Abstract
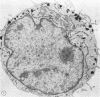
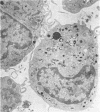
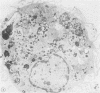
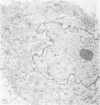
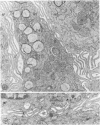
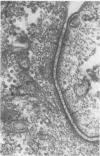
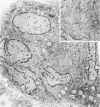
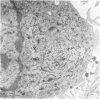
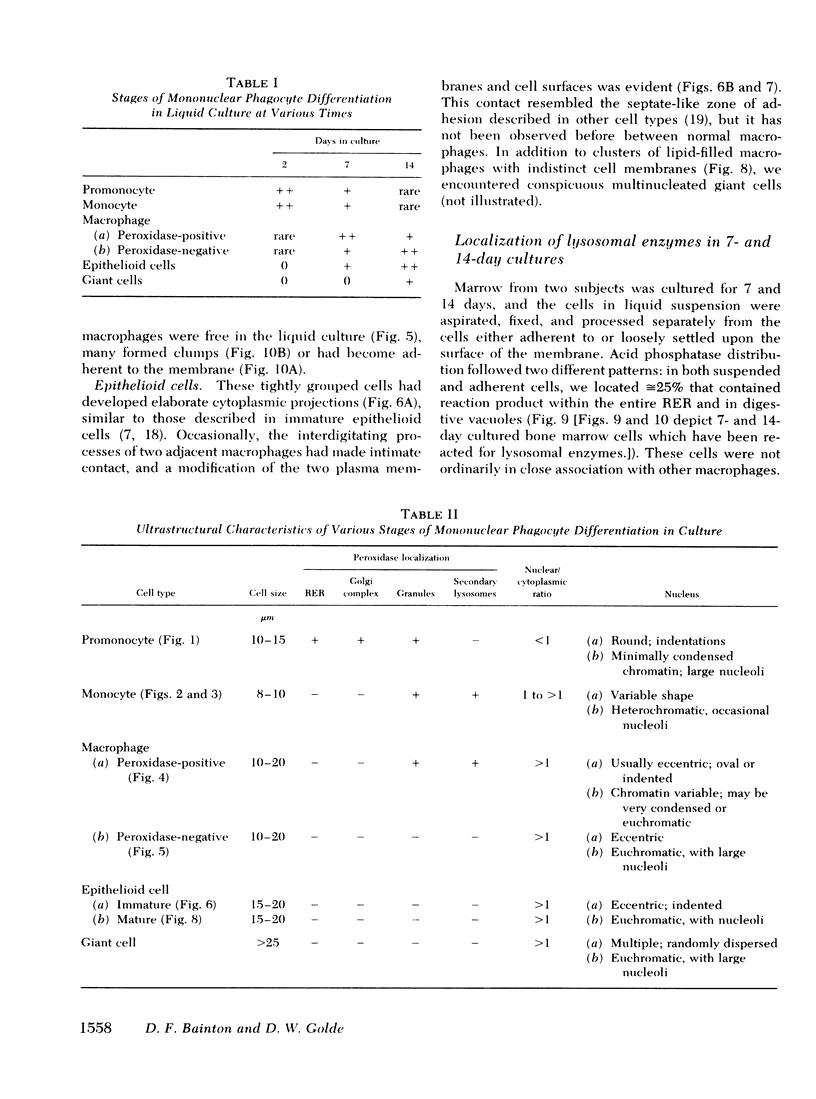
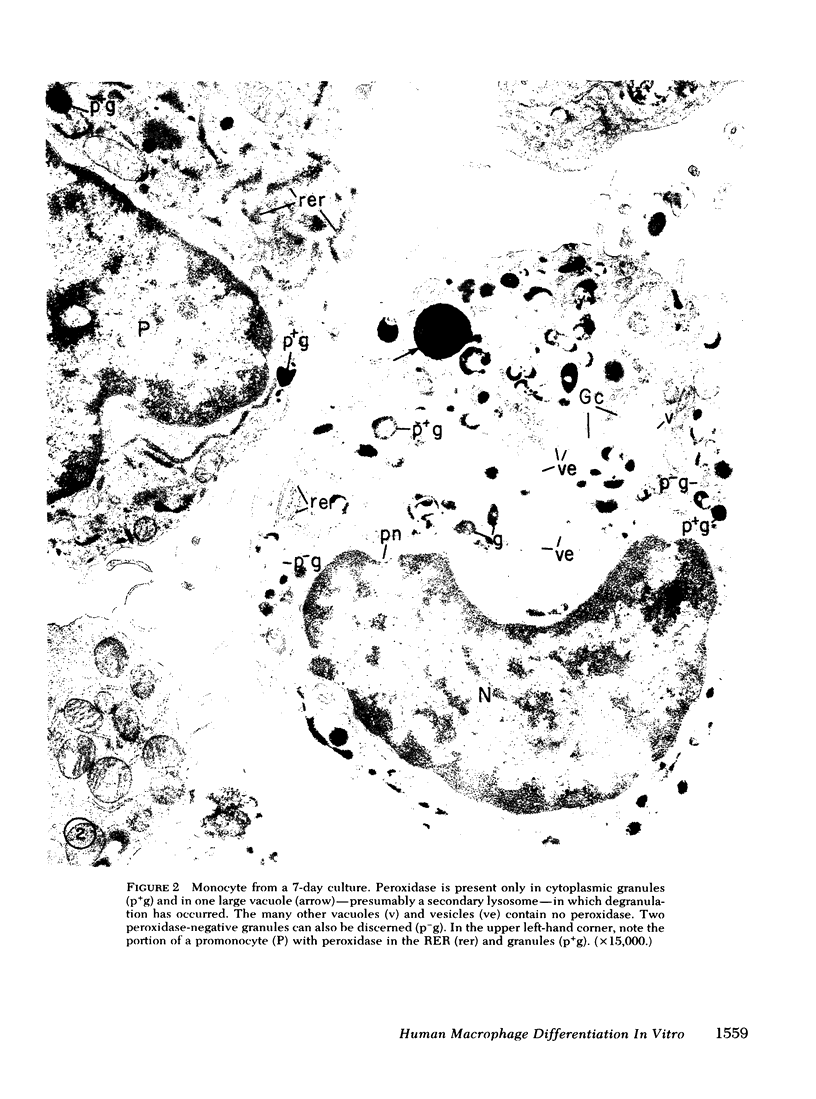
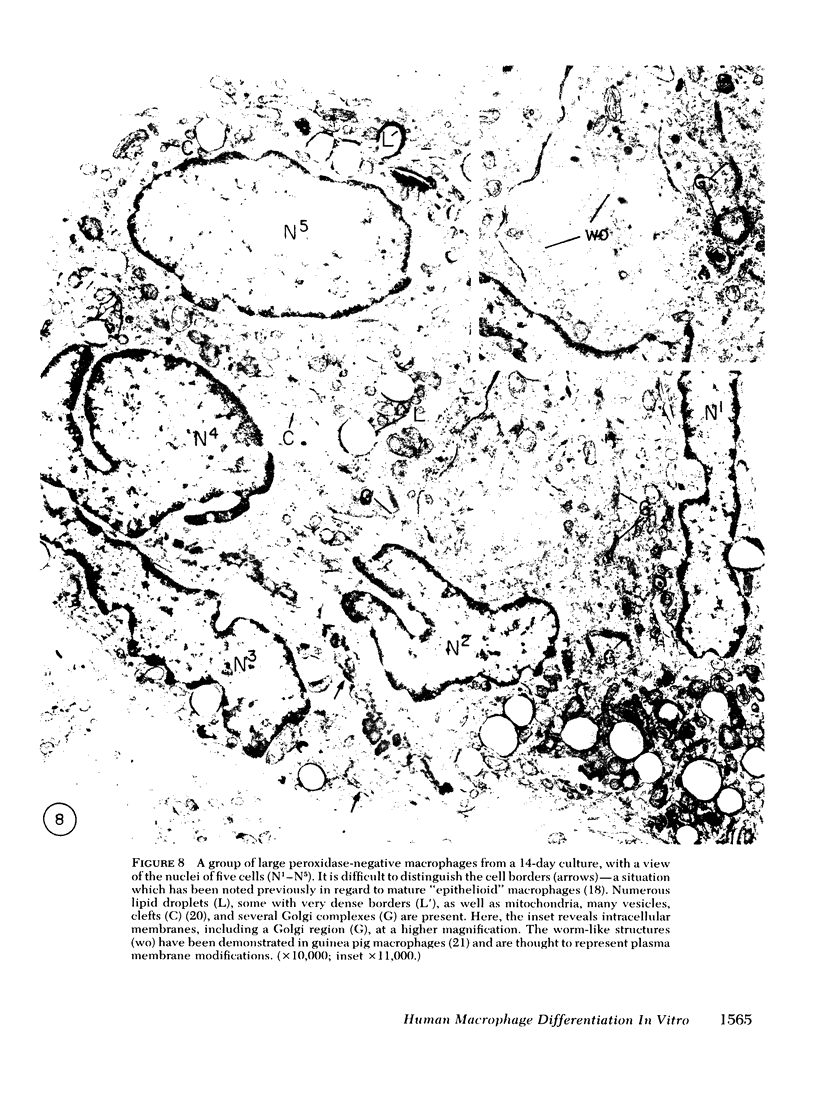
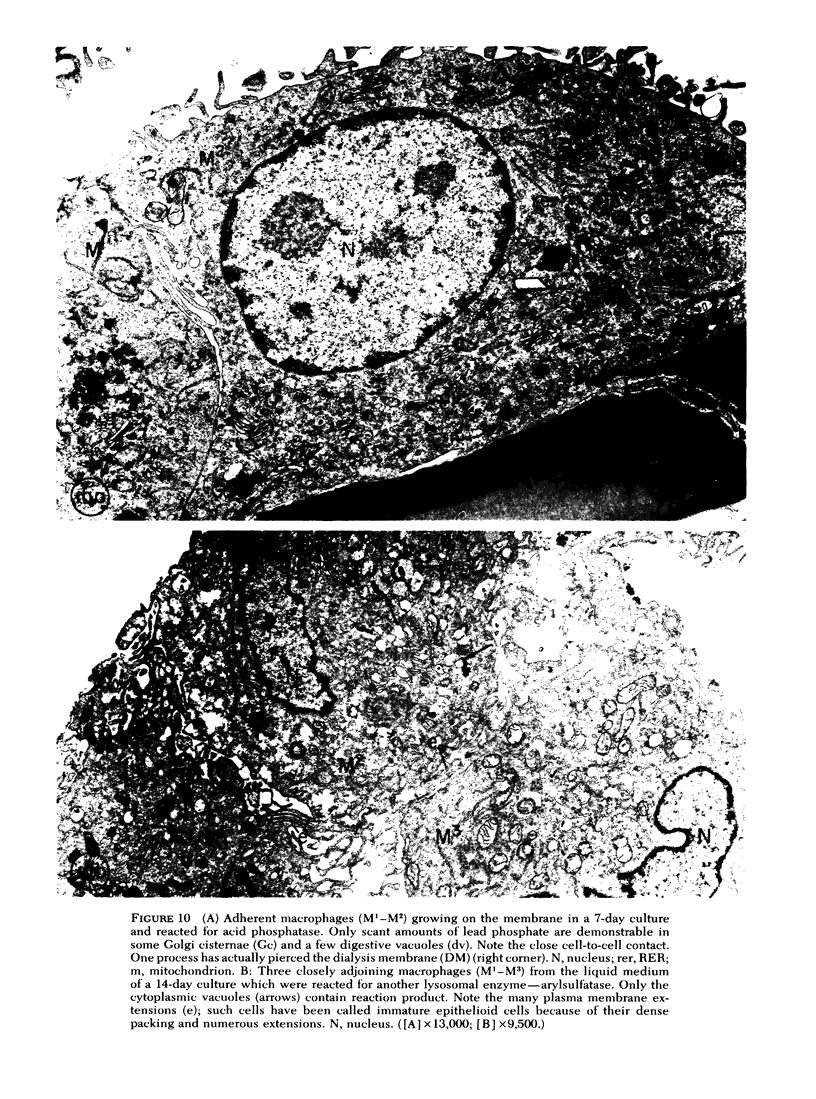
To study the various stages of human mononuclear phagocyte maturation, we cultivated bone marrow in an in vitro diffusion chamber with the cells growing in suspension and upon a dialysis membrane. At 2, 7, and 14 days, the cultured cells were examined by electron microscopy and cytochemical techniques for peroxidase and for more limited analysis of acid phosphatase and arylsulfatase. Peroxidase was being synthesized in promonocytes of 2- and 7-day cultures, as evidenced by reaction product in the rough-surfaced endoplasmic reticulum, Golgi complex, and storage granules. Peroxidase synthesis had ceased in monocytes and the enzyme appeared only in some granules. By 7 days, large macrophages predominated, containing numerous peroxidase-positive storage granules, and heterophagy of dying cells was evident. By 14 days, the most prevalent cell type was the large peroxidase-negative macrophage. Thus, peroxidase is present in high concentrations in immature cells but absent at later stages, presumably a result of degranulation of peroxidase-positive storage granules. Clusters of peroxidase-negative macrophages with indistinct borders (epithelioid cells), as well as obvious multinucleated giant cells, were noted. Frequently, the interdigitating plasma membranes of neighboring macrophages showed a modification resembling a septate junction--to our knowledge, representing the first documentation of this specialized cell contact between normal macrophages. We suggest that such junctions may serve as zones of adhesion between epithelioid cells.
Full text
PDF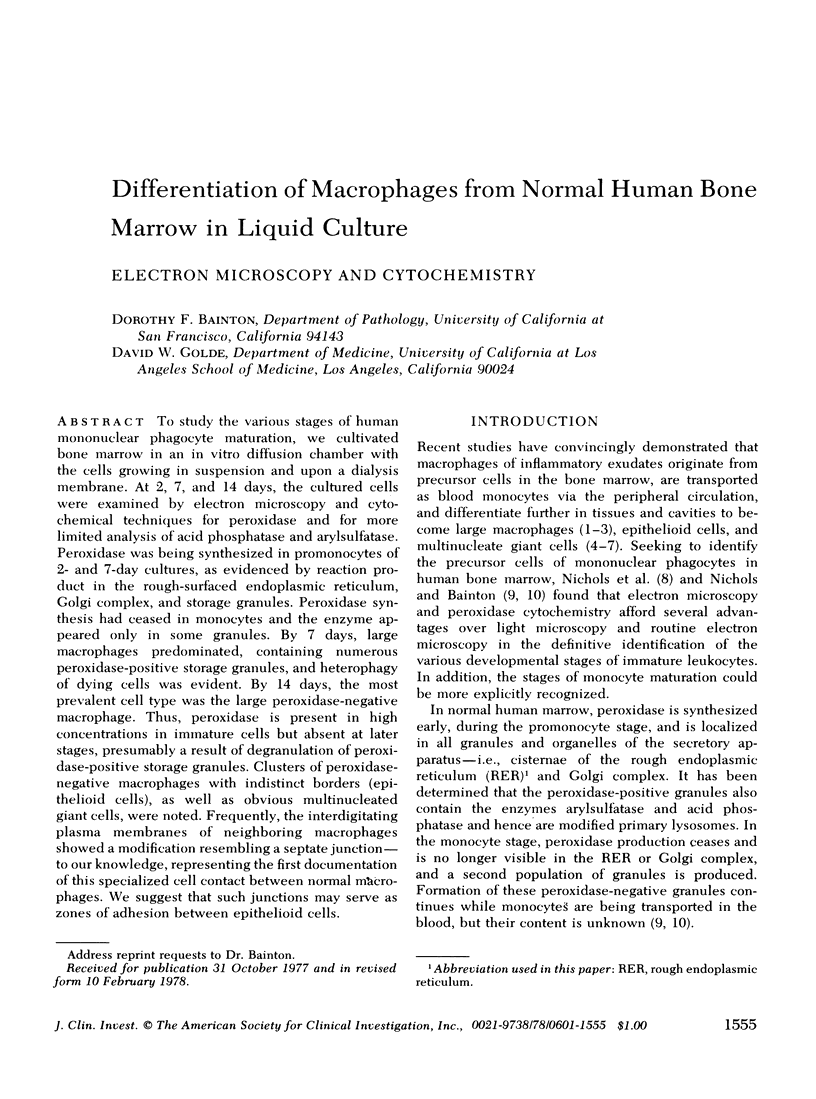





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med. 1977 Feb 1;145(2):264–274. doi: 10.1084/jem.145.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brederoo P., Daems W. T. Cell coat, worm-like structures, and labyrinths in guinea pig resident and exudate peritoneal macrophages, as demonstrated by an abbreviated fixation procedure for electron microscopy. Z Zellforsch Mikrosk Anat. 1972;126(1):135–156. doi: 10.1007/BF00306785. [DOI] [PubMed] [Google Scholar]
- Brenton-Gorius J., Flandrin G., Daniel M. T., Chevalier J., Lebeau M., Sanel F. T. Septate-like junctions in abnormal erythroblasts: cytochemical, ultrastructural and freeze-etch studies. Virchows Arch B Cell Pathol. 1975 Jul 18;18(3):165–180. doi: 10.1007/BF02889245. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Litt M. Ultrastructural localization of horseradish peroxidase and endogenous peroxidase activity in guinea pig peritoneal macrophages. J Immunol. 1970 Dec;105(6):1536–1546. [PubMed] [Google Scholar]
- Daems W. T., Brederoo P. Electron microscopical studies on the structure, phagocytic properties, and peroxidatic activity of resident and exudate peritoneal macrophages in the guinea pig. Z Zellforsch Mikrosk Anat. 1973 Nov 5;144(2):247–297. doi: 10.1007/BF00307305. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Epstein W. L. Ultrastructural observations on experimentally induced foreign-body and organized epithelioid-cell granulomas in man. Am J Pathol. 1968 Jun;52(6):1207–1223. [PMC free article] [PubMed] [Google Scholar]
- Fahimi H. D. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970 Oct;47(1):247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. A distinctive cell contact in the rat adrenal cortex. J Cell Biol. 1972 Apr;53(1):148–163. doi: 10.1083/jcb.53.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Growth of human bone marrow in liquid culture. Blood. 1973 Jan;41(1):45–57. [PubMed] [Google Scholar]
- Gordon S., Cohn Z. A. The macrophage. Int Rev Cytol. 1973;36:171–214. doi: 10.1016/s0074-7696(08)60218-1. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Dominitz R. Cytochemical studies of lysosomes, golgi apparatus and endoplasmic reticulum in secretion and protein uptake by adrenal medulla cells of the rat. J Histochem Cytochem. 1968 May;16(5):320–336. doi: 10.1177/16.5.320. [DOI] [PubMed] [Google Scholar]
- Lepper A. W., Hart P. D. Peroxidase staining in elicited and nonelicited mononuclear peritoneal cells from BCG-sensitized and nonsensitized mice. Infect Immun. 1976 Aug;14(2):522–526. doi: 10.1128/iai.14.2.522-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Weiss R. M., Dirksen E. R., Rosen M. R. Possible communication between murine macrophages oriented in linear chains in tissue culture. Exp Cell Res. 1976 Dec;103(2):375–385. doi: 10.1016/0014-4827(76)90273-1. [DOI] [PubMed] [Google Scholar]
- Liss R. H., Norman J. C. Visualization of doxycycline in lung tissue and sinus secretions by fluorescent techniques. Chemotherapy. 1975;21 (Suppl 1):27–35. doi: 10.1159/000221889. [DOI] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- McIntyre J. A., La Via M. F., Prater T. F., Niblack G. D. Studies of the immune response in vitro. I. Ultrastructural examination of cell types and cluster formation and functional evaluation of clusters. Lab Invest. 1973 Dec;29(6):703–713. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F. Differentiation of human monocytes in bone marrow and blood. Sequential formation of two granule populations. Lab Invest. 1973 Jul;29(1):27–40. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Spector W. G. The origin, properties and fate of epithelioid cells. J Pathol. 1971 Nov;105(3):187–203. doi: 10.1002/path.1711050305. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Ogawa M., Spicer S. S., Wright N. J. Ultrastructure and cytochemistry of bone marrow granulocytes in culture. Exp Hematol. 1976 Mar;4(2):75–89. [PubMed] [Google Scholar]
- Robbins D., Fahimi H. D., Cotran R. S. Fine structural cytochemical localization of peroxidase activity in rat peritoneal cells: mononuclear cells, eosinophils and mast cells. J Histochem Cytochem. 1971 Sep;19(9):571–575. doi: 10.1177/19.9.571. [DOI] [PubMed] [Google Scholar]
- Sanel F., Serpick A. A. Plasmalemmal and subsurface complexes in human leukemic cells: membrane bonding by zipperlike junctions. Science. 1970 Jun 19;168(3938):1458–1460. doi: 10.1126/science.168.3938.1458. [DOI] [PubMed] [Google Scholar]
- Shoham D., David E. B., Rozenszajn L. A. Cytochemical and morphologic identification of macrophages and eosinophils in tissue cultures of normal human bone marrow. Blood. 1974 Aug;44(2):221–233. [PubMed] [Google Scholar]
- Smyth A. C., Weiss L. Electron microscopic study of inhibition of macrophage migration in delayed hypersensitivity. J Immunol. 1970 Dec;105(6):1360–1374. [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice L. W. Effects of hypophysectomy and TSH replacement on the ultrastructural localization of thyroperoxidase. Endocrinology. 1974 Aug;95(2):421–433. doi: 10.1210/endo-95-2-421. [DOI] [PubMed] [Google Scholar]
- Willcox M. B., Golde D. W., Cline M. J. Cytochemical reactions of human hematopoietic cells in liquid culture. J Histochem Cytochem. 1976 Sep;24(9):979–983. doi: 10.1177/24.9.61241. [DOI] [PubMed] [Google Scholar]
- Williams W. J., James E. M., Erasmus D. A., Davies T. The fine structure of sarcoid and tuberculous granulomas. Postgrad Med J. 1970 Aug;46(538):496–500. doi: 10.1136/pgmj.46.538.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G., L'Esperance P. Granulocyte colonies derived from lymphocyte fractions of normal human peripheral blood. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2711–2714. doi: 10.1073/pnas.71.7.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G. Ultrastructural analysis of hematopoietic colonies derived from human peripheral blood. A newly developed method. J Cell Biol. 1974 Dec;63(3):855–863. doi: 10.1083/jcb.63.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Fedorko M. E. Ultrastructure of mouse mononuclear phagocytes in bone marrow colonies grown in vitro. Lab Invest. 1976 Apr;34(4):440–450. [PubMed] [Google Scholar]