Abstract
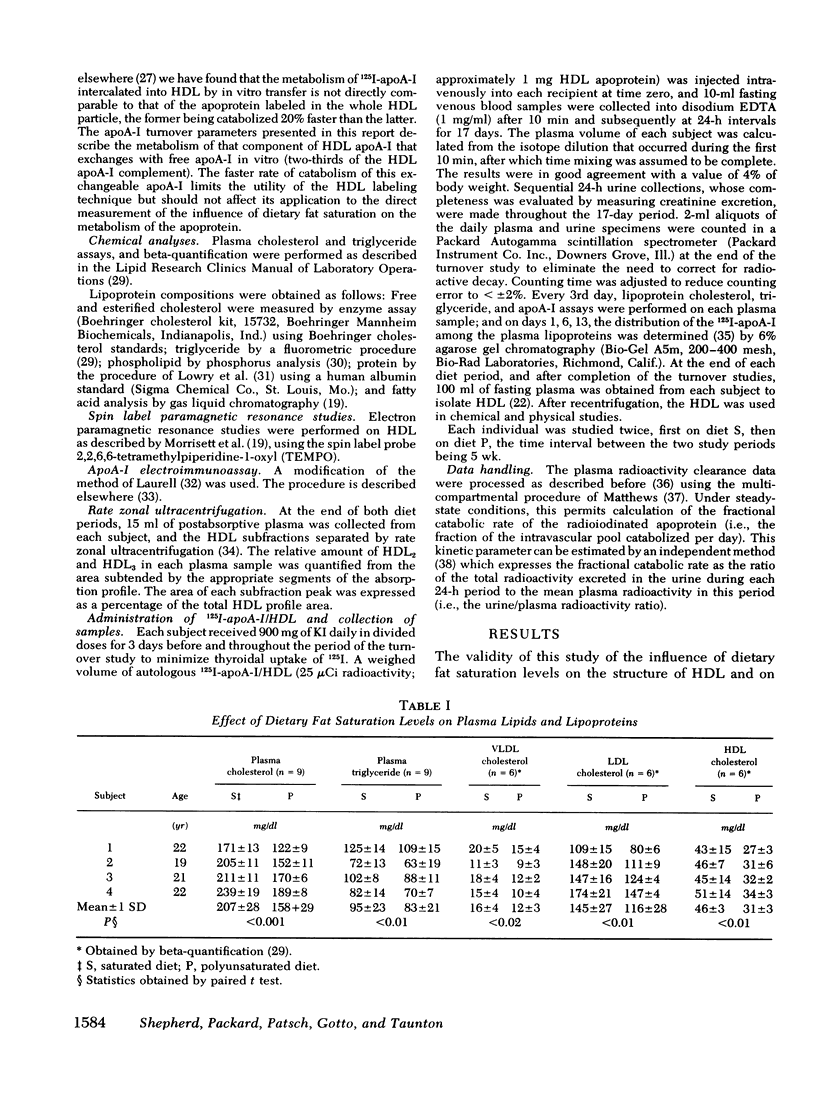
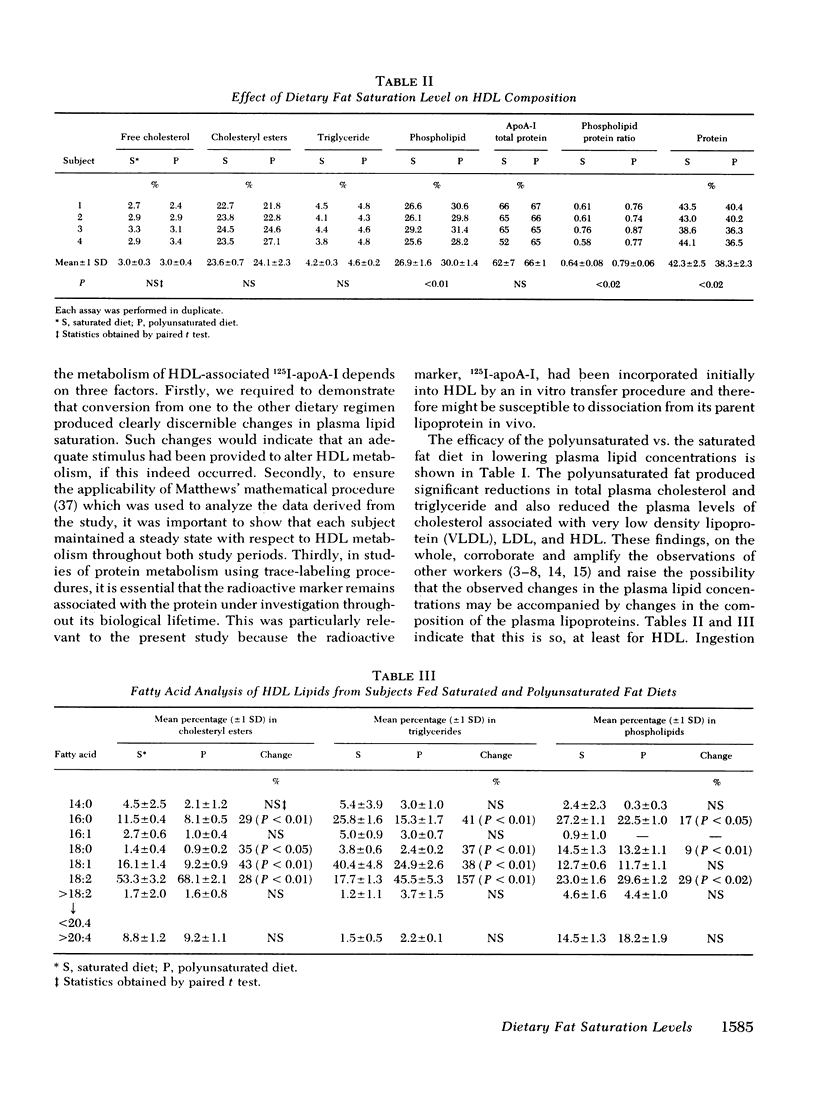
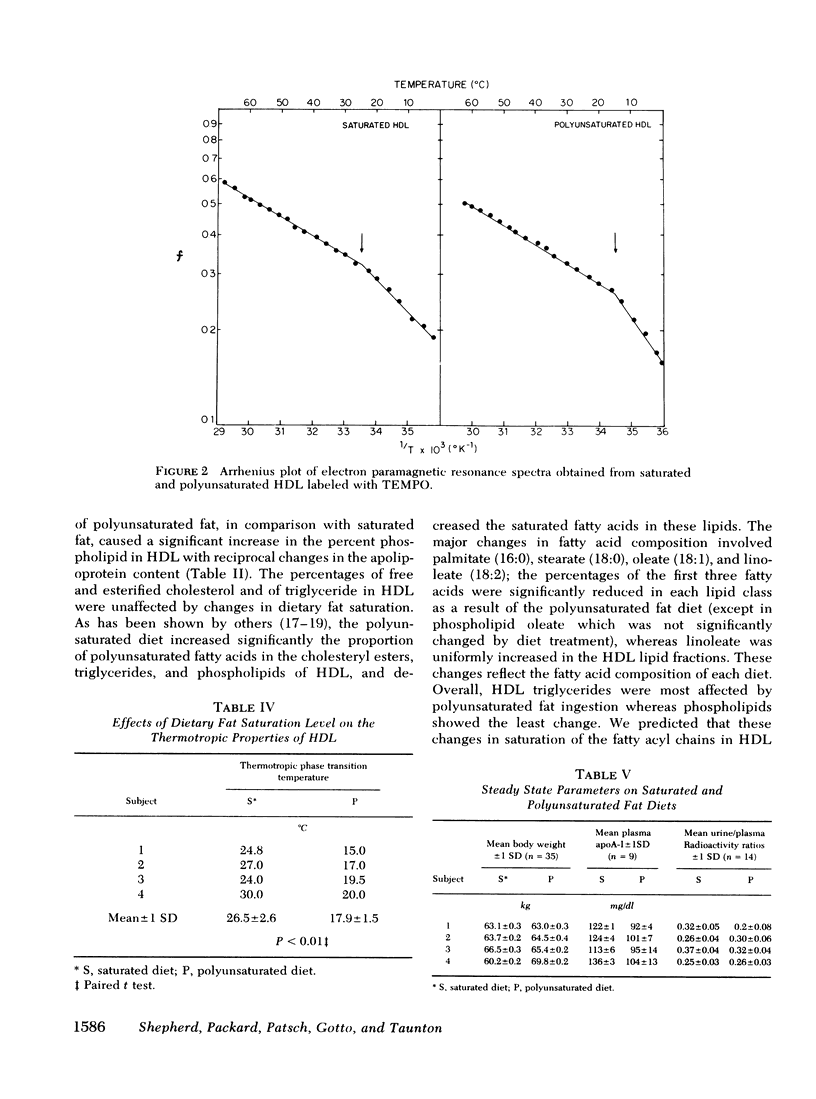
In this study we have investigated, in four normal males the effects of dietary saturated and polyunsaturated fat on the chemical composition and thermotropic properties of human high density lipoproteins (HDL) and have measured the influence of the diets on the metabolism of that fraction of HDL apolipoprotein A-I (apoA-I) that undergoes exchange in vitro and accounts for approximately two-thirds of the lipoprotein's apoA-I complement. When compared with the saturated fat diet, the polyunsaturated diet reduced plasma cholesterol (24%, P < 0.01) by affecting the cholesterol content in the very low density lipoprotein (↓25%, P < 0.02), low density lipoprotein (↓20%, P < 0.01), and high density lipoprotein fractions (↓33%, P < 0.01). Plasma triglyceride was also lowered (by 13%, P < 0.01). Furthermore, polyunsaturated fat ingestion caused a significant fall in the palmitate and stearate content of HDL triglyceride (41 and 37%, respectively), cholesteryl esters (29 and 35%), and phospholipids (17 and 9%) with a concomitant increase in the linoleate content of these moieties (157, 28, and 29%, respectively). The polyunsaturated diet also produced reciprocal changes in the percentage protein (↓9%, P < 0.02) and phospholipid (↓11.5%, P < 0.01) in HDl. These compositional changes were associated with an increase in the microscopic fluidity of the polyunsaturated HDL, although both diets had little effect on the fluidity parameters of HDL at body temperature. Rate zonal ultracentrifugation indicated that the HDL2/HDL3 ratio fell by 28% (P < 0.05) on the polyunsaturated fat diet. In addition to the above, this diet reduced plasma apoA-I by 21% (P < 0.01). No change was seen in the fractional catabolic rate or the distribution of the apoprotein between intravascular and extravascular compartments on the two diets. However, when compared with the saturated diet, the synthetic rate of apoA-I was reduced by 26% during polyunsaturated fat feeding. The results show that polyunsaturated fat alters the chemical composition, thermotropic properties, and subfraction distribution of HDL without changing the fractional rate of catabolism of their major protein, apoA-I.
These findings deserve careful consideration in determining the applicability and efficacy of polyunsaturated fat diet therapy in the prevention of atherosclerosis in man.
Full text
PDF
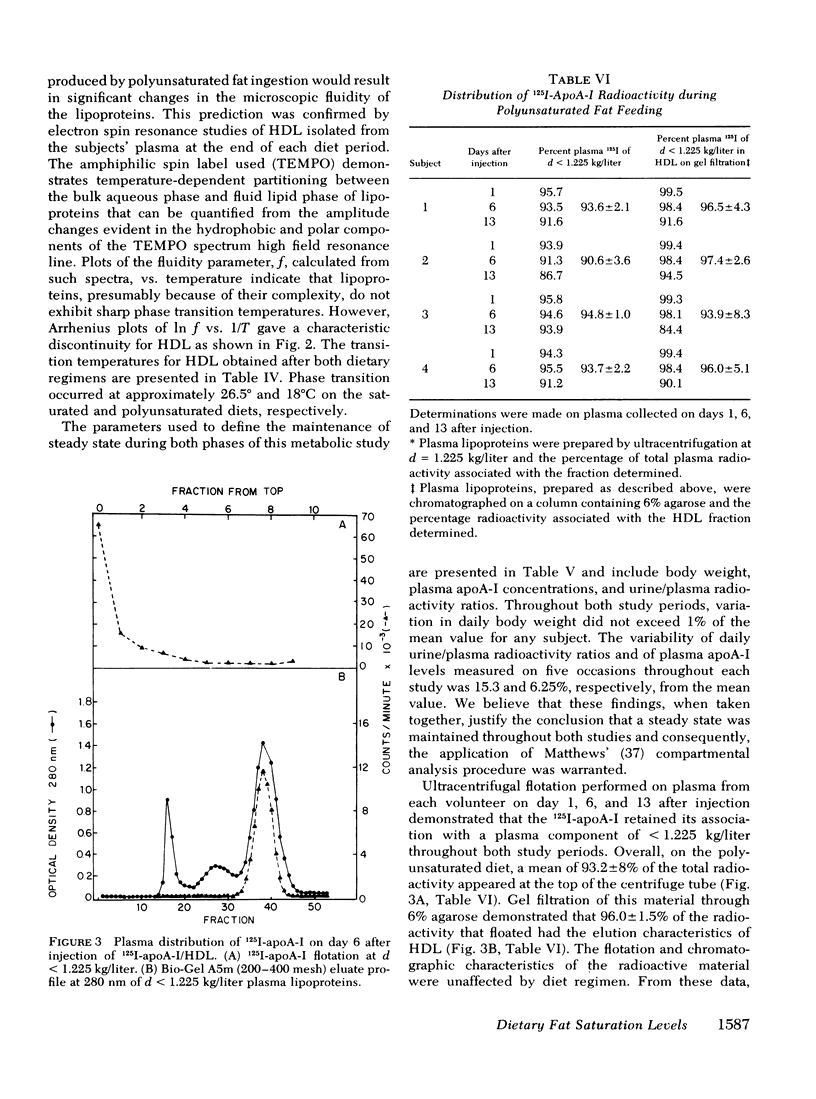
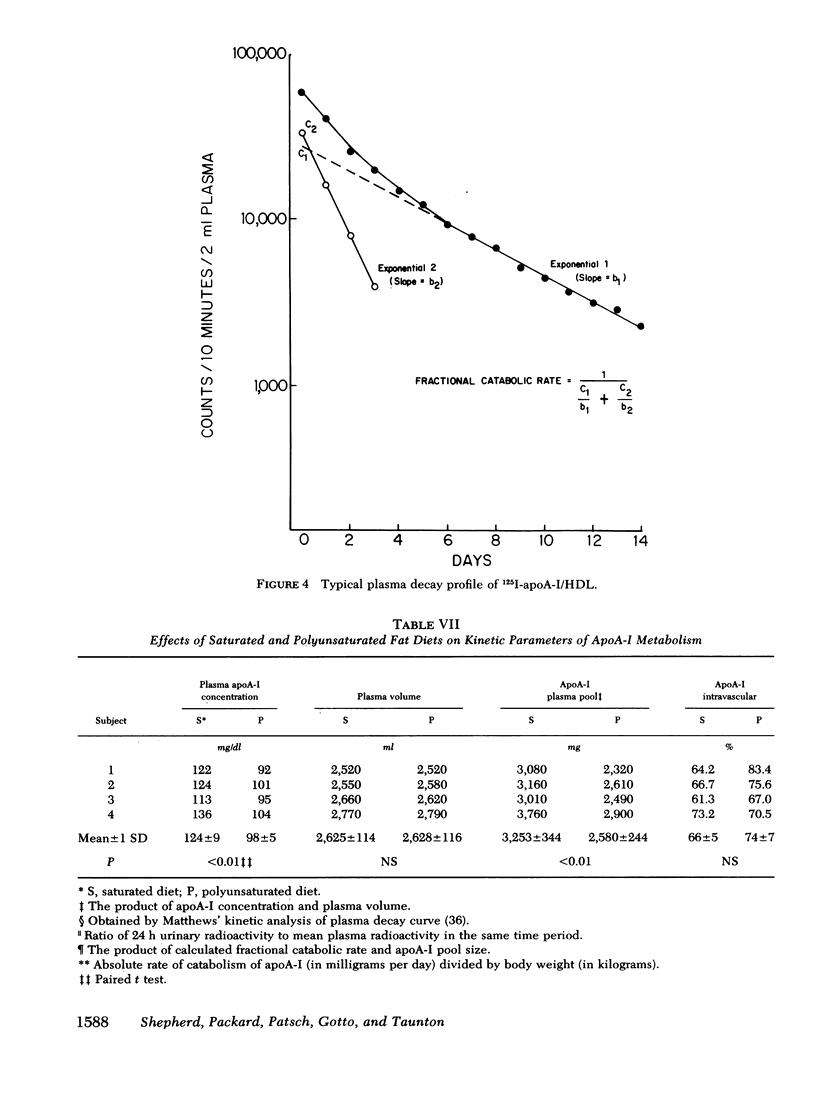



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHRENS E. H., Jr, INSULL W., Jr, BLOMSTRAND R., HIRSCH J., TSALTAS T. T., PETERSON M. L. The influence of dietary fats on serum-lipid levels in man. Lancet. 1957 May 11;272(6976):943–953. doi: 10.1016/s0140-6736(57)91280-1. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BRONTE-STEWART B., ANTONIS A., EALES L., BROCK J. F. Effects of feeding different fats on serum-cholesterol level. Lancet. 1956 Apr 28;270(6922):521–526. doi: 10.1016/s0140-6736(56)90592-x. [DOI] [PubMed] [Google Scholar]
- Baker H. N., Delahunty T., Gotto A. M., Jr, Jackson R. L. The primary structure of high density apolipoprotein-glutamine-I. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3631–3634. doi: 10.1073/pnas.71.9.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor W. E., Witiak D. T., Stone D. B., Armstrong M. L. Cholesterol balance and fecal neutral steroid and bile acid excretion in normal men fed dietary fats of different fatty acid composition. J Clin Invest. 1969 Aug;48(8):1363–1375. doi: 10.1172/JCI106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg H. Mechanisms involved in the reduction of serum triglycerides in man upon adding unsaturated fats to the normal diet. Metabolism. 1966 Sep;15(9):796–807. doi: 10.1016/0026-0495(66)90172-7. [DOI] [PubMed] [Google Scholar]
- FARQUHAR J. W., SOKOLOW M. Response of serum lipids and lipoproteins o man to beta-sitosterol and safflower oil; a longterm study. Circulation. 1958 May;17(5):890–899. doi: 10.1161/01.cir.17.5.890. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Gordon D. T., Pitas R. E., Jensen R. G. Effects of diet and type IIa hyperlipoproteinemia upon structure of triacylglycerols and phosphatidyl cholines from human plasma lipoproteins. Lipids. 1975 May;10(5):270–283. doi: 10.1007/BF02532700. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Gotto A. M. A study of the cystine-containing apolipoprotein of human plasma high density lipoproteins: characterization of cyanogen bromide and tryptic fragments. Biochim Biophys Acta. 1972 Nov 28;285(1):36–47. doi: 10.1016/0005-2795(72)90178-x. [DOI] [PubMed] [Google Scholar]
- KINSELL L. W., MICHAELS G. D., PARTRIDGE F. W., BOLING L. A., BALCH H. E., COCHRANE G. C. Effect upon serum cholesterol and phospholipids of diets containing large amounts of vegetable fat. J Clin Nutr. 1953 Mar-Apr;1(3):224–231. doi: 10.1093/ajcn/1.3.224. [DOI] [PubMed] [Google Scholar]
- KINSELL L. W., PARTRIDGE J., BOLING L., MARGEN S., MICHAELS G. Dietary modification of serum cholesterol and phospholipid levels. J Clin Endocrinol Metab. 1952 Jul;12(7):909–913. doi: 10.1210/jcem-12-7-909. [DOI] [PubMed] [Google Scholar]
- LAMBERT G. F., MILLER J. P., OLSEN R. T., FROST D. V. Hypercholesteremia and atherosclerosis induced in rabbits by purified high fat rations devoid of cholesterol. Proc Soc Exp Biol Med. 1958 Mar;97(3):544–549. doi: 10.3181/00379727-97-23800. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Windmueller H. G., Fredrickson D. S. Comparison of the triglyceride lipase of liver, adipose tissue, and postheparin plasma. J Lipid Res. 1972 May;13(3):356–363. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- MALMROS H., WIGAND G. The effect on serum-cholesterol of diets containing different fats. Lancet. 1957 Jul 6;273(6984):1–7. doi: 10.1016/s0140-6736(57)90568-8. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Nichaman M. Z., Sweeley C. C., Olson R. E. Plasma fatty acids in normolipemic and hyperlipemic subjects during fasting and after linoleate feeding. Am J Clin Nutr. 1967 Oct;20(10):1057–1069. doi: 10.1093/ajcn/20.10.1057. [DOI] [PubMed] [Google Scholar]
- Packard C. J., Third J. L., Shepherd J., Lorimer A. R., Morgan H. G., Lawrie T. D. Low density lipoprotein metabolism in a family of familial hypercholesterolemic patients. Metabolism. 1976 Sep;25(9):995–1006. doi: 10.1016/0026-0495(76)90129-3. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Sailer S., Kostner G., Sandhofer F., Holasek A., Braunsteiner H. Separation of the main lipoprotein density classes from human plasma by rate-zonal ultracentrifugation. J Lipid Res. 1974 Jul;15(4):356–366. [PubMed] [Google Scholar]
- Pearson A. M. Some factors that may alter consumption of animal products. A review. J Am Diet Assoc. 1976 Nov;69(5):522–530. [PubMed] [Google Scholar]
- Rhoads G. G., Gulbrandsen C. L., Kagan A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med. 1976 Feb 5;294(6):293–298. doi: 10.1056/NEJM197602052940601. [DOI] [PubMed] [Google Scholar]
- STEINER A., VARSOS A., SAMUEL P. Effect of saturated and unsaturated fats on the concentration of serum cholesterol and experimental atherosclerosis. Circ Res. 1959 May;7(3):448–453. doi: 10.1161/01.res.7.3.448. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Pfleger B. The structure of human high density lipoprotein and the levels of apolipoprotein A-I in plasma as determined by radioimmunoassay. J Clin Invest. 1974 Aug;54(2):236–246. doi: 10.1172/JCI107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. Diagnosis of dyslipoprotein aemia by molecular sieve chromatography. Clin Chim Acta. 1976 Jun 1;69(2):161–173. doi: 10.1016/0009-8981(76)90493-9. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Gotto A. M., Jr, Taunton O. D., Caslake M. J., Farish E. The in vitro interaction of human apolipoprotein A-I and high density lipoproteins. Biochim Biophys Acta. 1977 Dec 21;489(3):486–501. doi: 10.1016/0005-2760(77)90169-2. [DOI] [PubMed] [Google Scholar]
- Soutar A. K., Pownall H. J., Hu A. S., Smith L. C. Phase transitions in bilamellar vesicles. Measurements by pyrene excimer fluorescence and effect on transacylation by lecithin: cholesterol acyltransferase. Biochemistry. 1974 Jul 2;13(14):2828–2836. doi: 10.1021/bi00711a008. [DOI] [PubMed] [Google Scholar]
- Spritz N., Mishkel M. A. Effects of dietary fats on plasma lipids and lipoproteins: an hypothesis for the lipid-lowering effect of unsaturated fatty acids. J Clin Invest. 1969 Jan;48(1):78–86. doi: 10.1172/JCI105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. R., Jadhav A., Nava M., Gotto A. M., Jr Effects of intravenous phospholipid on low density lipoprotein turnover in man. Eur J Clin Invest. 1976 Jun 21;6(3):241–248. doi: 10.1111/j.1365-2362.1976.tb00516.x. [DOI] [PubMed] [Google Scholar]
- WIGAND G. Production of hypercholesterolemia and atherosclerosis in rabbits by feeding different fats without supplementary cholesterol. Acta Med Scand Suppl. 1959;351:1–91. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


