Abstract
Neurological abnormalities are a major cause of morbidity in patients with renal failure. The pathophysiology of these neurological changes is unclear, and the effects on them of dialysis and return of renal function have not been well studied. Studies were done in 31 patients who had acute renal failure (ARF), all of whom were either treated with dialysis within 5 days or did not survive. Studies on these patients included the electroencephalogram (EEG), motor nerve conduction velocity, and plasma Ca++ and parathyroid hormone (PTH) levels. Studies were done at the time ARF was diagnosed, after stabilization on dialysis, during the diuretic phase of ARF, and 3 mo after recovery from ARF. In 16 patients with acute or chronic renal failure who did not survive and in nine patients without renal disease who died, measurements were made in brain of content of Na+, K+, Cl−, Ca++, Mg++, and water.
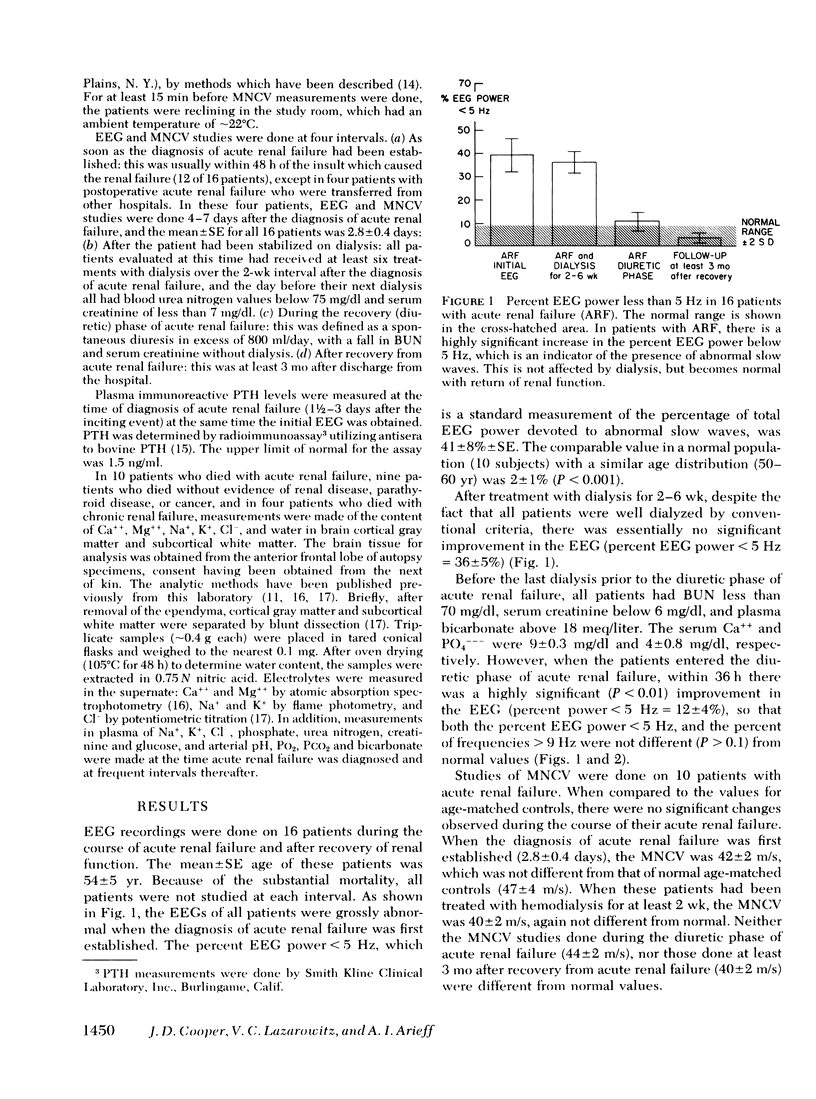
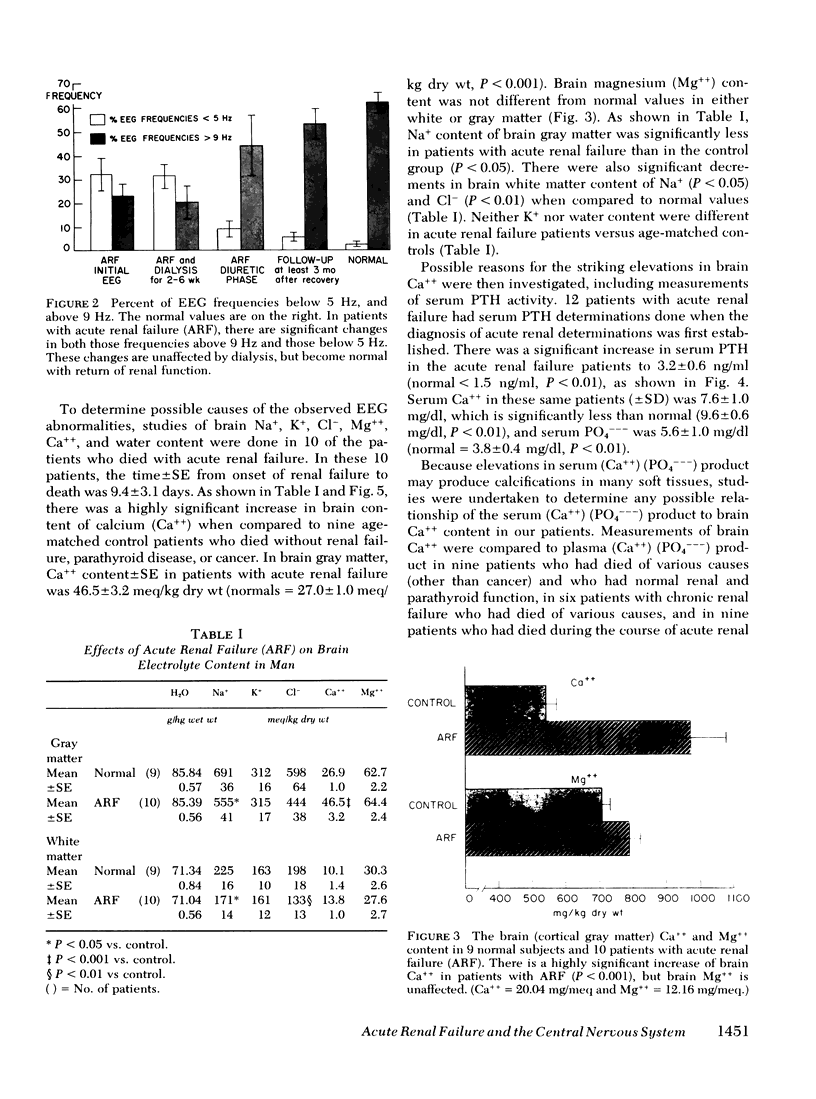
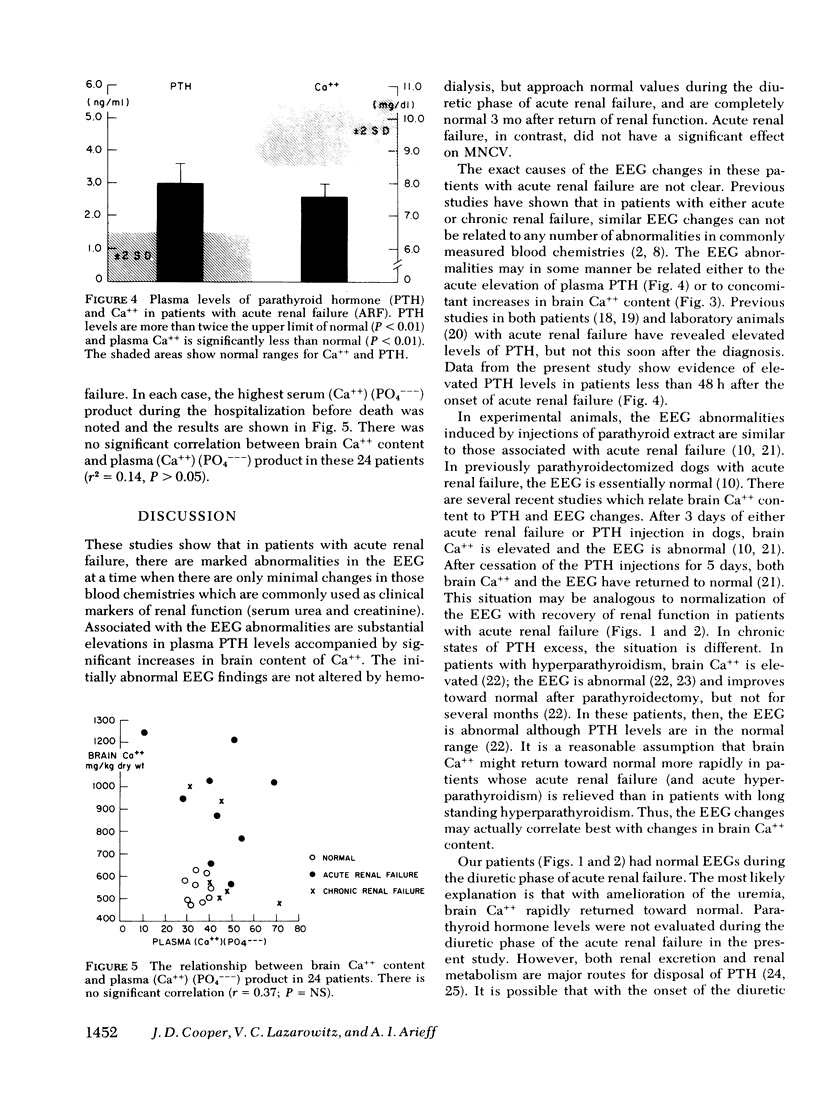
In patients with ARF for less than 48 h, despite the fact that there were only modest increases in plasma urea and creatinine, there were striking abnormalities in the EEG. The percent EEG power < 5 Hz±SE was 41±8% (normal = 2±1%), whereas the percent of frequencies > 9 Hz was only 22±6% (normal = 62±3%). These changes were unaffected by dialysis, but became normal with return of renal function and remained normal at 3 mo follow-up. The motor nerve conduction velocity was unaffected by either ARF or dialysis. In patients with ARF, the brain Ca++ was 46.5±3.2 meq/kg dry wt, almost twice the normal value of 26.9±1.0 meq/kg dry wt (P < 0.001). The plasma PTH level was 3.2±0.6 ng/ml (normal < 1.5 ng/ml, P < 0.01). The increased brain Ca++ was not related to an increased plasma (Ca++) (PO4−−−) product (r2 = 0.14, P > 0.05). There was a small but significant decrement in brain Na+ (P < 0.05), but brain water, K+, and Mg++ were unaffected by ARF.
Thus, in patients with ARF for less than 48 h, the EEG is grossly abnormal and there are elevated levels of PTH in plasma. The PTH appears to have a direct effect on the brain, resulting in an increased brain Ca++ content. The EEG abnormalities are unaffected by dialysis, but they become normal with return of renal function and remain normal after 3 mo follow-up. Thus, PTH may be a major uremic toxin, demonstrating evidence for central nervous system toxicity when there are only minimal abnormalities of other biochemical markers of ARF.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey A. C., Mishell J. M., Burks J., Contiguglia S. R., Rudolph H., Lewin E., Holmes J. H. Syndrome of dyspraxia and multifocal seizures associated with chronic hemodialysis. Trans Am Soc Artif Intern Organs. 1972;18(0):257-61, 266-7. doi: 10.1097/00002480-197201000-00064. [DOI] [PubMed] [Google Scholar]
- Allen E. M., Singer F. R., Melamed D. Electroencephalographic abnormalities in hypercalcemia. Neurology. 1970 Jan;20(1):15–22. doi: 10.1212/wnl.20.1.15. [DOI] [PubMed] [Google Scholar]
- Arieff A. I., Massry S. G., Barrientos A., Kleeman C. R. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973 Sep;4(3):177–187. doi: 10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- Arieff A. I., Massry S. G. Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Invest. 1974 Feb;53(2):387–392. doi: 10.1172/JCI107571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow J. W., Fine B. S., Zimmerman L. E. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968 Nov;66(5):812–824. doi: 10.1016/0002-9394(68)92795-5. [DOI] [PubMed] [Google Scholar]
- Bernstein D. S., Pletka P., Hattner R. S., Hampers C. L., Merrill J. P. Effect of total parathyroidectomy and uremia on the chemical composition of bone, skin and aorta in the rat. Isr J Med Sci. 1971 Mar;7(3):513–514. [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in cell cultures. IV. Effects of phosphate and parathyroid hormone in kidney cells. Endocrinology. 1970 Jun;86(6):1389–1393. doi: 10.1210/endo-86-6-1389. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Leeman C. R., Bagdoyanh, Berberian A. The calcium and magnesium content of skeletal muscle, brain, and cerebrospinal fluid as determined by atomic bsorption flame photometry. J Lab Clin Med. 1968 May;71(5):884–892. [PubMed] [Google Scholar]
- Conger J. D., Hammond W. S., Alfrey A. C., Contiguglia S. R., Stanford R. E., Huffer W. E. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med. 1975 Sep;83(3):330–336. doi: 10.7326/0003-4819-83-3-330. [DOI] [PubMed] [Google Scholar]
- Dayan A. D., Gardner-Thorpe C., Down P. F., Gleadle R. I. Peripheral neuropathy in uremia. Pathological studies on peripheral nerves from 6 patients. Neurology. 1970 Jul;20(7):649–658. doi: 10.1212/wnl.20.7.649. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Johnson W. J., Lambert E. H., O'Brien P. C. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo Clin Proc. 1971 Jun;46(6):400–431. [PubMed] [Google Scholar]
- Guisado R., Arieff A. I., Massry S. G., Lazarowitz V., Kerian A. Changes in the electroencephalogram in acute uremia. Effects of parathyroid hormone and brain electrolytes. J Clin Invest. 1975 Apr;55(4):738–745. doi: 10.1172/JCI107984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstam K. E. EEG frequency content related to chemical blood parameters in chronic uraemia. Scand J Urol Nephrol. 1971;7:1–56. [PubMed] [Google Scholar]
- Hruska K. A., Martin K., Mennes P., Greenwalt A., Anderson C., Klahr S., Slatopolsky E. Degradation of parathyroid hormone and fragment production by the isolated perfused dog kidney. The effect of glomerular filtration rate and perfusate CA++ concentrations. J Clin Invest. 1977 Sep;60(3):501–510. doi: 10.1172/JCI108802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB J. C., GLOOR P., ELWAN O. H., DOSSETOR J. B., PATERS V. R. ELECTROENCEPHALOGRAPHIC CHANGES IN CHRONIC RENAL FAILURE. Neurology. 1965 May;15:419–429. doi: 10.1212/wnl.15.5.419. [DOI] [PubMed] [Google Scholar]
- Jastak J. T., Morrison A. B., Raisz L. G. Effects of renal insufficiency on the parathyroid gland and calcium homeostasis. Am J Physiol. 1968 Jul;215(1):84–89. doi: 10.1152/ajplegacy.1968.215.1.84. [DOI] [PubMed] [Google Scholar]
- KILEY J., HINES O. ELECTROENCEPHALOGRAPHIC EVALUATION OF UREMIA. WAVE FREQUENCY EVALUATIONS ON 40 UREMIC PATIENTS. Arch Intern Med. 1965 Jul;116:67–73. doi: 10.1001/archinte.1965.03870010069009. [DOI] [PubMed] [Google Scholar]
- Kiley J. E., Woodruff M. W., Pratt K. I. Evaluation of encephalopathy by EEG frequency analysis in chronic dialysis patients. Clin Nephrol. 1976 Jun;5(6):245–250. [PubMed] [Google Scholar]
- Kovithavongs T., Becker F. O., Ing T. S. Parathyroid hyperfunction in acute renal failure. Serial studies in man. Nephron. 1972;9(6):349–355. doi: 10.1159/000180168. [DOI] [PubMed] [Google Scholar]
- LAIDLAW J. The application in general medical conditions of a visual method of assessing and representing generalized electroencephalographic abnormalities. J Neurol Neurosurg Psychiatry. 1959 Feb;22(1):69–76. doi: 10.1136/jnnp.22.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindall A. W., Wong E. T. Column chromatography of human serum parathyroid immunoreactive peptides. Proc Soc Exp Biol Med. 1975 Mar;148(3):799–804. doi: 10.3181/00379727-148-38635. [DOI] [PubMed] [Google Scholar]
- Mallick N. P., Berlyne G. M. Arterial calcification after vitamin-D therapy in hyperphosphatemic renal failure. Lancet. 1968 Dec 21;2(7582):1316–1320. doi: 10.1016/s0140-6736(68)91816-3. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Hruska K. A., Lewis J., Anderson C., Slatopolsky E. The renal handling of parathyroid hormone. Role of peritubular uptake and glomerular filtration. J Clin Invest. 1977 Oct;60(4):808–814. doi: 10.1172/JCI108834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massry S. G., Arieff A. I., Coburn J. W., Palmieri G., Kleeman C. R. Divalent ion metabolism in patients with acute renal failure: studies on the mechanism of hypocalcemia. Kidney Int. 1974 Jun;5(6):437–445. doi: 10.1038/ki.1974.62. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Stein R., Garty J., Arieff A. I., Coburn J. W., Norman A. W., Friedler R. M. Skeletal resistance to the calcemic action of parathyroid hormone in uremia: role of 1,25 (OH)2 D3. Kidney Int. 1976 Jun;9(6):467–474. doi: 10.1038/ki.1976.60. [DOI] [PubMed] [Google Scholar]
- Nielsen V. K. The peripheral nerve function in chronic renal failure. VI. The relationship betweeen sensory and motor nerve conduction and kidney function, azotemia, age, sex, and clinical neuropathy. Acta Med Scand. 1973 Nov;194(5):455–462. [PubMed] [Google Scholar]
- Raskin N. H., Fishman R. A. Neurologic disorders in renal failure (first of two parts). N Engl J Med. 1976 Jan 15;294(3):143–148. doi: 10.1056/NEJM197601152940306. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Caglar S., Pennell J. P., Taggart D. D., Canterbury J. M., Reiss E., Bricker N. S. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971 Mar;50(3):492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman D. S., Alfrey A. C., Hammond W. S., Donndelinger T., Ogden D. A., Holmes J. H. Cardiac calcification in uremia. A clinical, biochemical and pathologic study. Am J Med. 1971 Jun;50(6):744–755. doi: 10.1016/0002-9343(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. K., Hollinrake K., Lascelles R. G., O'Sullivan D. J., Baillod R. A., Moorhead J. F., Mackenzie J. C. The polyneuropathy of chronic renal failure. Brain. 1971;94(4):761–780. doi: 10.1093/brain/94.4.761. [DOI] [PubMed] [Google Scholar]
- Tyler H. R. Neurologic disorders in renal failure. Am J Med. 1968 May;44(5):734–748. doi: 10.1016/0002-9343(68)90255-6. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M., DICKERSON J. W. The effect of growth and function on the chemical composition of soft tissues. Biochem J. 1960 Oct;77:30–43. doi: 10.1042/bj0770030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Calcium-binding phosphoprotein from pig brain: identification as a calcium-dependent regulator of brain cyclic nucleotide phosphodiesterase. Arch Biochem Biophys. 1974 Jul;163(1):349–358. doi: 10.1016/0003-9861(74)90486-x. [DOI] [PubMed] [Google Scholar]


