Abstract
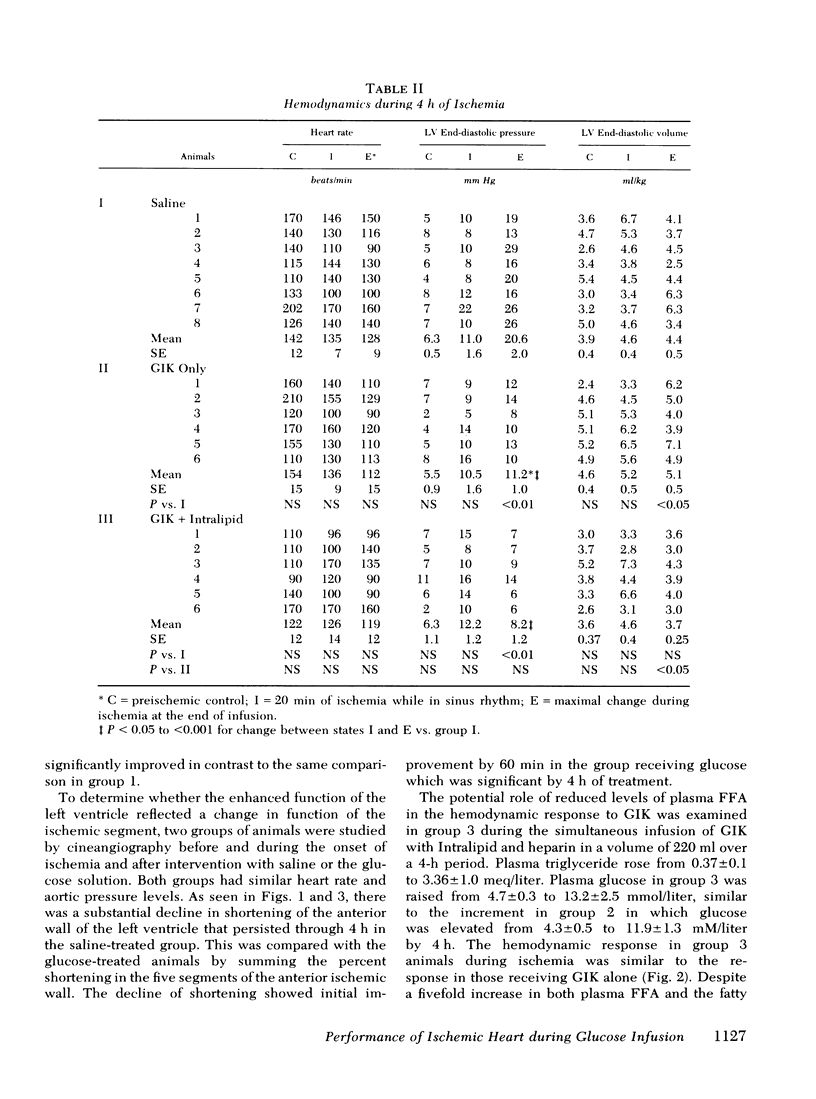
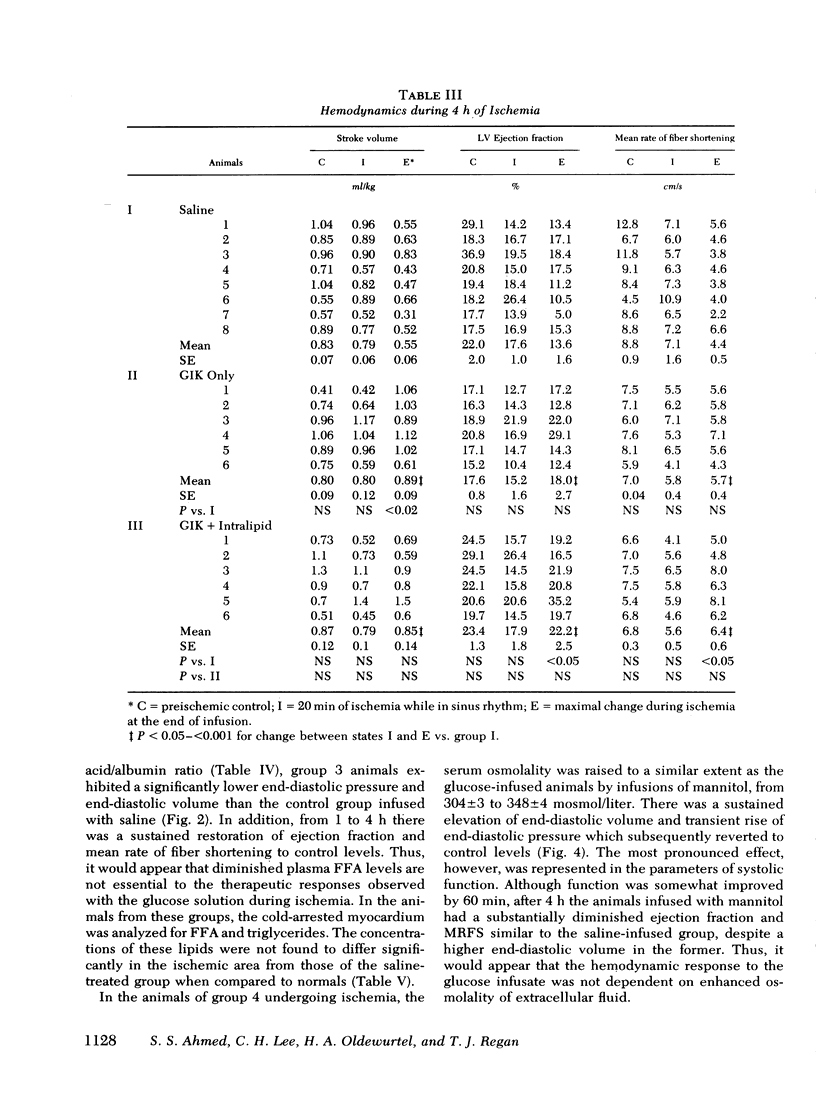
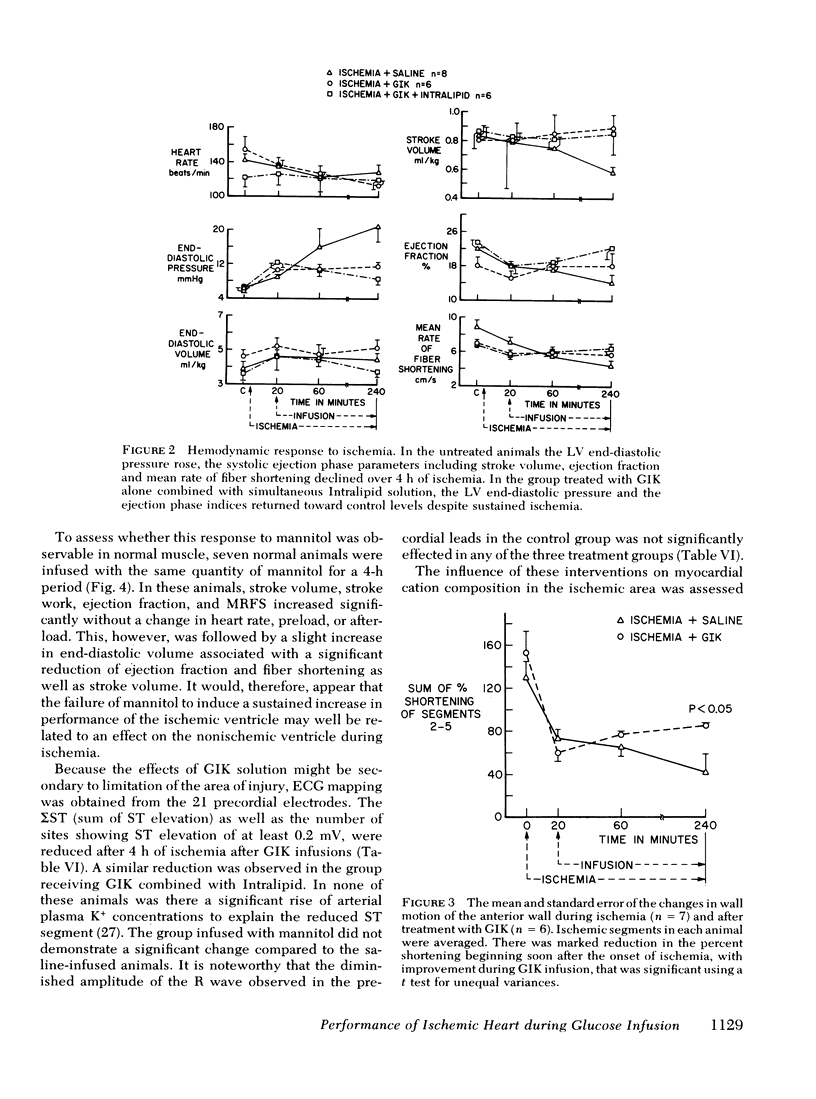
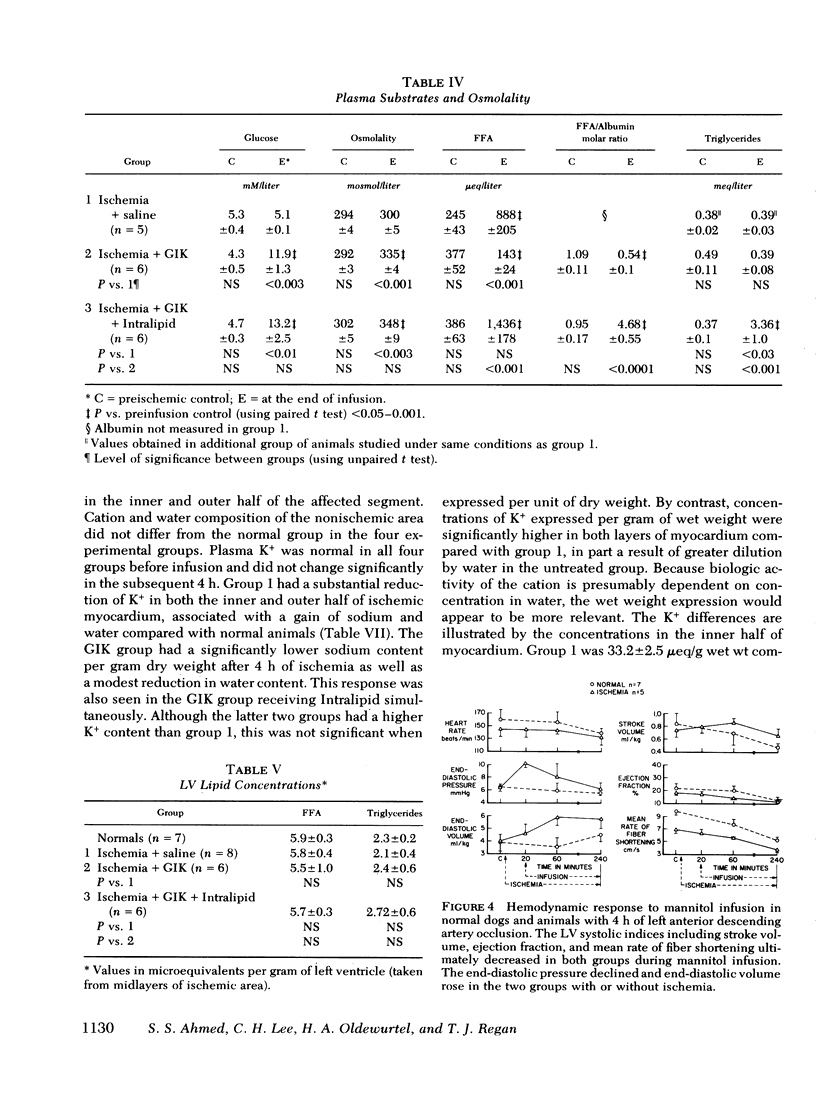
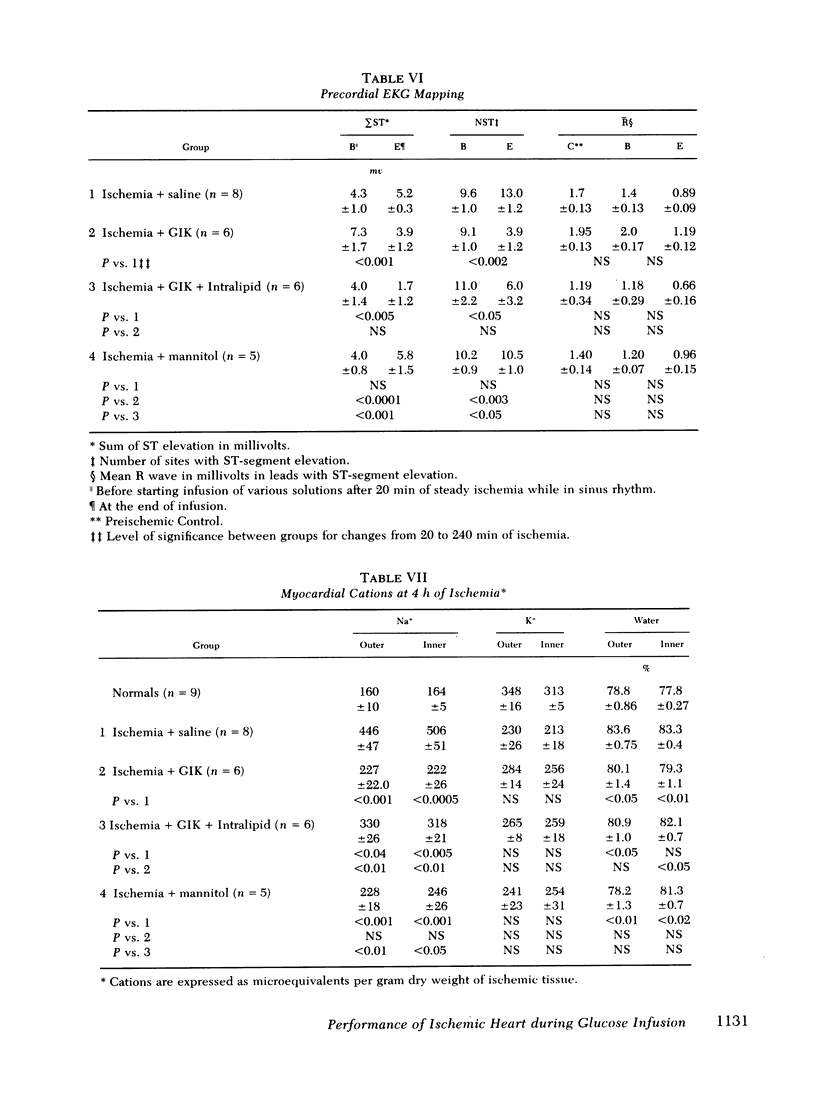
To evaluate the influence of glucose infusate administered with insulin and potassium on left ventricular function during 4 h of ischemia, as well as mechanism of action, four groups of intact anesthetized dogs were studied. Acute regional ischemia was induced with a balloon tip catheter in the left anterior descending artery and infusates were begun after 20 min of ischemia. A threefold increase of plasma glucose concentration was associated with improved left ventricular function during ischemia, compared to animals receiving isovolumic saline. There was a significant decline of left ventricular end-diastolic pressure associated with elevation of stroke volume and ejection fraction to control levels, as determined by indicator dilution. In a separate subgroup studied by cineangiography, shortening of the ischemic anterior wall, after an initial decline, was increased in response to glucose but there was no evidence of extension of injury. Ischemic tissue exhibited a smaller gain of water as well as Na+ per gram dry weight as compared to ischemic controls. On precordial electrocardiogram mapping there was a significant decrease in the sigmaST (sum of ST elevation) as well as NST (number of ST segment elevations), but the reduction of R wave amplitude was not different from controls. To further evaluate long-term effects, eight controls and six treated animals underwent myocardial ischemia and were sacrificed after 4 mo. Calculated area and weight of scar, as well as degree of wall thinning, were similar in both groups. The glucose-treated animals had a significant decrease of plasma FFA in contrast to controls which manifested a significant rise. To examine the postulate that the decrease in FFA was important to therapeutic action, a third group was infused with Intralipid (Cutter Laboratories, Inc., Berkeley, Calif.) and heparin, simultaneously with the glucose infusate, to effect an elevation of plasma FFA during ischemia. Changes in myocardial function and electrolyte composition, as well as precordial electrocardiogram mapping, were similar to that of animals receiving glucose alone. Because serum osmolality was increased approximately 40 mosmol during the glucose infusion, the potential role of hyperosmolality was assessed by infusion of 20% mannitol during acute ischemia in a fourth group. After a transient small increase, there was a moderate decline in function by 4 h, suggesting that the response to glucose is not dependent upon extracellular osmolality. Thus, it is concluded that during the initial hours after the onset of myocardial ischemia the glucose infusate improves ventricular performance without evidence of arrhythmia induction or intensification of ischemic injury. Evolution of irreversible necrosis appears to be delayed rather than prevented under the circumstances of this study.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apstein C. S., Bing O. H., Levine H. J. Cardiac muscle function during and after hypoxia: effects of glucose concentration, mannitol and isoproternol. J Mol Cell Cardiol. 1976 Aug;8(8):627–640. doi: 10.1016/0022-2828(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Burke W. M., Asokan S. K., Moschos C. B., Oldewurtel H. A., Regan T. J. Effects of glucose and nonglucose infusions on myocardial potassium ion transfers and arrhythmias during ischemia. Am J Cardiol. 1969 Nov;24(5):713–722. doi: 10.1016/0002-9149(69)90459-7. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A., WADSTROM L. B. Determination of glycerides in blood serum. Clin Chim Acta. 1959 Mar;4(2):197–205. doi: 10.1016/0009-8981(59)90130-5. [DOI] [PubMed] [Google Scholar]
- Chiariello M., Gold H. K., Leinbach R. C., Davis M. A., Maroko P. R. Comparison between the effects of nitroprusside and nitroglycerin on ischemic injury during acute myocardial infarction. Circulation. 1976 Nov;54(5):766–773. doi: 10.1161/01.cir.54.5.766. [DOI] [PubMed] [Google Scholar]
- Cox J. L., Daniel T. M., Boineau J. P. The electrophysiologic time-course of acute myocardial ischemia and the effects of early coronary artery reperfusion. Circulation. 1973 Nov;48(5):971–983. doi: 10.1161/01.cir.48.5.971. [DOI] [PubMed] [Google Scholar]
- De Leiris J., Opie L. H., Lubbe W. F. Effects of free fatty acid and enzyme release in experimental glucose on myocardial infarction. Nature. 1975 Feb 27;253(5494):746–747. doi: 10.1038/253746a0. [DOI] [PubMed] [Google Scholar]
- Falsetti H. L., Mates R. E., Greene D. G., Bunnell I. L. Vmax as an index of contractile state in man. Circulation. 1971 Apr;43(4):467–479. doi: 10.1161/01.cir.43.4.467. [DOI] [PubMed] [Google Scholar]
- Forrester J. S., Ganz W., Diamond G., McHugh T., Chonette D. W., Swan H. J. Thermodilution cardiac output determination with a single flow-directed catheter. Am Heart J. 1972 Mar;83(3):306–311. doi: 10.1016/0002-8703(72)90429-2. [DOI] [PubMed] [Google Scholar]
- Frank M. J., Cundey P. E., Jr, Crews T. L., Lewis W. J., 3rd Comparison of left ventricular volumes by single-plane cineangiography and by indicator dilution. J Lab Clin Med. 1971 Apr;77(4):580–593. [PubMed] [Google Scholar]
- Frank M. J., Levinson G. E. An index of the contractile state of the myocardium in man. J Clin Invest. 1968 Jul;47(7):1615–1626. doi: 10.1172/JCI105853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. M., Jr, Nakamura Y. Effect of coronary constriction on myocardial distribution of iodoantipyrine-131-I. Am J Physiol. 1968 Nov;215(5):1082–1086. doi: 10.1152/ajplegacy.1968.215.5.1082. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S., Fenton J. C., Wolf P. L., Bing R. J. Stimulation of reparative processes following experimental myocardial infarction. Arch Intern Med. 1966 Jul;118(1):33–40. [PubMed] [Google Scholar]
- HILL J. B., KESSLER G. An automated determination of glucose utilizing a glucose oxidase-peroxidase system. J Lab Clin Med. 1961 Jun;57:970–980. [PubMed] [Google Scholar]
- Hillis L. D., Askenazi J., Braunwald E., Radvany P., Muller J. E., Fishbein M. C., Maroko P. R. Use of changes in the epicardial QRS complex to assess interventions which modify the extent of myocardial necrosis following coronary artery occlusion. Circulation. 1976 Oct;54(4):591–598. doi: 10.1161/01.cir.54.4.591. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P. G., Wagner H. R., Sandler H. The in vivo determination of left ventricular volume. Comparison of the fiberoptic-indicator dilution and the angiocardiographic methods. Circulation. 1968 Apr;37(4):489–508. doi: 10.1161/01.cir.37.4.489. [DOI] [PubMed] [Google Scholar]
- Keroes J., Rapaport E. Ventricular volume measurement in the awake dog using implanted thermistor beads. J Appl Physiol. 1972 Mar;32(3):404–408. doi: 10.1152/jappl.1972.32.3.404. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Yates P. A., Oliver M. F. The role of free fatty acids in the production of ventricular arrhythmias after acute coronary artery occlusion. Eur J Clin Invest. 1971 Jan;1(4):225–241. doi: 10.1111/eci.1971.1.4.225. [DOI] [PubMed] [Google Scholar]
- Leighton R. F., Wilt S. M., Lewis R. P. Detection of hypokinesis by a quantitative analysis of left ventricular cineangiograms. Circulation. 1974 Jul;50(1):121–127. doi: 10.1161/01.cir.50.1.121. [DOI] [PubMed] [Google Scholar]
- Levinson G. E., Frank M. J., Nadimi M., Braunstein M. Studies of cardiopulmonary blood volume. Measurement of left ventricular volume by dye dilution. Circulation. 1967 Jun;35(6):1038–1048. doi: 10.1161/01.cir.35.6.1038. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J., Hughes H. C., Neely J. R. Effects of excess glucose and insulin on glycolytic metabolism during experimental myocardial ischemia. Am J Cardiol. 1976 Jul;38(1):17–27. doi: 10.1016/0002-9149(76)90057-6. [DOI] [PubMed] [Google Scholar]
- Lochner A., Van Der Walt J. J., Bajusz E., Brink A., Kotze J. C. Effects of potassium-glucose-insulin treatment on the histology, protein synthesis, and mechanical activity of the myopathic hamster heart. Recent Adv Stud Cardiac Struct Metab. 1973;2:543–555. [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Covell J. W., Sobel B. E., Ross J., Jr, Braunwald E. Precordial S-T segment elevation mapping: an atraumatic method for assessing alterations in the extent of myocardial ischemic injury. The effects of pharmacologic and hemodynamic interventions. Am J Cardiol. 1972 Feb;29(2):223–230. doi: 10.1016/0002-9149(72)90633-9. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Sobel B. E., Bloor C. M., Sybers H. D., Shell W. E., Covell J. W., Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972 Jun;45(6):1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- Mullins C. B., Leshin S. J., Mierzwiak D. S., Alsobrook H. D., Mitchell J. H. Changes in left ventricular function produced by the injection of contrast media. Am Heart J. 1972 Mar;83(3):373–381. doi: 10.1016/0002-8703(72)90439-5. [DOI] [PubMed] [Google Scholar]
- NEWMAN E. V., MERRELL M., GENECIN A., MONGE C., MILNOR W. R., McKEEVER W. P. The dye dilution method for describing the central circulation. An analysis of factors shaping the time-concentration curves. Circulation. 1951 Nov;4(5):735–746. doi: 10.1161/01.cir.4.5.735. [DOI] [PubMed] [Google Scholar]
- Norris R. M., Smith H. J., Singh B. N., Nisbet H., John M. B., Hurley P. J. The effects of isoprenaline on epicardial ST-segment elevation, lactate production, and myocardial blood flow following coronary artery ligation. Cardiovasc Res. 1975 Nov;9(6):770–778. doi: 10.1093/cvr/9.6.770. [DOI] [PubMed] [Google Scholar]
- Oliver M. F., Yates P. A. Induction of ventricular arrhythmias by elevation of arterial free fatty acids in experimental myocardial infarction. Cardiology. 1971;56(1):359–364. doi: 10.1159/000169385. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Owen P. Effect of glucose-insulin-potassium infusions on arteriovenous differences of glucose of free fatty acids and on tissue metabolic changes in dogs with developing myocardial infarction. Am J Cardiol. 1976 Sep;38(3):310–321. doi: 10.1016/0002-9149(76)90173-9. [DOI] [PubMed] [Google Scholar]
- PRINZMETAL M., TOYOSHIMA H., EKMEKCI A., MIZUNO Y., NAGAYA T. Myocardial ischemia. Nature of ischemic electrocardiographic patterns in the mammalian ventricles as determined by intracellular electrographic and metabolic changes. Am J Cardiol. 1961 Oct;8:493–503. doi: 10.1016/0002-9149(61)90123-0. [DOI] [PubMed] [Google Scholar]
- REGAN T. J., BINAK K., GORDON S., DEFAZIO V., HELLEMS H. K. Myocardial blood flow and oxygen consumption during postprandial lipemia and heparin-induced lipolysis. Circulation. 1961 Jan;23:55–63. doi: 10.1161/01.cir.23.1.55. [DOI] [PubMed] [Google Scholar]
- Regan T. J., Harman M. A., Lehan P. H., Burke W. M., Oldewurtel H. A. Ventricular arrhythmias and K+ transfer during myocardial ischemia and intervention with procaine amide, insulin, or glucose solution. J Clin Invest. 1967 Oct;46(10):1657–1668. doi: 10.1172/JCI105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. G., Opie L. H., Bricknell O. L. Early experimental myocardial infarction. Evaluation of histologic criteria and comparison with biochemical and electrocardiographic measurements. Arch Pathol Lab Med. 1976 Oct;100(10):516–521. [PubMed] [Google Scholar]
- SODI-PALLARES D., BISTENI A., MEDRANO G. A., TESTELLI M. R., DE MICHELI A. The polarizing treatment of acute myocardial infarction. Possibility of its use in other cardiovascular conditions. Dis Chest. 1963 Apr;43:424–432. doi: 10.1378/chest.43.4.424. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Brachfeld N. Myocardial uptake and fractional distribution of palmitate-1 C14 by the ischemic dog heart. Metabolism. 1966 Oct;15(10):945–954. doi: 10.1016/0026-0495(66)90165-x. [DOI] [PubMed] [Google Scholar]
- Serur J. R., Urschel C. W., Sonnenblick E. H., Laraia P. J. Experimental myocardial ischemia. III. Protective effect of glucose of myocardial function. J Mol Cell Cardiol. 1976 Jul;8(7):521–531. doi: 10.1016/0022-2828(76)90053-5. [DOI] [PubMed] [Google Scholar]
- Stanley A. W., Jr, Moraski R. E., Russell R. O., Rogers W. J., Mantle J. A., Kreisberg R. A., McDaniel H. G., Rackley C. E. Effects of glucose-insulin-potassium on myocardial substrate availability and utilization in stable coronary artery disease. Studies on myocardial carbohydrate, lipid and oxygen arterial-coronary sinus differences in patients with coronary artery disease. Am J Cardiol. 1975 Dec;36(7):929–937. doi: 10.1016/0002-9149(75)90085-5. [DOI] [PubMed] [Google Scholar]
- Sybers H. D., Maroko P. R., Ashraf M., Libby P., Braunwald E. The effect of glucose-insulin-potassium on cardiac ultrastructure following acute experimental coronary occlusion. Am J Pathol. 1973 Mar;70(3):401–420. [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. The effect of glucose and fructose ingestion on the adrenaline and noradrenaline levels in human plasma. J Endocrinol. 1954 Oct;11(3):298–303. doi: 10.1677/joe.0.0110298. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Watson J. T., Hutton I., Fixler D. E., Curry G. C., Templeton G. H. The influence of hypertonic mannitol on regional myocardial blood flow during acute and chronic myocardial ischemia in anesthetized and awake intact dogs. J Clin Invest. 1975 May;55(5):892–902. doi: 10.1172/JCI108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk M. J., Scheidt S., Killip T. Heart failure complicating acute myocardial infarction. Circulation. 1972 May;45(5):1125–1138. doi: 10.1161/01.cir.45.5.1125. [DOI] [PubMed] [Google Scholar]


