Abstract
As a result of the burgeoning growth of disease-specific neural autoantibody markers available for diagnostic patient evaluation, there has been increasing awareness of autoimmune central nervous system (CNS) disorders in hospital practice. Hospital-based neurologists have also taken great interest in these disorders since many occur in the setting of an occult systemic cancer which can be detected and treated at an early stage, and many affected patients are responsive to immunotherapy. Associated neurological disorders are typically subacute in onset, some are common or classic (eg, limbic encephalitis, cerebellar degeneration), but others have atypical or multifocal presentations. For patients with a suspected paraneoplastic disorder, many and costly oncological evaluations may be required for diagnosis. Comprehensive serological and cerebrospinal fluid (CSF) evaluation for neural autoantibodies may permit a focused cancer evaluation (eg, antineuronal nuclear antibody type 1 [ANNA-1] is associated with small cell lung carcinoma), and in some circumstances may indicate the likelihood of a good response to therapy (eg, voltage-gated potassium channel complex antibody) or poor neurological prognosis (eg, purkinje cell cytoplasmic antibody type 1 [antiYo]). Positron-emission tomography–computed tomography (PET-CT) imaging of trunk may increase the diagnostic yield for certain cancers where other modalities have been negative. For some patients, rapid treatment with immunotherapy may facilitate marked improvement, or full recovery; multiple sequential trials of one or more of steroids, intravenous immunoglobulin or plasma exchange, or combination therapy are often required. For patients with N-methyl-d-aspartate receptor antibody encephalitis, early treatment with immunosuppressants and weeks or months of supportive intensive care may additionally be required. One or more of clinical examination, electroencephalogram (including video telemetry), and imaging provide objective parameters to which posttreatment outcomes can be compared.
Keywords: neuromyelitis optica, Stiff-Person syndrome, myelitis, transverse
Introduction
Central nervous system (CNS) autoimmunity is increasingly recognized in patients hospitalized with acute or subacute neurological presentations. Diagnosis of these disorders is frequently based on the detection of 1 or more markers of autoimmunity (usually neural-specific antibodies). These disorders are distinct from inflammatory, demyelinating diseases such as multiple sclerosis or acute disseminated encephalomyelitis (ADEM), for which there are no specific biomarkers, other than pathological examination. Autoimmune CNS disorders may be paraneoplastic or idiopathic in etiology. Improvements in existing methodologies1,2 and new laboratory techniques3,4 for detecting neural-specific antibody markers have greatly enhanced the evaluation of suspected cases. Novel pathophysiological insights have been gained through the discovery of many of these antibodies, some of which likely play a central role in disease pathogenesis.5 There is growing interest in these disorders among general and subspecialist neurologists since they are often multifocal and rapidly progressive, but expedited initiation of immunotherapy frequently permits clinical improvement. Herein, autoimmune CNS disorders will be described, focusing on clinical presentation, evaluation, and management of patients.
Pathophysiology
In broad terms, autoimmune neurological disorders arise as a peripheral immune response against 1 or more autoantigens in the nervous system, which are also expressed in tumors (paraneoplastic) or microbial organisms (parainfectious). In many instances, an inciting cause is not identified.
Pathophysiological mechanisms for paraneoplastic neurological disorders are well described. Tumor immune surveillance in affected patients usually results in neoplasia being confined to the primary organ and to regional lymph nodes.5 The neurological attack, in contrast, can be devastating and can affect the central, peripheral somatic, or autonomic nervous systems. Tumor-targeted immune responses are initiated by onconeural proteins expressed in the plasma membrane, nucleus, cytoplasm, or nucleolus of certain cancers. These antigens are also expressed in neurons or glia and thus are coincidental targets. Antibodies directed at neural cell plasma membrane antigens (Table 1; eg N-methyl-d-aspartate [NMDA] receptors, aquaporin 4) are effectors through multiple mechanisms.6,7 Consistent with this, in vitro experiments and autopsy studies have demonstrated perivascular and parenchymal plasmablast, antibody, and complement deposition. The response to one or more of steroids, intravenous immunoglobulin (IVIg), and plasma exchange is usually excellent.8
Table 1.
Characteristic Neurological and Oncological Findings Among Patients With Plasma Membrane Protein Antibodies.
| Antibody | Antigen | Oncological Association | Neurological Presentation |
|---|---|---|---|
| VGKC complex antibody | LGI1, CASPR2 | Small cell lung carcinoma; thymoma; adenocarcinoma of breast, prostate | Limbic encephalitis, amnestic syndrome, executive dysfunction, personality change, disinhibition hypothalamic disorder, brain stem encephalitis, ataxia, extrapyramidal disorders, myoclonus, peripheral, and autonomic neuropathy |
| NMDA receptor antibody | NR1 | Ovarian teratoma | Anxiety, psychosis, seizures, amnestic syndrome, extrapyramidal disorders |
| AMPA receptor antibody | GluR1,2 | Thymic tumors, lung carcinomas, breast carcinoma | Limbic encephalitis, nystagmus, seizures |
| GABA-B receptor antibody | GABA-B | Small cell lung carcinoma, other neuroendocrine neoplasia | Limbic encephalitis, orolingual dyskinesias |
| P/Q and N type calcium channel antibody | P/Q and N-type calcium channels | Small cell carcinoma, breast, or gynecological adenocarcinoma | Encephalopathies, myelopathies, neuropathies, Lambert-Eaton syndrome |
| Muscle AChR antibody | Muscle AChR | Thymoma, thymic carcinoma, lung carcinoma | Myasthenia gravis. Also sometimes observed in paraneoplastic CNS contexts |
| Neuronal ganglionic AChR antibody | Neuronal ganglionic AChR | Adenocarcinoma, thymoma, small cell carcinoma | Dysautonomia, peripheral somatic neuropathies, encephalopathies. |
| NMO-IgG | Aquaporin-4 | Some reports of thymoma and other solid tumors | Relapsing optic neuritis, transverse myelitis, encephalopathies |
| Glycine receptor antibody | α1 subunit GlyR | Lymphoma and thymoma reported | Stiff-man syndrome and variants |
| Metabotropic glutamate receptor 5 antibody | mGluR5 | 2 patients with Hodgkin lymphoma | Limbic encephalitis |
Abbreviations: AchR, acetylcholine receptor; AMPA, alpha-amino-3-hydroxy-5-methyl-4- isoxazole-propionic acid; CASPR, contactin-associated protein; GABA, gamma amino butyric acid; GluR, glutamate receptor; GlyR, glycine receptor; LGI1, leucine-rich, glioma inactivated 1; NMDA, N-methyl-d-aspartate; NMO, neuromyelitis optica; VGKC, voltage-gated potassium channel; IGg, immunoglobulin G.
Reproduced with permission from Springer.5
In contrast, intracellular antigens are not accessible to immune attack in situ, but peptides derived from intracellular proteins are displayed on upregulated MHC class I molecules after breakdown in the proteasome and in turn are targeted by peptide-specific cytotoxic T cells. Antibodies (eg, Purkinje cell cytoplasmic antibody type 1 [PCA-1, anti-Yo]) targeting these intracellular antigens (Table 2) are not pathogenic.9 Nonetheless, these antibodies serve as diagnostic markers in clinical practice of a T cell predominant effector process. Studies of autopsied tissues and experimental studies suggest that these types of paraneoplastic neurological disorders are caused by CD8+ cytotoxic T cells.9 The neurological deficits usually do not improve with treatment, although sometimes the clinical picture stabilizes post oncological therapy.10,11 Deaths from the complications of neurological disease (rather than from metastatic cancer) are common.
Table 2.
Characteristic Findings Among Patients With Neuronal Nuclear or Cytoplasmic Antibodies.a
| Antibody | Antigen | Oncological Association | Neurological Presentations |
|---|---|---|---|
| ANNA-1 | ELAVL (Hu) | Small cell carcinoma | Limbic encephalitis, brain stem encephalitis, autonomic neuropathies, sensory neuronopathy, other peripheral neuropathies |
| ANNA-2 | NOVA 1, 2 (Ri) | Small cell carcinoma, breast adenocarcinoma | Dementia, limbic encephalitis, brain stem encephalitis, myelopathy, peripheral neuropathy |
| ANNA-3 | Unknown | Aerodigestive carcinomas | Brain stem encephalitis, limbic encephalitis, myelopathy, peripheral neuropathy |
| AGNA | SOX-1 | Small cell carcinoma | Neuropathy, Lambert-Eaton syndrome, limbic encephalitis |
| Ma1, Ma2 | PNMA1, PNMA2 (Ma1, Ma2) | Testicular (Ma2); breast, colon, testicular (Ma1) | Limbic encephalitis, hypothalamic disorder, brain stem encephalitis |
| PCA-1 | CDR2 | Mullerian adenocarcinoma, breast adenocarcinoma | Cerebellar ataxia, brain stem encephalitis, myelopathy, radiculopathies, peripheral neuropathies |
| PCA-2 | Unknown | Small cell carcinoma | Limbic encephalitis, ataxia, brain stem encephalitis, Lambert-Eaton syndrome, peripheral and autonomic neuropathies |
| PCA-Tr | Unknown | Hodgkin lymphoma | Cerebellar ataxia |
| CRMP-5 IgG | CRMP-5 | Small cell carcinoma, thymoma | Subacute onset dementia, personality change, aphasia, depression, chorea, ataxia, myelopathy, radiculopathy, neuropathy, Lambert-Eaton syndrome |
| Amphiphysin IgG | Amphiphysin | Small cell carcinoma, breast adenocarcinoma | Limbic encephalitis, aphasia, other subacute onset dementias, stiff-person phenomena, myelopathy, neuropathy |
| GAD65 antibody | GAD65 | Thymoma; renal cell, breast or colon adenocarcinoma | Stiff-man syndrome, stiff-man phenomena, ataxia, seizures, limbic encephalitis, brain stem encephalitis, ophthalmoplegia, parkinsonism, myelopathy |
| ZIC-4 antibody | ZIC-4 | Small cell carcinoma | Cerebellar ataxia |
Abbreviations: AGNA, antiglial/neuronal nuclear antibody; ANNA, antineuronal antibody; CDR, cerebellar degeneration-related protein; ELAVL, embryonic lethal, abnormal vision, Drosophila-like 1; GAD65, 65 kDa isoform of glutamic acid decarboxylase; NOVA, neuro-oncological ventral antigen; PCA, Purkinje cell cytoplasmic antibody; PNMA, paraneoplastic Ma antigen; SOX, sex determining region Y-box 1; ZIC, zinc finger transcription factor; NMDA, N-methyl-d-aspartate; NMO, neuromyelitis optica; IgG, immunoglobulin G.
aReproduced with permission from Springer.5
Neurological Presentation
General
Patients usually present with subacute onset of neurological dysfunction, which may include classic syndromes (Table 3) or multifocal disorders (Tables 1 and 2).12 Classic syndromes include limbic encephalitis, opsoclonus–myoclonus syndrome (OMS), cerebellar degeneration, paraneoplastic myelopathy, and sensory ganglionopathy.
Table 3.
Paraneoplastic and Other Autoimmune Neurological Disorders Organized by Anatomic Level.a
| Neuraxis Level | Disorder | Examples of Salient Antibodies |
|---|---|---|
| Eye | Autoimmune retinopathy, optic neuropathy | CRMP-5-IgG, NMO-IgG |
| Cerebral cortex | Limbic encephalitis, encephalitides presenting primarily with seizures or cognitive dysfunction, myoclonus | ANNA-1, VGKC complex Ab, NMDA receptor Ab |
| Diencephalon | Hypothalamic dysfunction, sleep disorders | Ma1/Ma2 Ab |
| Basal ganglia | Chorea, dystonia | CRMP-5 IgG |
| Cerebellum | Cerebellar ataxia/degeneration | PCA-1, P/Q type calcium channel Ab |
| Brain stem | Brain stem encephalitis, jaw dystonia Stiff-man phenomena (including stiffness, spasms, exaggerated startle, if widespread referred to as progressive encephalomyelitis with rigidity and myoclonus [PERM]), opsoclonus–myoclonus syndrome | ANNA-2, Ma1/Ma2, glycine receptor ab |
| Cranial nerves | Special senses, bulbar, motor neuropathies | ANNA-1, CRMP-5 IgG |
| Spinal cord | Myelopathy, myoclonus Stiff-man phenomena | CRMP-5 IgG, NMO-IgG, GAD65 Ab |
| Peripheral somatic nerves and ganglia | Sensory neuronopathy, sensorimotor neuropathies | ANNA-1, VGKC complex Ab |
| Neuromuscular junction | Lambert-Eaton syndrome, Myasthenia gravis | P/Q-type calcium channel Ab, muscle AChR Ab |
| Muscle | Polymyositis dermatomyositis, necrotizing myopathy | Jo1 Ab, SRP-54 Ab |
| Autonomic and enteric nervous system | Dysautonomias (pandysautonomia or limited dysautonomia, which includes gastrointestinal dysmotilities) | ANNA-1, alpha-3 ganglionic AChR |
Abbreviations: AchR, acetylcholine receptor; ANNA, antineuronal nuclear antibody; CRMP5, collapsin-response mediator protein 5; PCA-1, purkinje cell cytoplasmic antibody type 1; VGKC, voltage-gated potassium channel; NMDA, N-methyl-d-aspartate; GABA-B, gamma-aminobutyric acid-B; Jo-1 Abs, anti-histidyl-tRNA synthetase Abs; NMO, neuromyelitis optica; IgG, immunoglobulin G; PERM=progressive encephalomyelitis with rigidity and myoclonus; SRP=signal recognition particle.
aMany of these disorders can be multifocal.
While certain disorders have been syndromically associated with certain autoantibody markers (eg, limbic encephalitis and voltage-gated potassium channel [VGKC] complex autoantibodies),13,14 a broader neurologic spectrum inevitably emerges over time through individual cases reported or by systematic serologic evaluation of large numbers of patients not selected by neurologic syndrome. For example, VGKC complex antibodies were initially considered to be specific for autoimmune limbic encephalitis or disorders of peripheral nervous hyperexcitability, but over time other presentations were reported, including rapidly progressive dementias mimicking frontotemporal dementia15 or Creutzfeldt-Jakob disease.16 The neurological presentation is often the first clue to the existence, or limited recurrence, of a cancer. Cancer may remain unsuspected and undetectable both clinically and by conventional radiology for long intervals after neurological symptom onset. Central nervous system dysfunction sometimes reflects an effective immune response to a systemic cancer that has devastating neurological consequences.
Some Common Neurological Disorders
Any anatomical region of the CNS can be affected, and sometimes multiple regions are affected simultaneously. In some circumstances, a particular neurological disorder is encountered frequently with a specific autoantibody (eg, cerebellar ataxia in patients with PCA-1 [anti-yo])17 However, the neurological manifestations associated with any 1 paraneoplastic antibody are protean (in one series 60% of PCA-1-seropositive patients had neurological features in addition to ataxia, 23% did not have ataxia at presentation, and 11% never developed ataxia).11 Thus, testing for a single antibody (individually rare) on the premise of a particular neurological syndrome is likely to have low diagnostic yield, and comprehensive serological evaluations are usually preferred.12 An example of an exception to this rule is the NMDA receptor antibody encephalitis (described below), which has a fairly stereotyped sequence of neuropsychiatric and neurological events that characterize its course.8
Limbic encephalitis
Limbic encephalitis is the classic CNS autoimmune disorder (Figure 1). Patients typically present with delirium, mood change, memory and personality disturbance, and focal seizures of mesial temporal type with or without secondary generalization. Potential antibody and cancer associations are protean, and thus a comprehensive serological evaluation is required. Information regarding cancer type and likely treatment responsiveness can be obtained from a serological diagnosis. For example, a patient with ANNA-1 would likely have small cell lung carcinoma (90% of cases)18 and is unlikely to improve much with immunotherapy. In contrast, a patient with VGKC complex antibody would be unlikely to have cancer detected (<20% of patients) but would stand a good chance of full recovery or near full recovery if treated early and aggressively with immunotherapy.19 Morvan syndrome is a rare multifocal manifestation of VGKC complex autoimmunity (limbic encephalitis, sleep disturbance, and peripheral nerve hyperexcitability).
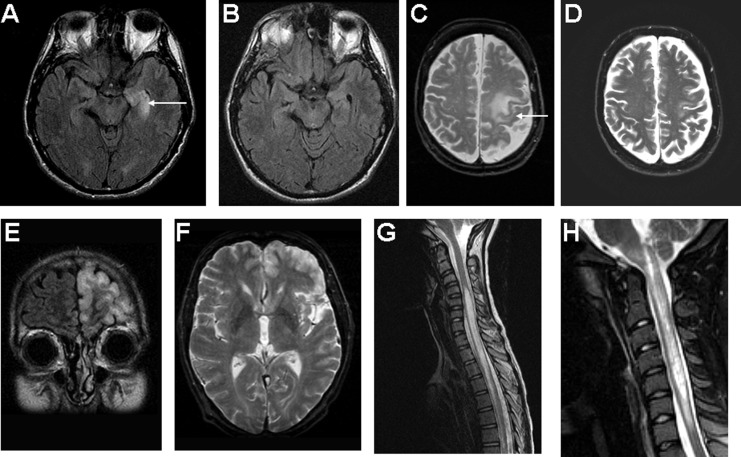
Figure 1.
Characteristic MRI brain and spinal cord findings. A, T2 FLAIR axial of brain demonstrating left hippocampal T2 abnormality typical of limbic encephalitis, which resolved with immunotherapy, B. C, T2 FLAIR axial image of brain demonstrating a superior–posterior left frontal abnormality in a patient who presented with right upper extremity apraxia and myoclonus. D, Postimmunotherapy improvement. E, T2 coronal FLAIR demonstrated extensive, predominantly left frontal cortical abnormality in a patient with ANNA-1 (anti-Hu), also seen on T2 axial image F. G, Sagittal T2 MRI of spinal cord demonstrates longitudinally extensive myelitis typical of NMO. H, Sagittal T2 MRI image of a patient with NMO who presented with intractable vomiting and myelitis. The longitudinally extensive lesion extends into the brain stem. Reproduced with permission from American Medical Association (C-F)20 and Lippincott Williams & Wilkins (H).36 MRI indicates magnetic resonance imaging; NMO, neuromyelitis optica; ANNA, antineuronal nuclear antibody.
Some patients present with more limited encephalitic forms and may mimic a primary seizure disorder or rapidly progressive dementia (including mimics of Creutzfeldt-Jakob disease).16 Other unusual clinical and radiological localizations include frontal, parietal, occipital lobe distributions (Figure 1).20
A Common and Stereotyped CNS Disorder: NMDA Receptor Antibody Encephalitis
Patients with NMDA receptor antibody (targeting the GluN1 [NR1] subunit) have a fairly stereotyped neurological disorder, as described below by Dalmau and colleagues.8 This often develops in several stages. Prodromal symptoms include headache, fever, nausea, vomiting, diarrhea, or upper respiratory tract symptoms. Shortly afterward, patients develop psychiatric symptoms that in many cases are brought to to the attention of psychiatrists. Anxiety, insomnia, fear, delusions, mania, and paranoia occur frequently. Other behavioral changes include social withdrawal and stereotyped behavior. Amnesia and an unusual language disorder (noncortical aphasia) are common. The behavioral changes in children may be less specific and include temper tantrums and hyperactivity. Neurological disorders including seizures and dystonia are frequent and may be the presenting symptom. After these initial symptoms, in both adults and children, decreased responsiveness ensues. Movement disorders include orolingual-facial dyskinesias, generalized dyskinesias, oculogyric crisis, dystonia, and rigidity. Frequent autonomic manifestations include hyperthermia, tachycardia, hypersalivation, hypertension, bradycardia, hypotension, urinary incontinence, and erectile dysfunction. Hypoventilation, necessitating prolonged ventilatory support (often months) may be required. Dissociative responses similar to those caused by NMDA receptor antagonists (eg, phencyclidine or ketamine) have been observed (eg, resisting eye opening despite lack of response to painful stimuli).
Movement disorders
Rapidly progressive cerebellar ataxia is a common presentation of autoimmune neurological disorders. Hyperkinetic movement disorders are commonly encountered in patients with autoimmune CNS disorders. Chorea has historically been described in patients with Sydenham chorea (poststreptococcal infection), patients with chorea gravidarum, and in patients with serological markers of antiphospholipid syndrome. More recently, chorea has been recognized in a paraneoplastic context, including patients with collapsin-response mediator protein 5 (CRMP5)-IgG and small cell carcinoma21 and in patients with NMDA receptor antibody and teratoma.8
Myoclonus is a frequent neurological manifestation of autoimmunity, either paraneoplastic or idiopathic. A well-known disorder encountered in children and adults is OMS.22,23 Patients with OMS present with vertigo, dizziness, nausea and vomiting, tremulousness, and imbalance. Neurological examination reveals generalized myoclonic jerks, and lightning-like haphazard eye movements, consistent with myoclonus of the extraocular muscles (opsoclonus). Commonly encountered neoplasms include neuroblastoma (in children)24 and lung carcinoma and breast carcinoma (in adults).25 The most common paraneoplastic antibody encountered in adult-onset OMS is ANNA-2 (anti-Ri).25 Patients may present with opsoclonus or myoclonus in isolation, with or without accompanying neurological disorders, usually encephalopathy.23 Some patients present with tremulousness only, and on examination have small amplitude myoclonus in isolation without opsoclonus; these cases can be diagnostically challenging and are frequently misdiagnosed as having essential tremor. Clues include a subacute onset and a widespread distribution of symptoms and signs, including trunk and cranium as well as extremities.
More rarely encountered movement phenomena include parkinsonism and dystonia. Again, subacute onset and a rapid progression should prompt consideration of an autoimmune cause.
Brain stem encephalitis
Presenting symptoms include one or more cranial neuropathies, eye movement disorders, vertigo, nausea and vomiting (sometimes intractable), postural instability, and parkinsonism.26,27 These symptoms are common for patients with Ma2 antibody (anti-Ta, usually young men with testicular carcinoma) or both Ma1 and Ma2 antibodies (anti-Ma, men and women with heterogenous cancer types). One unusual manifestation of brain stem encephalitis reported in ANNA-2-seropositive patients is jaw dystonia.28
Myelopathy
Spinal cord disorders may be acute or subacute (myelitis) or progressive (myelopathy) in an autoimmune context. An example of autoimmune inflammatory myelitis includes the longitudinally extensive transverse myelitis of neuromyelitis optica (NMO [aquaporin 4 autoimmunity] described below, from which patients may recover with steroids or plasma exchange).29 In contrast, paraneoplastic myelopathies are frequently progressive and patients rarely improve with immunotherapy. In a recent series of 31 patients from 1 institution (two-thirds were women), common cancers found were of lung, breast, kidney, thyroid and ovary, and melanoma.30 Paraneoplastic autoantibodies found included amphiphysin IgG,31 CRMP 5 IgG,32 and PCA-1.11 Common magnetic resonance imaging (MRI) cord abnormalities included longitudinally extensive T2 signal change, symmetric tract, or gray matter–specific signal abnormality (usually enhancing).30 Over half were wheelchair dependent and one-third had died after 3 years of follow-up, despite oncological and immunological therapies.30 Idiopathic inflammatory transverse myelitides include monophasic transverse myelitis, multiple sclerosis, and sarcoidosis.
Neuromyelitis optica
Historically this disorder was classified as an unusual form of multiple sclerosis. In recent times, it has been recognized that patients with the relapsing form of NMO have an autoimmune channelopathy, with antibody targeting the most abundant CNS water channel, aquaporin 4,3,33 which can be detected in patient serum or cerebrospinal fluid (CSF) in approximately 80% of cases.4 Patients typically present with optic neuritis or transverse myelitis but usually not simultaneously.29 Approximately 12% present with manifestations of area postrema dysfunction (intractable hiccup, nausea, and vomiting) or circumventricular organ (hyponatremia) before developing optic neuritis or transverse myelitis.34–36 Typically, patients have a relapsing course and disability accrues with successive attacks.37 Aside from the inaugural symptoms described above, brain involvement is uncommon in adults but occurs in nearly half of the children, including presentations that can mimic the usually monophasic disorder, ADEM.36,38 Inevitably, NMO-IgG-seropositive children have relapsing disease, requiring acute treatment (steroids and plasma exchange) and remission-maintaining immunosuppression (azathioprine, mycophenolate mofetil, or rituximab).
Stiff-Man syndrome and related disorders
Patients with classic stiff-man syndrome (SMS; aka Moersch-Woltman syndrome) present with low back and proximal lower extremity stiffness, with superimposed spasms, and are prone to frequent falls.39 Characteristic clinical findings include a fixed hyperlordotic lumbar spine and increased limb tone. Many improve with benzodiazepines.40 More restricted forms are also encountered, often involving one limb in isolation.41 The spectrum of disease also extends to a more severe and widespread form that may be accompanied by encephalopathy and prominent myelopathic findings (progressive encephalomyelitis and rigidity with myoclonus).42 Antibody targeting the 65 kDA isoform of glutamic acid decarboxylase (GAD65) is detected in approximately 80% of patients.41 In recent times, antibody targeting the α1 subunit of αβ-heteromeric glycine receptors has been detected in serum of patients with the progressive encephalomyelitis with rigidity and myoclonus (PERM) phenotype.43 Symptomatic treatments (benzodiazepines and baclofen) and immunotherapies (steroids and IVIg) are frequently beneficial.
Risk Factors
Risk factors for a paraneoplastic or other autoimmune CNS disorder include a personal or family history of cancer or autoimmune disease and a smoking history.
Evaluation
As well as serving a diagnostic purpose, testing provides a pretreatment objective baseline. Resolution of a clinical electroencephalograph (EEG), MRI, or functional imaging abnormality after immunotherapy serves as an objective marker supporting subjective improvements.
Neurological
Clinical examination
Careful documentation of objective abnormalities with careful neurological examination and paraclinical testing serves not only to aid localization of neurological disorders but also to establish a baseline before trial of immunotherapy. A bedside evaluation of cognitive function, such as the Mini-Mental State Examination or Kokmen Short Test of Mental Status, should reveal impairments in one or more categories of attention, memory, reasoning, calculation, and praxis. The presence of multifocal neurologic symptoms and signs is a clue to a possible autoimmune etiology. Rapid evolution of a cognitive disorder, with accompanying epilepsy, ataxia, parkinsonism, brain stem signs, myelopathy, or a peripheral nervous system disorder, should trigger consideration of an autoimmune cause as well as toxic, nutritional, metabolic causes, or other inflammatory disorders. The neurological examination also provides a baseline for comparison when reevaluating the patient after a trial of immunotherapy.44
The MRI
MRI may reveal abnormalities in T2 signal characteristics atypical for neurodegenerative disorders (Figure 1). Mesial temporal lobes are a common location for T2-signal abnormalities in brain disorders, for example in patients with VGKC complex autoantibodies.13 However, unusual extratemporal hemispheric abnormalities may also be observed,20 including subtle white matter or gyriform enhancement. Neuroimaging of affected CNS regions may be normal early in the disease course; in some cases (such as NMDA receptor antibody encephalitis) imaging remains normal throughout the clinical course.8
Electrophysiological testing
Although abnormalities detected are not specific for an autoimmune etiology, EEG, like functional imaging, may aid the characterization of the disorder in an individual patient and provide a pretreatment baseline. Common abnormalities detected on EEG include focal or generalized slowing or spike-and-slow-wave epileptiform discharges. Mesial temporal abnormalities are often observed in autoimmune dementias, but extratemporal abnormalities also have been reported. Some patients may present with an isolated seizure disorder, and thus EEG, and sometimes video EEG monitoring is required to establish the diagnosis and provide an objective pretreatment baseline. It has recently been reported that patients who present with seizures had localization-related disorders, were refractory to antiepileptic drugs, and the common antibodies encountered were VGKC complex, GAD65 Ab, and CRMP-5 IgG; two-thirds became seizure free with immunotherapy.45
Neuropsychology
Detailed neuropsychological testing is more appropriate to the outpatient practice, where patients often present with more subtle abnormalities than patients on an inpatient clinical service. Again, neuropsychometrics can provide an objective measure of dysfunction prior to treatment and follow-up testing.
Routine CSF Parameters
Further clues to an autoimmune etiology for a cognitive disorder may be found on CSF evaluation. Elevated protein concentration (>100 mg/dL), mild CSF pleocytosis, abnormal numbers of CSF-exclusive oligoclonal bands, and elevated IgG index and synthesis rate all support an autoimmune etiology. These CSF findings are encountered in many inflammatory (possibly autoimmune) CNS disorders, including MS. Specificity may be an issue in some cases since oligoclonal bands are detected in a small minority of patients with pathologically proven primary neurodegenerative disorders or Creutzfeldt-Jakob disease.46
Autoantibody Testing
Antibody profiles include neural (Tables 1 and 2) and nonneural IgG markers. Seropositivity for nonneural autoantibodies does not confirm a diagnosis of an autoimmune CNS disorder but does support consideration of an autoimmune pathogenesis and may trigger additional testing and a trial of treatment. For example, detection of thyroperoxidase (TPO) or thyroglobulin autoantibodies in a patient with cognitive symptoms does not confirm a diagnosis of an autoimmune cognitive disorder (so-called Hashimoto encephalopathy47 or steroid-responsive encephalopathy associated with autoimmune thyroiditis48) but is a supportive finding since it is indicative of autoimmunity affecting another organ. In a patient presenting with cognitive dysfunction, seropositivity for ANA and double-stranded DNA antibodies will raise suspicion for lupus cerebritis (or CNS lupus),49 a poorly understood disorder classified under the umbrella of neuropsychiatric lupus for which there are no reliable neural-specific antibody marker tests in clinical service.
The detection of a neural-specific antibody in serum or CSF aids the diagnostic process by increasing suspicion for an autoimmune neurologic disorder and raises the possibility of a paraneoplastic cause. An individual patient’s profile of coexisting autoantibodies may have high predictive value for a specific cancer. For example, amphiphysin antibody in a woman may be associated with breast carcinoma or small cell lung carcinoma, but, if coexisting ANNA-1 is detected, this narrows the search to small cell carcinoma, either pulmonary or extrapulmonary in origin.12 Antibody tests performed on serum alone are often sufficiently informative, but CSF testing sometimes increases the diagnostic yield (eg, CRMP-5-IgG).50 However, in the case of NMDA receptor antibodies, CSF is frequently more informative than serum. The opposite is true for VGKC complex and aquaporin-4 antibodies which (almost always) are more readily detectable in serum than in CSF.13,51
Other antibodies that target neural plasma membrane antigens that have been described recently in an autoimmune CNS context include (Table 1) glutamatergic alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor,52 gamma-aminobutyric acid (GABA)-B receptor,53 and proteins complexed with VGKC (leucine-rich, glioma inactivated 1 [LGI1] and contactin-associated protein [CASPR]2).54–56
Testing for Cancer
Suspicion for a paraneoplastic cause of an autoimmune cognitive disorder may arise because of a risk factor identified in the history (eg, smoking or a family history of cancer) or because one or more antibodies detected have a known cancer association. In this setting, screening for cancer is warranted. In addition to a general clinical examination, CT of chest, abdomen, and pelvis in all patients, mammography in women, and testicular ultrasound and prostate-specific antigen (PSA) in men should be performed where appropriate. Chest and abdominal computed tomography (CT) or MRI and urine testing for homovallinic acid metabolites should be undertaken in children where neuroblastoma is suspected. Certain antibodies with a particular specificity for cancer (eg, NMDA receptor antibody and teratoma) may further refine the oncological evaluation. For paraneoplastic neurologic disorders, positron-emission tomography (PET) imaging alone, or in combination with anatomic data (PET-CT), increases the cancer diagnostic yield by 20% when all standard evaluations (eg, whole-body CT scan) have been uninformative.57 However, PET imaging is not helpful for detecting gonadal tumors (ovary or testis), neuroblastoma, or thymoma. MRI has good sensitivity for both ovarian and thymic neoplasms.
Treatment
Randomized-controlled treatment trials of autoimmune CNS disorders are unavailable, and data pertaining to treatment are mostly derived from expert opinion, large case series, and anecdotal reports.
Based on a combination of available literature and clinical experience, a therapeutic approach is outlined, which has been used for a variety of autoimmune CNS disorders.44 The treatment of an autoimmune CNS disorder comprises oncological therapy (where appropriate) and immunotherapy. The treatment protocol must be individualized for the patient, dictated by clinical severity, type of antibody detected, presence or absence of cancer, and treatment response.
Cancer Treatment
Early cancer detection and treatment aims to achieve stabilization or improvement of the neurological symptoms, by removing the antigenic source driving the immune response. In some patients, tumor treatment alone may be dramatically effective (eg, teratoma removal in patients with NMDA receptor antibody).8 For other patients, cancer treatment may (at best) bring about stabilization (eg, small cell carcinoma treatment in a patient with ANNA-1 antibody). Neurological therapeutic benefit may occur as a result of tumor removal but also because of the immunosuppressant effects of chemotherapy. If there is no neurological improvement or stabilization after cancer treatment, the patient may benefit from a trial of immunotherapy.
Immunotherapy
Most of the recommendations discussed below are nonevidence based but are used by the author and colleagues at Mayo Clinic for managing autoimmune neurological disorders. The type of antibody identified may predict the neurologic response to immunotherapy. Detection of antibodies targeting intracellular antigens such as PCA-1 (anti-Yo) carries a poor prognosis because the underlying immune process is mediated by cytotoxic T cells, leading to neuronal degeneration.10 On the other hand, detection of antibodies targeting neural surface antigens (such as NMDA receptors) usually predicts a more favorable outcome, since the direct pathogenic effects of these antibodies may be amenable to immunotherapy (eg, removal by plasma exchange).58 The timing of immunotherapy is also important. Case series of patients with autoimmune CNS disorders has demonstrated that early immunotherapy confers a better treatment outcome than delayed therapy.45,59,60
The immunotherapy protocol may be subdivided into acute therapy and chronic therapy. Before starting immunotherapy, baseline evaluations should be performed to establish clinical parameters for objective monitoring of treatment response. In the hospital setting, this may be simply the neurological examination, or in some cases, EEG telemetry or MRI findings. Antibody titers have limited use in the acute phase of treatment, since values often reduce with treatment even if therapy is not clinically beneficial. Sometimes patients may have mild disorders responsive to symptomatic therapy alone (eg, diazepam therapy in SMS; salt, fluids, fludrocortisone, and compression stockings in a patient with mild orthostasis secondary to autoimmune dysautonomia) or are refractory to immunotherapy (eg, occasional patients with limbic seizure disorders may respond well to antiepileptic drug therapy).
Acute Therapy
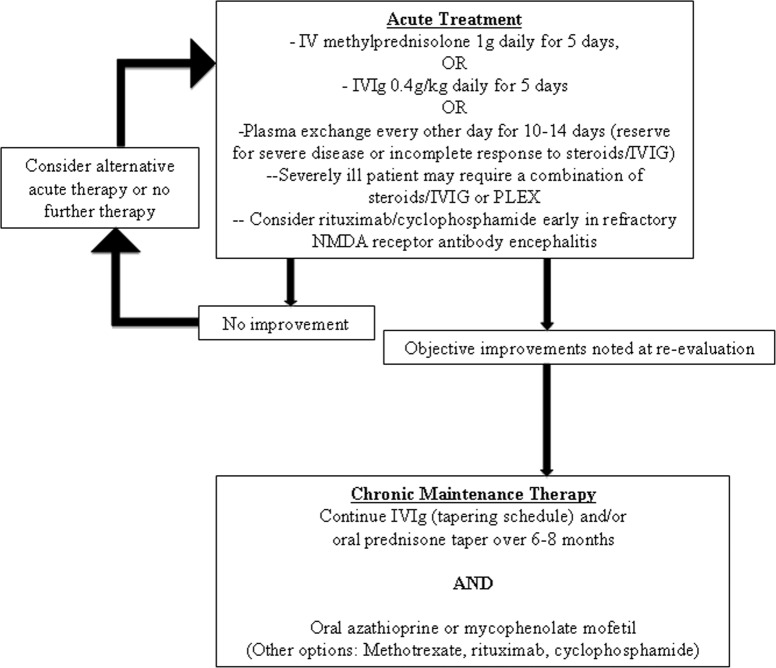
The treatment algorithm (Figure 2, Table 4) and description below is based largely on clinical experience, small retrospective case series, and individual case reports, rather than clinical trial data (which are largely nonexistent). For acute therapy, a trial of high-dose pulse intravenous (IV) corticosteroid therapy is initiated. Intravenous immunoglobulin is an alternative to consider in patients who cannot tolerate corticosteroids or are at risk of diabetes (including GAD65-seropositive patients). Typical treatment protocols in the hospital setting include 1000 mg of IV methylprednisolone daily or 0.4 g/kg of IVIg daily for 5 consecutive days. For patients with severe autoimmune encephalitis, for example VGKC complex antibody or NMDA receptor antibody encephalitis, continued high dose corticosteroid (IV or oral (PO) [eg, prednisone 60 mg daily]) is required. In patients with limited responses, or no response, plasma exchange given every other day for 10 to 14 days frequently proves beneficial. Timing of pursuing the second-line therapy varies depending on the severity of the clinical situation. This is true for patients with VGKC complex antibody, in patients with NMDA receptor antibody encephalitis, and in patients with an NMO spectrum disorder. After the initial trial of therapy, the patient should be reevaluated for objective evidence of improvement. If a rapid series of trials of immunotherapy is not immediately beneficial in an acutely ill hospitalized patient, continued combination therapy (steroids and plasma exchange or IVIg) may be required in addition. This is particularly true for patients with NMDA receptor antibody encephalitis who frequently require months of supportive care in addition to immunotherapy. For this latter group of patients, rituximab with or without cyclophosphamide are frequently introduced where early trials of steroid, IVIG, and plasma exchange do not prove beneficial.8
Figure 2.
Treatment algorithm.
Table 4.
Common Immunotherapies Used to Treat Autoimmune CNS Disorders.
| Treatment | Regimen | Route | Common Side Effects | Therapeutic Phase |
|---|---|---|---|---|
| Methylprednisolone | 1000 mg daily for 5 days (may require ongoing daily dosing in conjunction with other IV therapies, or conversion to oral prednisone 60 mg/d in severe cases) | Intravenous | Insomnia, psychiatric, hyperglycemia, electrolyte imbalances, fluid retention, hypertension, peptic ulcer, Cushing syndrome, cataracts, infection and osteoporosis; avascular necrosis of the hip is rare but has serious side effect. Beware of Addisonian crisis on rapid withdrawal of corticosteroid. | Acute and chronic taper |
| Intravenous immunoglobulin (IVIg) | 0.4/kg daily for 5 days | Intravenous | Headache, aseptic meningitis, thromboembolic events, acute renal failure and anaphylaxis due to IgA deficiency (rare). | Acute and chronic taper |
| Plasma exchange | 1 exchange every other day for 10-14 days | Intravenous (usually through a central line) | Hypotension, electrolyte imbalance (hypocalcemia-related perioral paresthesia). Related to central line: infection, hemorrhage, thrombosis, and pneumothorax. | Acute |
| Rituximab | 375 mg/m2 weekly for 4 doses | Intravenous | Peripheral edema, fever, headache, cytopenias, rash, infusion reaction, opportunistic infection | Acute or chronic |
| Cyclophosphamide | IV: 500-1000 mg/m2 monthly for 3-6 months PO: 1-2 mg/kg per d | Intravenous (IV) or oral (PO) | Gastrointestinal (nausea, vomiting), alopecia, mucositis, hemorrhagic cystitis (administer mesna prophylaxis), infertility and myelosuppression | Acute or chronic |
| Azathioprine | Initially 1.5 mg/kg per d, target 2.5-3 mg/kg per d (Guided by 5-point MCV increase from baseline, and TPMT value) | Oral | Gastrointestinal symptoms (nausea, vomiting, diarrhea), hypersensitivity reactions, alopecia, cytopenia and hepatotoxicity. Rare complications are lymphoma and infection. | Chronic |
| Mycophenolate mofetil | Initially 500 mg twice daily, target 1000 mg twice daily. | Oral | Gastrointestinal (diarrhea, nausea, vomiting), hypertension, peripheral edema, infections, myelosuppression, lymphoma and other malignancies. | Chronic |
Abbreviations: CNS, central nervous system; IgA, immunoglobulin A; TPMT, thiopurine methyltransferase; MCV, mean corpuscular volume.
For patients who ultimately improve and are in a position to be discharged home from a hospital service, with well-defined disorders (such as VGKC complex antibody or NMDA receptor antibody) the author favors 1 mg/kg per d of oral prednisone for 3 months (and starting an oral steroid-sparing agent simultaneously, see chronic therapy below) followed by taper.
For patients with antibodies targeting intracellular antigens, such as ANNA-1, results from immunotherapy are variable, but improvements are rarely dramatic. Intravenous corticosteroids and IVIg may stabilize the neurological symptoms. If neurological symptoms persist despite oncological treatment, IV cyclophosphamide (given as 6 monthly IV pulses or daily oral therapy for 6-12 months) could be considered in those with a good oncological performance status.
Chronic Therapy
If objective assessments clearly establish response to initial immunotherapeutic measures, chronic “maintenance” immunosuppressive therapy should be considered and initiated prior to discharge from hospital, to maintain remission and reduce glucocorticoid or IVIg dependence. In general, azathioprine or mycophenolate mofetil are the agents of choice, having been used in a wide range of autoimmune neurological diseases. Alternatives include methotrexate, hydroxychloroquine, and oral cyclophosphamide. For NMDA receptor antibody encephalitis, azathioprine is often added in as a maintenance therapy in patients who have completed courses of rituximab and/or cyclophosphamide.8 Cyclophosphamide may also bring about stabilization in certain patients with classical paraneoplastic neurological disorders (eg, PCA-1 or ANNA-1) where oncological therapy has been completed.61
The initiation of a chronic oral agent should overlap with gradual taper of oral corticosteroids and/or IVIg infusions over a period of approximately 6 to 8 months. Relapses frequently occur during rapid immunotherapy taper or discontinuation; these have been noted to occur in patients with NMDA receptor, AMPA-R, and VGKC complex antibody–associated encephalitis. The approach of slow, conservative tapering permits the oral immunosuppressants with slow onset of effect, especially azathioprine, to take effect before the steroids or IVIg are fully discontinued. A small minority of patients relapse despite this approach and are dependent on corticosteroid therapy to varying degrees. In these patients, a low dose of oral prednisone (10-20 mg/d or every other day) may be required.
Careful monitoring for side effects of long-term immunotherapy is critical. Surveillance include monitoring of blood counts, liver, and renal function. Where azathioprine is the oral agent chosen, thiopurine methyltransferase (TPMT) deficiency must be excluded prior to treatment initiation. Experience from the use of azathioprine in patients with NMO suggests that monitoring for a rise of mean corpuscular volume (MCV) of at least 5 points from baseline measurement is a good and reliable indicator to ensure optimum efficacy.62
Patients on glucocorticoids must also take elemental calcium, at least 1500 mg/d and vitamin D 1000 IU/d, either through diet or as supplements, as the deterioration of bone mineral density may occur soon after starting steroid use. Baseline and follow-up bone densitometry and bisphosphonate treatment should be considered in patients requiring >3 months of glucocorticoid treatment. Pneumocystis jiroveci pneumonia (PCP) prophylaxis should consist of trimethoprim/sulfamethoxazole 1 double-strength table 3 times per week for patients on chronic corticosteroid or azathioprine. Alternatives for sulfa-allergic patients include daily oral dapsone or monthly inhaled pentamidine.
The correct duration of chronic immunotherapy has not been established since there is no supportive data. The author recommends a trial of withdrawal of chronic therapy after 3 to 5 years of immunotherapy, where no relapses of symptoms have occurred during that time.
Conclusions
Autoimmune CNS disorders are increasingly recognized in the hospital setting. Neural (including paraneoplastic) antibody testing on serum and CSF helps stratify patients diagnostically (neurological and oncological) and prognostically (likely antibody-mediated disorder [immunotherapy responsive] or likely T cell-mediated process [poor prognosis]).
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Wilkinson PC, Zeromski J. Immunofluorescent detection of antibodies against neurones in sensory carcinomatous neuropathy. Brain. 1965;88(3):529–583 [DOI] [PubMed] [Google Scholar]
- 2. Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia: a model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975;141(6):1365–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta Neuropathol. 2011;122(4):381–400 [DOI] [PubMed] [Google Scholar]
- 6. Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010;168(4):1009–1018 [DOI] [PubMed] [Google Scholar]
- 7. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77(2):179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4(11):1321–1324 [DOI] [PubMed] [Google Scholar]
- 10. Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann Neurol. 2000;47(1):9–17 [PubMed] [Google Scholar]
- 11. McKeon A, Tracy JA, Pittock SJ, Parisi JE, Klein CJ, Lennon VA. Purkinje cell cytoplasmic autoantibody type 1 accompaniments: the cerebellum and beyond. Arch Neurol. 2011;68(10):1282–1289 [DOI] [PubMed] [Google Scholar]
- 12. Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56(5):715–719 [DOI] [PubMed] [Google Scholar]
- 13. Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(pt 3):701–712 [DOI] [PubMed] [Google Scholar]
- 14. Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62(7):1177–1182 [DOI] [PubMed] [Google Scholar]
- 15. McKeon A, Marnane M, O'Connell M, Stack JP, Kelly PJ, Lynch T. Potassium channel antibody associated encephalopathy presenting with a frontotemporal dementia like syndrome. Arch Neurol. 2007;64(10):1528–1530 [DOI] [PubMed] [Google Scholar]
- 16. Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch Neurol. 2008;65(10):1341–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42(10):1931–1937 [DOI] [PubMed] [Google Scholar]
- 18. Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50(3):652–657 [DOI] [PubMed] [Google Scholar]
- 19. Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70(20):1883–1890 [DOI] [PubMed] [Google Scholar]
- 20. McKeon A, Ahlskog JE, Britton JW, Lennon VA, Pittock SJ. Reversible extralimbic paraneoplastic encephalopathies with large abnormalities on magnetic resonance images. Arch Neurol. 2009;66(2):268–271 [DOI] [PubMed] [Google Scholar]
- 21. Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49(2):146–154 [PubMed] [Google Scholar]
- 22. Kinsbourne M. Myoclonic encephalopathy of infants. J Neurol Neurosurg Psychiatry. 1962;25(3):271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bataller L, Graus F, Saiz A, Vilchez JJ, Spanish Opsoclonus-Myoclonus Study Group. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001;124(pt 2):437–443 [DOI] [PubMed] [Google Scholar]
- 24. Antunes NL, Khakoo Y, Matthay KK, et al. Antineuronal antibodies in patients with neuroblastoma and paraneoplastic opsoclonus-myoclonus. J Pediatr Hematol Oncol. 2000;22(4):315–320 [DOI] [PubMed] [Google Scholar]
- 25. Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53(5):580–587 [DOI] [PubMed] [Google Scholar]
- 26. Dalmau J, Gultekin SH, Voltz R, et al. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain. 1999;122(pt 1):27–39 [DOI] [PubMed] [Google Scholar]
- 27. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127(pt 8):1831–1844 [DOI] [PubMed] [Google Scholar]
- 28. Pittock SJ, Parisi JE, McKeon A, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol. 2010;67(9):1109–1115 [DOI] [PubMed] [Google Scholar]
- 29. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999;53(5):1107–1114 [DOI] [PubMed] [Google Scholar]
- 30. Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology. 2011;76(24):2089–2095 [DOI] [PubMed] [Google Scholar]
- 31. Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58(1):96–107 [DOI] [PubMed] [Google Scholar]
- 32. Keegan BM, Pittock SJ, Lennon VA. Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann Neurol. 2008;63(4):531–534 [DOI] [PubMed] [Google Scholar]
- 33. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112 [DOI] [PubMed] [Google Scholar]
- 34. Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol. 2010;68(5):757–761 [DOI] [PubMed] [Google Scholar]
- 35. Iorio R, Lucchinetti CF, Lennon VA, et al. Syndrome of inappropriate antidiuresis may herald or accompany neuromyelitis optica. Neurology. 2011;77(17):1644–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKeon A, Lennon VA, Lotze T, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71(2):93–100 [DOI] [PubMed] [Google Scholar]
- 37. Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603–605 [DOI] [PubMed] [Google Scholar]
- 38. Eichel R, Meiner Z, Abramsky O, Gotkine M. Acute disseminating encephalomyelitis in neuromyelitis optica: closing the floodgates. Arch Neurol. 2008;65(2):267–271 [DOI] [PubMed] [Google Scholar]
- 39. Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm (“stiff-man” syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin. 1956;31(15):421–427 [PubMed] [Google Scholar]
- 40. Howard FM., Jr A new and effective drug in the treatment of the stiff-man syndrome: preliminary report. Proc Staff Meet Mayo Clin. 1963;38:203–212 [PubMed] [Google Scholar]
- 41. McKeon A, Robinson MT, McEvoy KM, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol. 2012;69(2):230–238 [DOI] [PubMed] [Google Scholar]
- 42. Whiteley AM, Swash M, Urich H. Progressive encephalomyelitis with rigidity. Brain. 1976;99(1):27–42 [DOI] [PubMed] [Google Scholar]
- 43. Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71(16):1291–1292 [DOI] [PubMed] [Google Scholar]
- 44. McKeon A, Lennon VA, Pittock SJ. Immunotherapy-responsive dementias and encephalopathies. Continuum Lifelong Learning Neurol. 2010;16(2):80–101 [DOI] [PubMed] [Google Scholar]
- 45. Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janssen JC, Godbolt AK, Ioannidis P, Thompson EJ, Rossor MN. The prevalence of oligoclonal bands in the CSF of patients with primary neurodegenerative dementia. J Neurol. 2004;251(2):184–188 [DOI] [PubMed] [Google Scholar]
- 47. Brain L, Jellinek EH, Ball K. Hashimoto's disease and encephalopathy. Lancet. 1966;2(7462):512–514 [DOI] [PubMed] [Google Scholar]
- 48. Castillo P, Woodruff B, Caselli R, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63(2):197–202 [DOI] [PubMed] [Google Scholar]
- 49. Joseph FG, Lammie GA, Scolding NJ. CNS lupus: a study of 41 patients. Neurology. 2007;69(7):644–654 [DOI] [PubMed] [Google Scholar]
- 50. McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology. 2011;76(12):1108–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klawiter EC, Alvarez E, 3rd, Xu J, et al. NMO-IgG detected in CSF in seronegative neuromyelitis optica. Neurology. 2009;72(12):1101–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65(4):424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69(2):303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McKeon A, Apiwattanakul M, Lachance DH, et al. Positron emission tomography-computed tomography in paraneoplastic neurologic disorders: systematic analysis and review. Arch Neurol. 2010;67(3):322–329 [DOI] [PubMed] [Google Scholar]
- 58. Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85(10):881–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207–1214 [DOI] [PubMed] [Google Scholar]
- 61. Vernino S, O'Neill BP, Marks RS, O'Fallon JR, Kimmel DW. Immunomodulatory treatment trial for paraneoplastic neurological disorders. Neuro Oncol. 2004;6(1):55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Costanzi C, Matiello M, Lucchinetti CF, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77(7):659–666 [DOI] [PubMed] [Google Scholar]