Abstract
Background and Purpose
Observations in human interventional stroke treatment led us to hypothesize that iodinated radiographic contrast material use may contribute to intracerebral hemorrhage. Effects of intra-arterial iodinated radiographic contrast material on hemorrhagic transformation after middle cerebral artery occlusion and reperfusion were studied in a placebo-controlled, blinded preclinical study in rats.
Methods
Four groups of male Sprague-Dawley rats were studied: saline group (n=8), contrast group (n=12), heparin group (n=9), and contrast+heparin group (n=9). The middle cerebral artery was occluded for 5 hours using suture placement. Heparin was infused before suture removal and reperfusion. Saline and/or contrast were infused immediately during reperfusion. Incidence, location, and size of hemorrhage were determined by brain necropsy inspection at 24 hours.
Results
There was a significant increase in incidence of cortical hemorrhage from control (37.5%), contrast (75.0%), heparin (77.8%) to contrast+heparin (100%; Cochran-Mantel-Haenszel correlation, P<0.01). Both pooled contrast groups (85.7%) and pooled heparin groups (88.9%) had higher rates of cortical intracerebral hemorrhage compared with the control group (P<0.05). Similar trends for increased cortical intracerebral hemorrhage were seen in the contrast-only (P=0.18) and heparin-only (P=0.18) groups. There was a trend for decreased infarct edema in rats receiving contrast versus those without (P=0.06).
Conclusion
Intraarterial iodinated radiographic contrast material may increase cortical intracerebral hemorrhage, similar to heparin. Iodinated radiographic contrast material effect may be additive to heparin effect on the incidence of cortical intracerebral hemorrhage.
Keywords: animal model, arterial occlusion, contrast media, heparin, intracerebral hemorrhage
Acute ischemic stroke injures the blood–brain barrier causing edema and swelling as well as intracerebral hemorrhage (ICH) in some instances.1 Acute stroke intraarterial intervention trials have encountered ICH rates higher than expected compared with untreated patients and with patients treated with intravenous recombinant tissue plasminogen activator. The Prolyse in Acute Cerebral Thromboembolism (PROACT) II Trial and Interventional Management of Stroke Trials I and II were associated with symptomatic ICH rates of 10%, 6.3%, and 9.9% as well as asymptomatic ICH rates of 35%, 42.5%, and 32.1%, respectively. Heparin-treated controls in PROACT I exhibited a symptomatic ICH rate of 7.1%, prompting a decrease in heparin for the PROACT II Trial.2 Heparin and thrombolytics are likely additive in their ICH effects.
Iodinated radiographic contrast material (IRCM) use is integral to intra-arterial thrombolytics as a diagnostic aid and to monitor therapy progress. In hyperacute stroke, iodinated CT contrast and gadolinium MR contrast accumulate under a variety of circumstances, and their passage across the blood–brain barrier by rapid CT or MR methods predicts blood–brain barrier disruption and subsequent ICH.3–5 Among patients treated with intravenous recombinant tissue plasminogen activator, those having CT angiography fared clinically than those who did not, raising the possibility of an unspecified harmful effect of intravenous IRCM.6
IRCM may accumulate in tissue and be visible on CT after intra-arterial thrombolysis.7–10 We hypothesize that IRCM itself may contribute to ICH and edema in acute stroke therapy. This report summarizes our preliminary observations on the effects of IRCM injection on ICH in a rat middle cerebral artery (MCA) reperfusion model.
Materials and Methods
The animal protocol was approved by the University Animal Care Committee and conformed to the National Institute of Health Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats (provided by Department of Neurology, University of Cincinnati, Cincinnati, Ohio) had unrestricted access to food and water and were housed with a 12-hour light–dark cycle. Throughout the study, the investigators and veterinarian staff closely monitored the rats’ health status. All chemicals and reagents were of reagent grade unless otherwise specified.
Stroke Model and Drug Injection
This was a single-blind, placebo-controlled study. The left MCA was occluded using the intraluminal filament technique.11 Rats were anesthetized with 1.5% of isoflurane and the left common carotid artery, external carotid artery, and left internal carotid artery isolated through a ventral midline neck incision. A 3-0 monofilament nylon suture was inserted into the external carotid artery and advanced approximately 20 mm beyond the carotid bifurcation until mild resistance was felt. The wound was closed temporarily and the suture kept in place for 5 hours followed by removal of the suture for reperfusion. The ventral midline neck incision was opened again, and a polyethylene-10 tube was placed into the external carotid artery with its tip at the internal carotid artery origin immediately after removal of the occluding suture (reperfusion), for infusion. The internal carotid artery was not directly catheterized. Four groups (n=38) of male Sprague-Dawley rats (body weight=309.7±1.24 g) with different infusion regimens were studied: (1) saline+saline (control group, n=8); (2) saline+contrast (contrast group, n=12); (3) intravenous heparin+saline+saline (heparin group, n=9); and (4) intravenous heparin+saline+contrast (contrast+heparin group, n=9). All animals received a 10-minute intra-arterial saline infusion using a KD Scientific Syringe (Model 210) of 1 mL/kg. Fifteen minutes later, contrast groups received an infusion of nonionic low-osmolar (672 mOsm/kg H2O) iohexol contrast (Omnipaque 300 mgI/mL; Amersham Health, Inc, Princeton, NJ) at 30 μL/min (infusion time 622±7.4 seconds). The external carotid artery was ligated and the incisions sutured. Five minutes before reperfusion, the heparin groups (heparin and contrast+heparin) received bolus intravenous heparin (APP Pharmaceuticals, LLC, Schaumburg, Ill; 150 U/kg body weight) followed by 36 U/kg body weight (57.5 U average heparin group, 56.9 U average heparin+contrast group) through a hind-limb vein. During anesthesia, rectal temperature was monitored and body temperature maintained at 37±0.5°C with a heating pad. Following a small craniotomy near the vertex, cerebral blood flow (CBF) was estimated from the left MCA using laser Doppler perfusion measurements (PF-5001; Perimed, Inc, Järfälla, Sweden) before and after MCA occlusion and after reperfusion with saline and/or contrast infusion.12 After the operation, rats were transferred to a temperature-controlled incubator at 37°C for 30 minutes before transfer to normal housing.
Neurological Deficits and Mortality
Neurological examinations at 45 minutes and 6.5, 8, 12, and 24 hours after induction of ischemia were scored according to a 7-point scale modified from Zhang.13 Mortality was expressed as the number of rats dying within 24 hours, before euthanization, divided by the number in each group×100.
Determination of Hemorrhage and Edema
The animals were euthanized 24 hours after occlusion onset. Brains were harvested, immersed and fixed in 4% paraformaldehyde for 15 minutes, then serially sectioned (typically 6 2-mm coronal slices with 12 surfaces), and imaged for hemorrhage and brain edema using a MCID digital image analysis system (Imaging Research, Inc, St Catherines, Ontario, Canada). The surface of all brains was visually inspected for the presence of hemorrhage. Hemorrhage rate was calculated as: (rat number with hemorrhage in striatum or cortex)/(total rat number)×100%. Sections showing hemorrhage in the striatum or cortex were counted visually for each rat in each group. The hemorrhage area (mm2) on each section face was measured with the MCID imaging system. Brain edema percentage was calculated: [volume of infarcted hemisphere (mm3)−volume of contralateral normal hemisphere (mm3)]/volume of contralateral hemisphere (mm3)×100.14
Statistics
Data were analyzed using SAS, Version 9.1 (SAS Institute, Cary, NC) and expressed as mean±SEM, or number (%), as appropriate. The continuous data, cerebral blood flow, area of cortex and undercortex, and percent edema were examined for deviation from assumption of normality. Analysis was performed using a generalized linear or mixed model approach to account for the repeated measures, where necessary. Dunnett test was used to compare groups as appropriate and account for the multiple comparisons. Analysis of cortical hemorrhage incidence and mortality was done initially using an overall χ2 or Fisher exact test, as appropriate, and a Cochran-Mantel-Haenszel statistic to specifically examine the correlation over groups. Further analysis to examine overall contrast and heparin effects was done using logistic regression. Multiple comparisons, for categorical variables, were controlled for by use of a Bonferroni correction.
Results
CBF Measurements
CBF 5 minutes after MCA occlusion decreased to less than approximately 25.0% of preischemia baseline levels in all groups, indicating successful occlusion (Table 1). The contrast group exhibited numerically lower percent CBF after injection versus control and both heparin groups. The heparin and contrast+heparin groups exhibited higher percent CBF after injection versus the control group (nonsignificant).
Table 1.
Effect of Contrast and/or Heparin Injection on CBF After 5 Hours of MCA Occlusion in Rats
| After Ischemia/Before Ischemia* | After Injection/Before Ischemia† | |
|---|---|---|
| Control (n=8) | 20.5±3.20 | 103.9±11.49 |
| Contrast (n=12) | 20.6±2.25 | 96.4±9.70 |
| Heparin (n=9) | 22.9±2.85 | 124.1±17.08 |
| Contrast+heparin (n=9) | 24.0±2.65 | 119.5±14.06 |
Data are expressed as the mean±SEM of percent changes compared with control values (before ischemia as 100%). There was no significant difference between groups (overall *P=0.54 and †P=0.28).
Hemorrhage Incidence and Location
There was a significant increase in incidence of cortical hemorrhage from control (37.5%), contrast (75.0%), heparin (77.8%) to contrast+heparin (100%; Table 2; Cochran-Mantel-Haenszel correlation, P<0.01). The contrast+heparin group exhibited increased cortical petechial ICH compared with the control group (P=0.03). The contrast group (P=0.18) and the heparin group (P=0.18) exhibited similar trends toward increased cortical ICH compared with the control group (Figure). Using logistic regression to look at the effects of heparin and contrast, the odds of hemorrhage were higher in those given contrast compared with no contrast (OR, 6.76; 95% CI, 1.13 to 40.34; P=0.04) and the odds of hemorrhage was higher in those given heparin compared with those not given heparin (P=0.03) All animals exhibited deep, subcortical hemorrhage, including 1 parenchymal hematoma, the remainder of the hemorrhagic infarction (HI)-1 type.15
Table 2.
Effect of Contrast and/or Heparin Injection on Cortical Hemorrhage After 5 Hours of MCA Occlusion in Rats (Overall P=0.03)
| Rats With Hemorrhage (No.) | Rats With Hemorrhage (%) | P (Versus Control) | |
|---|---|---|---|
| Control (n=8) | 3 | 37.5 | N/A |
| Contrast (n=12) | 9 | 75.0 | 0.18 |
| Heparin (n=9) | 7 | 77.7 | 0.18 |
| Contrast+heparin (n=9) | 9 | 100.0 | 0.03 |
| Any contrast (contrast and contrast+heparin; n=21) | 18 | 85.7 | 0.04 |
| Any heparin (heparin and contrast+heparin; n=18) | 16 | 88.9 | 0.03 |
N/A indicates not applicable.
Figure.
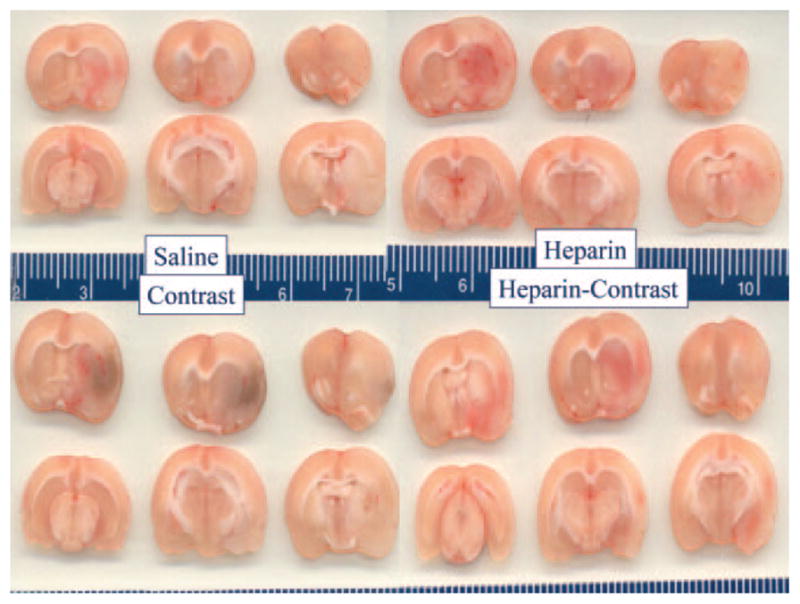
Postmortem brain sections from animals in the saline (top left), intravenous heparin (top right), contrast (lower left), and heparin+contrast (lower right) groups. Petechial hemorrhagic staining is visible in deep subcortical regions of all rats as well as in lateral cortical regions (reader’s right) of all animals. The blue color in the contrast group indicates greater petechial hemorrhagic change, including cortical regions.
Hemorrhage Area
The area of deep, undercortical hemorrhage for the heparin group was significantly larger than the control group (P=0.04; Table 3).
Table 3.
Effect of Contrast and/or Heparin Injection on Hemorrhage Area per Surface Number in the Cortex and Undercortex After 5 Hours of MCA Occlusion in Rats
| Cortex* (mm2) | Undercortex† (mm2) | |
|---|---|---|
| Control (n=8) | 2.97±1.63 | 9.19±0.97 |
| Contrast (n=12) | 4.66±1.25 | 10.94±1.32 |
| Heparin (n=9) | 4.71±1.30 | 14.44±1.45 |
| Contrast+heparin (n=9) | 3.10±0.86 | 10.26±1.18 |
Data are expressed as the mean±SEM.
P=0.61,
P=0.04 (control compared with heparin group; P=0.04).
Cerebral Edema
No difference was seen in edema between the individual groups (Table 4). Both groups administered contrast (contrast and contrast+heparin) exhibited a strong trend toward less edema compared with the saline control group (P=0.06).
Table 4.
Effect of Contrast and/or Heparin Injection on Percent Edema After 5 Hours of MCA Occlusion in Rats (Overall P=0.14)
| Percent Edema | |
|---|---|
| Control (n=8) | 26.11±1.44 |
| Contrast (n=12) | 22.90±3.01 |
| Heparin (n=9) | 24.78±1.38 |
| Contrast+heparin (n=9) | 18.83±3.36 |
| Contrast and contrast+heparin (n=21) | 21.00±1.68* |
| Heparin and contrast+heparin (n=18) | 21.80±1.81 |
Data are expressed as the mean±SEM.
P=0.06 compared with the control group.
Neurological Deficit and Mortality
After MCA occlusion, all rats had forelimb flexion, circling to the right, indicating successful suture placement. Statistically significant differences were seen between the saline control group and all other groups (P<0.05) and between the contrast and contrast+heparin groups and between heparin and contrast+heparin groups (P<0.05 for both), but not between contrast and heparin groups (P=0.16).
There was no overall difference in mortality between groups (P=0.12). Highest mortality rate was in the heparin group (77.8%), followed by saline control (50.0%) and contrast group (41.7%) and the contrast+heparin group (22.2%).
Discussion
Rats with transient MCA occlusion, followed by reperfusion, demonstrate significantly higher cortical ICH compared with those with permanent occlusion (81.8% versus 18.2%, P<0.05).16 Hemorrhage scores were also higher with transient occlusion, supporting the postulate that reperfusion contributes to ICH.17,18
If reperfusion leads to increased ICH, a trend for an agent to be associated with increased ICH after reperfusion implicates the agent in the etiologic mechanism. In our study, intra-arterial IRCM exhibited an effect on cortical ICH greater than saline control and similar to intravenous heparin. Pooled analysis of contrast groups exhibited a significant increase compared with saline, and when heparin and contrast were infused together, ICH effects appear additive, strongly suggesting IRCM contributes to ICH.
IRCMs differ in their ability to pass through a normal blood–brain barrier. Nonionic, low-osmolar iopromide and isosmolal iodixanol do not cross the blood–brain barrier.19,20 Non-ionic low-osmolal iohexol and iodixanol disrupt the blood–brain barrier with insignificant differences when injected intra-arterially in the rabbit.21 IRCMs may exhibit physiological effects mediated through leakage through an altered blood–brain barrier when administered intravenously for CT in the presence of enhancing intracranial tumors.22–24 Ionic hyperosmolar sodium iothalamate administration has been associated with increased infarct volume and worse neurological status compared with iopromide after permanent occlusion.25
IRCM deposition in the brain after intra-arterial injection has been demonstrated after uncomplicated aneurysm coiling procedures and after carotid angioplasty and stenting after repetitive injections of IRCM.26,27 Such deposition is usually transitory with local absorption decreasing local concentration with few clinical sequelae.28
IRCM may accumulate locally on CT after intra-arterial thrombolysis. Yoon defined deposition/accumulation as either contrast enhancement (any hyperdensity that disappeared within 24 hours) or contrast extravasation (a hyperdensity of Hounsfield units >90 that persisted on follow-up CT).29 Contrast enhancement appeared benign, whereas contrast extravasation was associated with increased symptomatic ICH as suggested by others.30,31 Khatri linked IRCM identification, subsequent hemorrhage, and local microcatheter injections during revascularization, suggesting local deposition might have important implications.9,10
In our model, IRCM may increase ICH, potentially associated with reduced CBF and infarct volume, effects that could be a result of diminished reperfusion. IRCM rats did exhibit lower CBF than control animals, and IRCM+heparin rats exhibited reduced CBF compared with heparin-only animals. IRCMs are known to have paradoxical prothrombotic, anticoagulant, and fibrinolytic effects32–38 in vitro.
Nonionic low-osmolar IRCMs and isosmolal iodixanol permit greater thrombin generation and platelet and fibrin deposition in animal models39–43 than ionic ioxaglate. Increased local thrombus formation during coronary intervention in humans occurs with nonionic compared with ionic low-osmolar contrast material.44,45 A local intra-arterial thrombotic occlusive effect may lead to a prolonged locally administered IRCM deposition in cerebral vessels, which in combination with local intracerebral IRCM anticoagulant effects, may contribute to increased cortical ICH in a contradictory, paradoxical fashion.
Problems exist in attempting to link ICH in this rat model to the human condition. Heparin dosage used in our study conformed to Food and Drug Administration animal study recommendations, which recognize the dose-equivalent effect of heparin and other drugs in rats differs from that in humans and requires more heparin.16,46,47 If one suggests our heparin dose was excessive, the equivalence of contrast with heparin in respect to ICH occurrence serves to further indict contrast as a potentially harmful agent, likely greater than lower doses of heparin. The arterial procedure in our model, including a constant 10-minute iopamidol injection, does not perfectly mimic a revascularization procedure in humans. However, it simulates prolonged deposition of high-concentration IRCM in narrow, short segments of occluded arteries as seen with intrathrombus microcatheter injections in acute stroke therapy. Although we hypothesize a possible contribution of a thrombotic effect of contrast to ICH, we have not excluded nonthrombotic vascular effects such as spasm as a contributor to reduced CBF in contrast-infused animals. Low-osmolal iopamidol has been reported to cause reduced blood flow due to vasoconstriction in the renal bed after intraarterial injection, but cerebral vasoconstrictive effects have not been similarly demonstrated.48
The significance of differences of deep versus cortical ICH in this rat model and implications for human stroke therapy remain unclear. It is not clear that petechial cortical ICH and deep basal ganglia ICH in the rat model have the same implications. However, some human data suggest hemorrhagic infarction of all types may be significant.49 Finally, this study does not yet confirm a direct link between the IRCM administered to the site of its deposition and to subsequent ICH at that site.
In summary, this study suggests IRCM itself contributes to cortical ICH in the setting of transient MCA occlusion and may be additive to heparin’s effect. This increased ICH may be promoted by a persistent occlusive effect diminished by heparin therapy, but at the risk of additional heparin-induced hemorrhage. Although any harmful effect of IRCM in humans is likely outweighed by the global benefit inherent in treatment, identifying, quantifying, and reducing such risk of different IRCM offers the opportunity to improve outcomes by optimizing treatment parameters, where possible.
Acknowledgments
Sources of Funding
This study was funded by The Neuroscience Institute, University of Cincinnati, Cincinnati, Ohio, and NS050569 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Disclosures
None.
References
- 1.Toni D, Fiorelli M, Bastianello S, Sacchetti ML, Sette G, Argentino C, Montinaro E, Bozzao L. Hemorrhagic transformation of brain infarct. Neurology. 1996;46:341–345. doi: 10.1212/wnl.46.2.341. [DOI] [PubMed] [Google Scholar]
- 2.Del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant prourokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Kendall BE, Pullicino P. Intravascular contrast injection in ischemic lesions. Neuroradiolgy. 1980;19:241–243. doi: 10.1007/BF00347802. [DOI] [PubMed] [Google Scholar]
- 4.Hayman LA, Evans RA, Bastion FO, Hinck VC. Delayed high dose contrast CT: identifying patients at risk of massive hemorrhagic infarction. AJNR Am J Neuroradiol. 1981;136:1151–1159. doi: 10.2214/ajr.136.6.1151. [DOI] [PubMed] [Google Scholar]
- 5.Dzialowski I, Puetz V, Demchuk AM. Does application of radio contrast material prior to thrombolysis impact thrombolytic effect in acute ischemic stroke? Stroke. 2008;39:601. [Google Scholar]
- 6.Aviv RI, d’Esterre CD, Murphy BD, Hopyan JJ, Buck B, Mallia G, Li V, Zhang L, Symons SP, Lee TY. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250:867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- 7.Wildenhain SL, Jungreis CA, Barr J, Mathis J, Wechsler L, Horton JA. CT after intraarterial thrombolysis for acute stroke. AJNR Am J Neuroradiol. 1994;15:487–492. [PMC free article] [PubMed] [Google Scholar]
- 8.Urbach H, Bendszus M, Brechtelsbauer D, Solymosi L. Extravastion of contrast medium in local intraarterial fibrinolysis of the carotid territory. Nervearzt. 1998;69:490–494. doi: 10.1007/s001150050302. [DOI] [PubMed] [Google Scholar]
- 9.Khatri P, Broderick JP, Khoury JC, Carrozzella JA, Tomsick TA IMS I and II Investigators. Microcatheter contrast injections during intraarterial thrombolysis increases intracranial hemorrhage risk. Stroke. 2008;39:3283–3287. doi: 10.1161/STROKEAHA.108.522904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khatri R, Khatri P, Broderick JP, Khoury JC, Carrozzella JA, Tomsick TA IMS I and II Investigators. Microcatheter contrast injections during intraarterial thrombolysis increase parenchymal hematoma risk: registry experience. Stroke. 2007;38:454. [Google Scholar]
- 11.Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood–brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1994;14:620–627. doi: 10.1038/jcbfm.1994.77. [DOI] [PubMed] [Google Scholar]
- 12.Nito C, Kamiya T, Ueda M, Arii T, Katayama Y. Mild hypothermia enhances the neuroprotective effects of FK506 and expands its therapeutic window following transient focal ischemia in rats. Brain Res. 2004;1008:179–185. doi: 10.1016/j.brainres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 14.Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia. Effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- 15.Tambasco N, Corea F, Luccioli R, Ciorba E, Parnetti L, Gallai V. Brain CT scan in acute ischemic stroke: early signs and functional outcome. Clin Exp Hypertens. 2002;24:687–696. doi: 10.1081/ceh-120015345. [DOI] [PubMed] [Google Scholar]
- 16.Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Khatri P, Tomsick T, Sharp FR. Mechanical reperfusion is associated with post-ischemic hemorrhage in rat brain. Exp Neurol. 2009;216:407–412. doi: 10.1016/j.expneurol.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin B, Ginsberg MD. Quantitative assessment of the normal cerebral microvasculature by endothelial barrier antigen (EBA) immunohistochemistry: application to focal cerebral ischemia. Brain Res. 2000;865:237–244. doi: 10.1016/s0006-8993(00)02228-9. [DOI] [PubMed] [Google Scholar]
- 18.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabín J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 19.Corot C, Perrin JM, Belleville J, Amiel M, Eloy R. Safety and efficacy of iopromide in cerebral arteriography. Invest Radiol. 1994;29:S94–S97. doi: 10.1097/00004424-199405001-00018. [DOI] [PubMed] [Google Scholar]
- 20.Spencer CM, Goa KL. Iodixanol. A review of its pharmacodynamic and pharmacokinetic properties and diagnostic use as of an x-ray contrast medium. Drugs. 1996;52:899–927. doi: 10.2165/00003495-199652060-00013. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox J, Wilson AJ, Evill CA, Sage MR. A comparison of blood–brain barrier disruption by intracarotid iohexol and iodixanol in the rabbit. AJNR Am J Neuroradiol. 1987;8:769–772. [PMC free article] [PubMed] [Google Scholar]
- 22.Drake TG, Kerr HD. Status epilepticus after cranial CT with contrast media. WMJ. 1997;96:46–48. [PubMed] [Google Scholar]
- 23.Avrahami E, Weiss-Peretz J, Cohn DE. Focal epileptic activity following intravenous contrast material injection in patients with metastatic brain disease. J Neurol Neurosurg Psychiatry. 1987;50:221–223. doi: 10.1136/jnnp.50.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sage MR. Blood–brain barrier: phenomenon of increasing importance to the imaging clinician. AJNR Am J Neuroradiol. 1982;138:887–898. doi: 10.2214/ajr.138.5.887. [DOI] [PubMed] [Google Scholar]
- 25.Doerfler A, Engelhorn T, von Kummer R, Weber J, Knauth M, Heiland S, Sartor K, Forsting M. Are iodinated contrast agents detrimental in acute cerebral ischemia? An experimental study in rats. Radiology. 1998;206:211–217. doi: 10.1148/radiology.206.1.9423675. [DOI] [PubMed] [Google Scholar]
- 26.Brisman JL, Jilani M, McKinney JS. Contrast enhancement hyperdensity after endovascular coiling of intracranial aneurysms. AJNR Am J Neuroradiol. 2008;29:588–593. doi: 10.3174/ajnr.A0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchiyama Y, Abe T, Hirohata M, Tanaka N, Kojima K, Nishimura H, Norbash AM, Hayabuchi N. Blood–brain barrier disruption of nonionic iodinated contrast medium following coil embolization of a ruptured intracerebral aneurysm. AJNR Am J Neuroradiol. 2004;25:1783–1786. [PMC free article] [PubMed] [Google Scholar]
- 28.De Wispelaere JF, Trigaux JP, Van Beers B, Gilliard C. Cortical and CSF hyperdensity after iodinated contrast medium overdose: CT findings. J Comput Assist Tomogr. 1992;16:998–1003. doi: 10.1097/00004728-199211000-00035. [DOI] [PubMed] [Google Scholar]
- 29.Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast Enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35:876–881. doi: 10.1161/01.STR.0000120726.69501.74. [DOI] [PubMed] [Google Scholar]
- 30.Mericle RA, Lopes DK, Fronckowiak MD, Wakhloo AK, Guterman LR, Hopkins LN. A grading scale to predict the outcomes after intraarterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery. 2000;46:1307–1314. doi: 10.1097/00006123-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke. 2001;32:2042–2048. doi: 10.1161/hs0901.095602. [DOI] [PubMed] [Google Scholar]
- 32.Morcos S, Thomsen HS, Exley CM. Contrast media: interactions with other drugs and clinical tests. Eur Radiol. 2005;15:1463–1468. doi: 10.1007/s00330-004-2600-1. [DOI] [PubMed] [Google Scholar]
- 33.Corot C, Perrin JM, Belleville J, Amiel M, Eloy R. Effect of iodinated contrast media on blood clotting. Invest Radiol. 1989;24:390–393. doi: 10.1097/00004424-198905000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ing JJ, Smith DC, Bull BS. Differing mechanisms of clotting inhibition by ionic and nonionic contrast agents. Radiology. 1989;172:345–348. doi: 10.1148/radiology.172.2.2748812. [DOI] [PubMed] [Google Scholar]
- 35.Jones CI, Goodall AH. Differential effects of the iodinated contrast agents ioxaglate, iohexol and iodixanol on thrombus formation and thrombolysis. Thromb Res. 2003;112:65–71. doi: 10.1016/j.thromres.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Kopko PM, Smith DC, Bull BS. Thrombin generation in nonclottable mixtures of blood and nonionic contrast agents. Radiology. 1990;174:459–461. doi: 10.1148/radiology.174.2.2296655. [DOI] [PubMed] [Google Scholar]
- 37.Dawson P, Hewitt P, Mackie IJ, Machin SJ, Amin S, Bradshaw A. Contrast, coagulation, and fibrinolysis. Invest Radiol. 1986;21:248–252. doi: 10.1097/00004424-198603000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Grabowski EF, Rodriguez M, McDonnell SL. Platelet adhesion/aggregation in an in vitro model of coronary artery stenosis. Catheter Cardiovasc Diagn. 1993;28:65–71. doi: 10.1002/ccd.1810280113. [DOI] [PubMed] [Google Scholar]
- 39.Markou CP, Chronos NAF, Hanson SR. Antithrombotic effects of ionic and non-ionic contrast media in nonhuman primates. Thromb Haemost. 2001;85:488–493. [PubMed] [Google Scholar]
- 40.Corot C, Stucker O, Duverger JP, Pons C, Vicaut E. Effect of iodinated contrast agents on the formation of platelet thrombi in the microarterioles of rats. Invest Radiol. 1994;29:S203–S205. doi: 10.1097/00004424-199406001-00067. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Tomasiek R, Fay WP. Do clinically relevant circulating concentrations of radiographic contrast agents inhibit platelet-dependent arterial thrombosis? Thromb Res. 2002;105:413–418. doi: 10.1016/s0049-3848(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 42.Grines CL, Mickelsen JK, Diaz C, DeMaria AN. Acute thrombosis in a canine model of arterial injury: effect of ionic versus non-ionic contrast media. J Invasive Cardiol. 1991;3(suppl):18B–23B. [Google Scholar]
- 43.Hwang MH, Piao ZE, Murdock DK, Giardina JJ, Pacold I, Loeb HS, Reyes CV, Scanlon PJ. Potential risk of thrombosis during coronary angiography. Radiology. 1990;174:209–213. doi: 10.1002/ccd.1810160318. [DOI] [PubMed] [Google Scholar]
- 44.Pislaru S, Pislaru C, Szilard M, Arnout J, Van de Werf F. In vivo effects of contrast media on coronary thrombolysis. J Am Coll Cardiol. 1998;32:1102–1110. doi: 10.1016/s0735-1097(98)00326-x. [DOI] [PubMed] [Google Scholar]
- 45.Esplugas E, Cequier A, Jara F, Mauri J, Soler T, Sala J, Sabate X. Risk of thrombosis during coronary angioplasty with low osmolarity contrast media. Am J Cardiol. 1991;68:1020–1024. doi: 10.1016/0002-9149(91)90489-8. [DOI] [PubMed] [Google Scholar]
- 46.Tang J, Li YJ, Li Q, Mu J, Yang DY, Xie P. Endogenous tissue plasminogen activator increases hemorrhagic transformation induced by heparin after ischemia reperfusion in rat brains. Neurol Res. 2009 Mar 23; doi: 10.1179/174313209X414560. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Guidance for Industry. [Accessed on December 26, 2009.];Estimating safe starting doses in initial clinical trials for therapeutics in adult healthy volunteers. Available at: www.fda.gov.cder/guidance/index.htm.
- 48.Treitl M, Rupprecht H, Wirth S, Korner M, Reiser M, Rieger J. Assessment of renal vasoconstriction in vivo after intra-arterial administration of the isosmotic contrast medium iodixanol compared to the low-osmotic contrast medium iopamidol. Nephrol Dial Transplant. 2009;24:1478–1485. doi: 10.1093/ndt/gfn638. [DOI] [PubMed] [Google Scholar]
- 49.Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill MD CASES Investigators. Asymptomatic hemorrhage after thrombolysis may not be benign. Prognosis by hemorrhage type in the Canadian Alteplase for Stroke Effectiveness Registry. Stroke. 2007;38:75–79. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]


