome can be expected when the bridging enzymes (such as Ku) or putative regulators (such as ATM, DNA-PK or Mre11 complex) are missing. The notion that hyperthermia is comparable to genetic repair defects gains support from their parallel effects on cell killing. We found that the ∼3-fold increase in non-rejoined and misrejoined dsb is associated with a 2.2-fold reduced survival after hyperthermia, which was similar to the observed increase in chromosomal aberrations (18). Even closer associations were found in ATM-, Ku- and DNA-PK-deficient strains for non-repairable dsb (2), chromosomal aberrations (51) and cell killing as compared to normal cells. Data for misrejoining have not yet been published.
In the present study we have found a dsb induction rate of 4.8 × 10–3 dsb/Gy/Mb in non-heated fibroblasts, in line with previous studies using the same technique (20,52). A recent paper reported a higher rate of 10 × 10–3 dsb/Gy/Mb (22), based on a different quantification procedure of band and smear intensities. In our hands this approach resulted in an induction rate of 9.2 × 10–3 dsb/Gy/Mb. The higher rate critically depends on the extent to which slightly smaller but yet broken NotI fragments contribute to the band signal. The assumption that this contribution increases linearly (as reflected by the line drawn under the 3.5 Mb band of the 50 Gy profile, Fig. 2B) might be an overestimation. For comparison we also applied graded field gel electrophoresis (GFGE) (53), which relies on fragment distribution in the whole genome, and found an induction of 5.2 ± 0.2 × 10–3 dsb/Gy/Mb averaged from seven fibroblast strains (data not shown). We conclude that the more conservative analysis might give more reasonable results, though in principle the new approach appears correct. As discussed recently by Kühne et al. (38), the number of chromosomal aberrations is generally lower by one order of magnitude than that of DNA misrejoining events, as recorded here. This may be partly due to the required size of aberrant chromosomal fragments (more than several Mb) to be visible under the light microscope. Smaller rearrangements (such as those measured here, >100 kb) will escape optical detection. However, chromosomal rearrangements involving only a few kb, as measured by inverse PCR, were found at a frequency of ∼5 × 10–3 rearrangements/Mb/50 Gy (recalculated from 54). This is also 10-fold lower than the present value (7 Gy equivalents of incorrectly joined dsb after 50 Gy corresponds to ∼34–64 × 10–3 rearrangements/Mb/50 Gy). This indicates that the deviations in the frequencies are not simply due to the sensitivity of the method. The underlying mechanisms, however, are not understood.
Two recent investigations using this assay for incorrect repair found rearranged NotI restriction fragments which were smaller and also larger than the original size (22, figs 3 and 6; 23, figs 1 and 2). In the present study we never observed NotI fragments >3.5 Mb, as illustrated in Figures 4A and 5A. At the start of these experiments it became obvious that harsh cell lysis and restriction conditions are required for complete NotI digestion of DNA embedded in agarose. Less stringency led to incomplete digestion, with hybridisation signals over the whole size range, including the well. The presumably slight overdigestion in our experiments may diminish the probability of detecting fragments of larger size than the original 3.5 Mb. Secondly, NotI digestion produces an average size of 1.2 Mb, hence, broken restriction fragments of high molecular weight (here 3.5 Mb) are more likely to find smaller partners, thus leaving new smaller restriction fragments. An additional bias towards reduced fragment sizes can be expected when SSA contributes significantly to repair. Although the experimental set-up does not favour homologous recombination, SSA (or BIR) cannot be excluded (i.e. between Alu sequences) and might be coupled with extensive degradation of intervening sequences.
In conclusion, dsb repair after IR leads to largely correct restitution of the chromosomal structure of G0 fibroblasts, presumably by the NHEJ pathway. Beside this correct repair, minor fractions of dsb were non-rejoined and misrejoined. When IR was combined with prior heating both aberrant repair products were increased, which might critically determine enhanced cell killing.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Dr M.Löbrich for introduction to and help with the NotI assay, Drs P.K.Cooper and A.Kronenberg for helpful advice. R.A.E.A. was supported by the Deutsche Akademische Austauschdienst, E.D. and J.D.D. were supported by the Deutsche Forschungsgemeinschaft (grant Di-457-2-2).
References
- 1.Dahm-Daphi J. and Dikomey,E. (1996) Rejoining of DNA double-strand breaks in X-irradiated CHO cells studied by constant- and graded-field gel electrophoresis. Int. J. Radiat. Biol., 69, 615–621. [DOI] [PubMed] [Google Scholar]
- 2.Dikomey E., Dahm-Daphi,J., Brammer,I., Martensen,R. and Kaina,B. (1998) Correlation between cellular radiosensitivity and non-repaired double-strand breaks studied in nine mammalian cell lines. Int. J. Radiat. Biol., 73, 269–278. [DOI] [PubMed] [Google Scholar]
- 3.Dikomey E., Brammer,I., Johansen,J., Bentzen,S.M. and Overgaard,J. (2000) Relationship between DNA double-strand breaks, cell killing and fibrosis studied in confluent skin fibroblasts derived from breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys., 46, 481–490. [DOI] [PubMed] [Google Scholar]
- 4.Dahm-Daphi J., Dikomey,E., Pyttlik,C. and Jeggo,P. (1993) Reparable and non-reparable DNA strand breaks induced by X-irradiation in CHO K1 cells and the radiosensitive mutants xrs1 and xrs5. Int. J. Radiat. Biol., 64, 19–26. [DOI] [PubMed] [Google Scholar]
- 5.Kasten U., Borgmann,K., Burgmann,P., Li,G. and Dikomey,E. (1999) Overexpression of human Ku70/Ku80 in rat cells resulting in reduced dsb repair capacity with appropriate increase in cell radiosensitivity but with no effect on cell recovery. Radiat. Res., 151, 532–539. [PubMed] [Google Scholar]
- 6.Dahm-Daphi J., Dikomey,E. and Pyttlik,C. (1994) Relationship between non-reparable strand breaks and cell survival studied in CHO, CHO K1 and the radiosensitive mutants xrs1 and xrs5. Int. J. Radiat. Biol., 65, 657–663. [DOI] [PubMed] [Google Scholar]
- 7.Giaccia A.J., Schwartz,J., Shieh,J. and Brown,J.M. (1992) The use of asymmetric field inversion gel electrophoresis to predict tumor cell radiosensitivity. Radiother. Oncol., 24, 231–238. [DOI] [PubMed] [Google Scholar]
- 8.Nunez M.I., McMillan,T.J., Valenzuela,M.T., Ruiz de Almodovar,J.M. and Pedraza,V. (1996) Relationship between DNA damage, rejoining and cell killing by radiation in mammalian cells. Radiother. Oncol., 39, 155–165. [DOI] [PubMed] [Google Scholar]
- 9.Ritter M.A., Cleaver,J.E. and Tobias,C.A. (1977) High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature, 266, 653–655. [DOI] [PubMed] [Google Scholar]
- 10.Rydberg B., Löbrich,M. and Cooper,P.K. (1994) DNA double-strand breaks induced by high-energy neon and iron ions in human fibroblasts. I. Pulsed-field gel electrophoresis method. Radiat. Res., 139, 133–141. [PubMed] [Google Scholar]
- 11.Iliakis G., Seaner,R. and Okayasu,R. (1990) Effects of hyperthermia on the repair of radiation-induced DNA single- and double-strand breaks in DNA double-strand break repair-deficient and repair-proficient cell lines. Int. J. Hyperthermia, 6, 813–833. [DOI] [PubMed] [Google Scholar]
- 12.Nevaldine B., Longo,J.A. and Hahn,P.J. (1994) Hyperthermia inhibits the repair of DNA double-strand breaks induced by ionizing radiation as determined by pulsed-field gel electrophoresis. Int. J. Hyperthermia, 10, 381–388. [DOI] [PubMed] [Google Scholar]
- 13.Dikomey E. and Jung,H. (1995) Correlation between thermal radiosensitization and slowly-rejoining DNA strand breaks in CHO cells. Int. J. Radiat. Biol., 68, 227–233. [DOI] [PubMed] [Google Scholar]
- 14.Dahm-Daphi J. Brammer,I. and Dikomey,E. (1997) Heat effects on the repair of DNA double-strand breaks in CHO cells. Int. J. Radiat. Biol., 72, 171–179. [DOI] [PubMed] [Google Scholar]
- 15.Kampinga H.H., Hiemstra,Y.S., Konings,A.W.T. and Dikomey,E. (1997) Correlation between slowly repairable double-strand breaks and thermal radiosensitization in the human HeLa S3 cell line. Int. J. Radiat. Biol., 72, 293–301. [DOI] [PubMed] [Google Scholar]
- 16.Overgaard J., Gonzalez Gonzalez,D., Hulshof,M.C.C.H., Arcangeli,G., Dahl,O., Mella,O. and Bentzen,S.M. (1996) Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int. J. Hyperthermia, 12, 3–20. [DOI] [PubMed] [Google Scholar]
- 17.van der Zee J., Gonzalez-Gonzalez,D., van Rhoon,G.C., van Dijk,J.D., van Putten,W.L. and Hart,A.A. (2000) Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours. A prospective, randomized, multicentre trial. Dutch Deep Hyperthermia Group. Lancet, 355, 1119–1125. [DOI] [PubMed] [Google Scholar]
- 18.Dewey W.C., Sapareto,S.A. and Betten,D.A. (1978) Hyperthermic radiosensitization of synchronous Chinese hamster cells: relationship between lethality and chromosomal aberrations. Radiat. Res., 76, 48–59. [PubMed] [Google Scholar]
- 19.Löbrich M., Rydberg,B. and Cooper,P.K. (1994) DNA double-strand breaks induced by high-energy neon and iron ions in human fibroblasts. II. Probing individual NotI fragments by hybridization. Radiat. Res., 139, 142–151. [PubMed] [Google Scholar]
- 20.Löbrich M., Rydberg,B. and Cooper,P.K. (1995) Repair of X-ray-induced DNA double-strand breaks in specific NotI restriction fragments in human fibroblasts: joining of correct and incorrect ends. Proc. Natl Acad. Sci. USA, 92, 12050–12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Löbrich M., Cooper,P.K. and Rydberg,B. (1998) Joining of correct and incorrect DNA ends at double-strand breaks produced by high-linear energy transfer radiation in human fibroblasts. Radiat. Res., 150, 619–626. [PubMed] [Google Scholar]
- 22.Rothkamm K. and Löbrich,M. (1999) Misrejoining of DNA double-strand breaks in primary and transformed human and rodent cells: a comparison between the HPRT region and other genomic locations. Mutat. Res., 433, 193–205. [DOI] [PubMed] [Google Scholar]
- 23.Fouladi B., Waldren,C.A., Rydberg,B. and Cooper,P.K. (2000) Comparison of repair of DNA double-strand breaks in identical sequences in primary human fibroblast and immortal hamster-human hybrid cells harbouring a single copy of human chromosome 11. Radiat. Res., 153, 795–804. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickson E.A. (1997) Insights from model systems. Cell cycle regulation of mammalian DNA double-strand break repair. Am. J. Hum. Genet., 61, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y., Lukacsovich,T. and Waldman,A.S. (1999) Multiple pathways for repair of DNA double-strand breaks in mammalian chromosoms. Mol. Cell. Biol., 19, 8353–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer P., Goedecke,W. and Obe,G. (2000) Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis, 15, 289–302. [DOI] [PubMed] [Google Scholar]
- 27.Morris T. and Thacker,J. (1993) Formation of large deletions by illegitimate recombination in the HPRT gene of primary human fibroblasts. Proc. Natl Acad. Sci. USA, 90, 1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronenberg A., Gauny,S., Criddle,K., Vannais,D., Ueno,A., Kraemer,S. and Waldren,S.A. (1995) Heavy ion mutagenesis: linear energy transfer effects and genetic linkage. Radiat. Environ. Biophys., 34, 73–78. [DOI] [PubMed] [Google Scholar]
- 29.Haber J.E. and Leung,W.Y. (1996) Lack of chomosome territoriality in yeast: promiscous rejoining of broken chromosome ends. Proc. Natl Acad. Sci. USA, 93, 13949–13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haber J.E. (1999) DNA recombination: the replication connection. Trends Biochem. Sci., 24, 271–275. [DOI] [PubMed] [Google Scholar]
- 31.Brenneman M.A., Weiss,A.E., Nickoloff,J.A. and Chen,D.J. (2000) XRCC3 is required for efficient repair of chromosome breaks by homologous recombination. Mutat. Res., 459, 89–97. [DOI] [PubMed] [Google Scholar]
- 32.Richardson C. and Jasin,M. (2000) Frequent chromosomal translocations induced by DNA double-strand breaks. Nature, 405, 697–700. [DOI] [PubMed] [Google Scholar]
- 33.Moynahan M.E. and Jasin,M. (1997) Loss of heterozygosity induced by a chromosomal doube-strand break. Proc. Natl Acad. Sci. USA, 94, 8988–8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothstein R., Michel,B. and Gangloff,S. (2000) Replication fork pausing and recombination or “gimme a break”. Genes Dev., 14, 1–10. [PubMed] [Google Scholar]
- 35.Oberle I., Camerino,G., Kloepfer,C., Moisan,J.P., Grzeschik,K.H., Hellkuhl,B., Hors-Cayla,M.C., Van Cong,N., Weil,D. and Mandel,J.L. (1986) Characterization of a set of X-linked sequences and a panel of somatic cell hybrids useful for the regional mapping of the human X chromosome. Hum. Genet., 72, 43–49. [DOI] [PubMed] [Google Scholar]
- 36.Lippert M.J., Albertini,R.J. and Nicklas,J.A. (1995) Physical mapping of the human hprt chromosomal region (Xq26). Mutat. Res., 326, 39–49. [DOI] [PubMed] [Google Scholar]
- 37.Sak A. and Stuschke,M. (1998) Repair of ionizing radiation induced DNA double-strand breaks (dsb) at the c-myc locus in comparison to the overall genome. Int. J. Radiat. Biol., 73, 35–43. [DOI] [PubMed] [Google Scholar]
- 38.Kühne M., Rothkamm,K. and Löbrich,M. (2000) No dose-dependence of DNA double-strand break misrejoining following α-particle irradiation. Int. J. Radiat. Biol., 76, 891–900. [DOI] [PubMed] [Google Scholar]
- 39.Corry P.M., Robinson,S. and Getz,S. (1977) Hyperthermic effects on DNA repair mechanisms. Radiology, 123, 475–482. [DOI] [PubMed] [Google Scholar]
- 40.Mills M.D. and Meyn,R.E. (1981) Effects of hyperthermia on repair of radiation-induced DNA strand breaks. Radiat. Res., 87, 314–328. [PubMed] [Google Scholar]
- 41.Wong R.S.L., Dynlacht,J.R., Cedervall,B. and Dewey,W.C. (1995) Analysis by pulsed-field gel electrophoresis of DNA double-strand breaks induced by heat and/or x-irradiation in bulk and replicating DNA of CHO cells. Int. J. Radiat. Biol., 68, 141–152. [DOI] [PubMed] [Google Scholar]
- 42.Jeggo P.A., Taccioli,G.E. and Jackson,S.P. (1995) Menage a’trois: double strand break repair, V(D)J recombination and DNA-PK. Bioessays, 17, 949–957. [DOI] [PubMed] [Google Scholar]
- 43.Pang D., Yoo,S., Dynan,W.S., Jung,M. and Dritschilo,A. (1997) Ku proteins join DNA fragments as shown by atomic force microscopy. Cancer Res., 57, 1412–1415. [PubMed] [Google Scholar]
- 44.Burgman P., Ouyang,H., Peterson,S., Chen,D.J. and Li,G.C. (1997) Heat inactivation of Ku autoantigen: possible role in hyperthermic radiosensitization. Cancer Res., 57, 2847–2850. [PubMed] [Google Scholar]
- 45.Woudstra E.C., Konings,A.W.T., Jeggo,P.A. and Kampinga,H.H. (1999) Role of DNA-PK subunits in radiosensitization by hyperthermia. Radiat. Res., 152, 214–218. [PubMed] [Google Scholar]
- 46.Kojis T.L., Gatti,R.A. and Sparkes,R.S. (1989) The cytogenetics of ataxia telangiectasia. Cancer Genet. Cytogenet., 56, 143–156. [DOI] [PubMed] [Google Scholar]
- 47.Antoccia A., Stumm,M., Saar,K., Ricordy,R., Maraschio,P. and Tanzarella,C. (1999) Impaired p53-mediated DNA damage response, cell-cycle disturbance and chromosome aberrations in Nijmegen breakage syndrome lymphoblastoid cell lines. Int. J. Radiat. Biol., 75, 583–591. [DOI] [PubMed] [Google Scholar]
- 48.Kemp L.M. and Jeggo,P.A. (1986) Radiation-induced chromosome damage in X-ray-sensitive mutants (xrs) of the Chinese hamster ovary cell line. Mutat. Res., 166, 255–263. [DOI] [PubMed] [Google Scholar]
- 49.Difilippantonio M.J., Zhu,J., Chen,H.T., Meffre,E., Nussenzweig,M.C., Max,E.E., Roed,T. and Nussenzweig,A. (2000) DNA repair protein Ku80 suppress chromosomal aberrations and malignant transformation. Nature, 404, 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C. and Kolodner,R.D. (1999) Gross chromosomal rearrangements in S. cerevisiae replication and recombination defective mutants. Nat. Genet., 23, 81–85. [DOI] [PubMed] [Google Scholar]
- 51.Borgmann K. and Dikomey,E. (1997) Relationship between PCC-fragments and cell killing studied in X-irradiated CHO, CHO K1 cells and two radiosensitive mutants xrs1 and xrs5. Int. J. Radiat. Biol., 72, 667–674. [DOI] [PubMed] [Google Scholar]
- 52.Sak A., Stuschke,M., Stapper,N. and Streffer,C. (1996) Induction of DNA double-strand breaks by ionizing radiation at the c-myc locus compared with the whole genome: a study using pulsed-field gel electrophoresis and gene probing. Int. J. Radiat. Biol., 69, 679–685. [DOI] [PubMed] [Google Scholar]
- 53.Dahm-Daphi J. and Dikomey,E. (1995) Separation of DNA fragments induced by ionizing irradiation using a graded-field gel electrophoresis. Int. J. Radiat. Biol., 67, 161–168. [DOI] [PubMed] [Google Scholar]
- 54.Forrester H.B., Yeh,R.-F. and Dewey,W.C. (1999) A dose response for radiation-induced intrachromosomal DNA rearrangements detected by inverse polymerase chain reaction. Radiat. Res., 152, 232–238. [PubMed] [Google Scholar]