Abstract
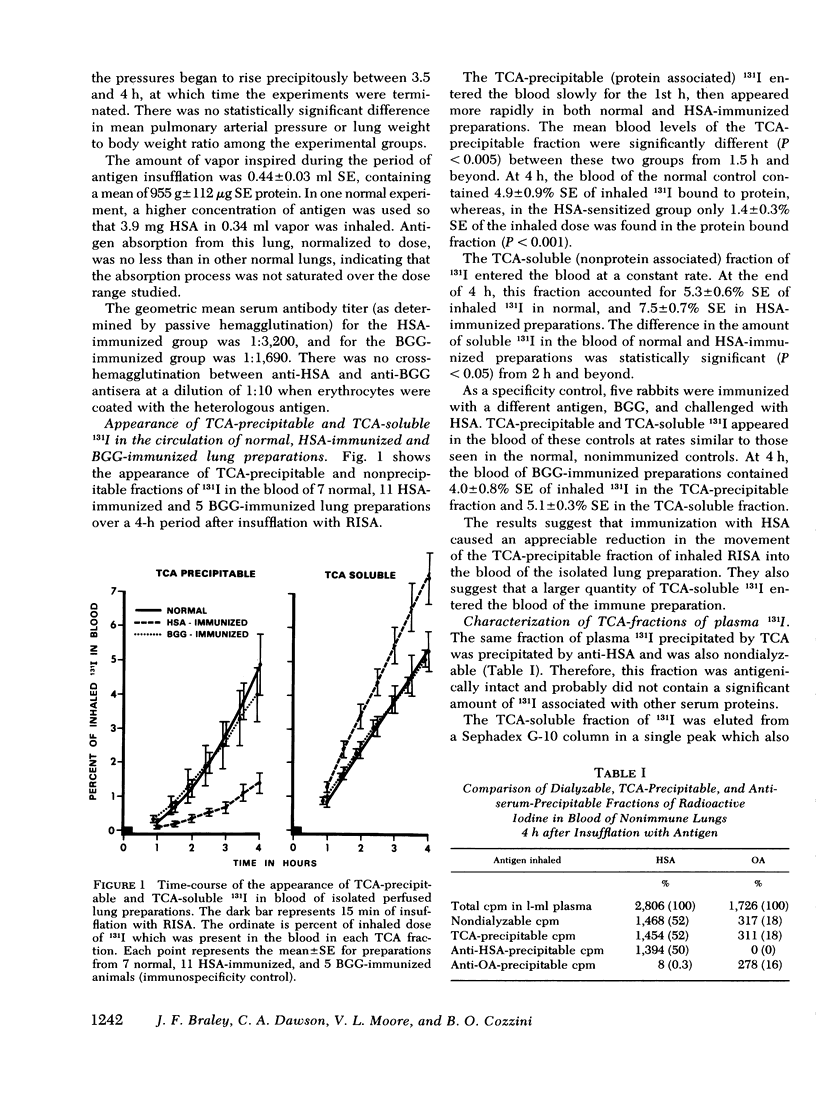
The purpose of this study was to determine whether the absorption of inhaled antigen (Ag) across the pulmonary air-blood barrier of the isolated perfused lung can be modulated by immunologic mechanisms. Lungs from immunized or nonimmunized rabbits were removed, ventilated, and perfused with autochthonous blood. Radioiodinated Ag (human serum albumin or ovalbumin) was introduced as an aerosol into the isolated lung for 15 min and blood samples were taken over a 4-h period. The results showed that radioactivity fom inhaled Ag entered the perfusing blood as two fractions. One fraction was precipitable by 5% trichloroacetic acid or antiserum. The TCA-soluble fraction chromatographed differently from iodide and may have represented metabolites of the Ag. Immunization specifically reduced the amount of antigenically intact protein entering the blood. On the other hand, the metabolite reached higher concentrations in the blood of immunized lungs. We conclude that the alveolar capillary barrier of the normal rabbit lung could provide a significant route of entry for inhaled antigen into the systemic circulation and that immunization reduces absorption via this route and enhances pulmonary metabolism of the Ag.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale W. F., Helmkamp R. W., Davis T. P., Izzo M. J., Goodland R. L., Contreras M. A., Spar I. L. High specific activity labeling of protein with I-131 by the iodine monochloride method. Proc Soc Exp Biol Med. 1966 Jun;122(2):407–414. doi: 10.3181/00379727-122-31148. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Bensch K. G., Dominguez E. A. Studies on the pulmonary air-tissue barrier. IV. Cytochemical tracing of macromolecules during absorption. Yale J Biol Med. 1971 Feb-Apr;43(4-5):236–241. [PMC free article] [PubMed] [Google Scholar]
- Bensch K. G., Dominguez E., Liebow A. A. Absorption of intact protein molecules across the pulmonary air-tissue barrier. Science. 1967 Sep 8;157(3793):1204–1206. doi: 10.1126/science.157.3793.1204. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973 Jun;28(6):693–698. [PubMed] [Google Scholar]
- Dominguez E. A., Liebow A. A., Bensch K. G. Studies on the pulmonary air-tissue barrier. I. Absorption of albumin by the alveolar wall. Lab Invest. 1967 Jun;16(6):905–911. [PubMed] [Google Scholar]
- Kaltreider H. B. Expression of immune mechanisms in the lung. Am Rev Respir Dis. 1976 Mar;113(3):347–379. doi: 10.1164/arrd.1976.113.3.347. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Durbin W. A., Reynolds H. Y. Kinetics of immunoglobulin transport into canine bronchial secretions. Proc Soc Exp Biol Med. 1976 Sep;152(4):493–498. doi: 10.3181/00379727-152-39425. [DOI] [PubMed] [Google Scholar]
- Mercer T. T. Production and characterization of aerosols. Arch Intern Med. 1973 Jan;131(1):39–50. [PubMed] [Google Scholar]
- Morrow P. E. Lymphatic drainage of the lung in dust clearance. Ann N Y Acad Sci. 1972 Dec 29;200:46–65. doi: 10.1111/j.1749-6632.1972.tb40177.x. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Niemeier R. W., Bingham E. An isolated perfused lung preparation for metabolic studies. Life Sci II. 1972 Aug 22;11(16):807–820. doi: 10.1016/0024-3205(72)90129-4. [DOI] [PubMed] [Google Scholar]
- Richardson J., Bouchard T., Ferguson C. C. Uptake and transport of exogenous proteins by respiratory epithelium. Lab Invest. 1976 Oct;35(4):307–314. [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- SCHULTZ A. L., GRISMER J. T., GRANDE F. Absorption of radioactive albumin from the lungs of normal dogs. J Lab Clin Med. 1963 Mar;61:494–500. [PubMed] [Google Scholar]
- SCHULTZ A. L., GRISMER J. T., WADA S., GRANDE F. ABSORPTION OF ALBUMIN FROM ALVEOLI OF PERFUSED DOG LUNG. Am J Physiol. 1964 Dec;207:1300–1304. doi: 10.1152/ajplegacy.1964.207.6.1300. [DOI] [PubMed] [Google Scholar]
- Stokes C. R., Soothill J. F., Turner M. W. Immune exclusion is a function of IgA. Nature. 1975 Jun 26;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Wu M., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules. III. Studies on the mechanism by which immunization interferes with antigen uptake. J Immunol. 1975 Sep;115(3):854–861. [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Isselbacher K. J. Protein uptake by the intestine: evidence for absorption of intact macromolecules. Gastroenterology. 1974 May;66(5):987–992. [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Aglycosylantibody. Effects of exoglycosidase treatments on autochthonous antibody survival time in the circulation. J Biol Chem. 1976 Feb 25;251(4):1074–1080. [PubMed] [Google Scholar]


