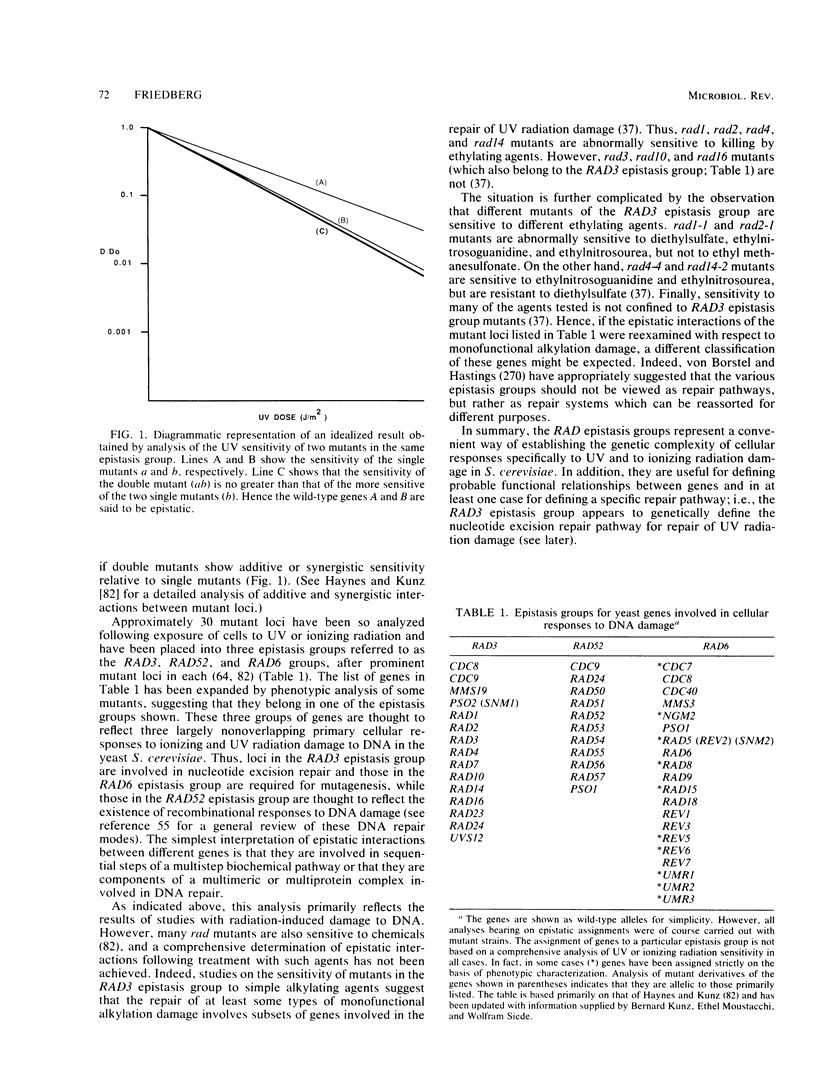
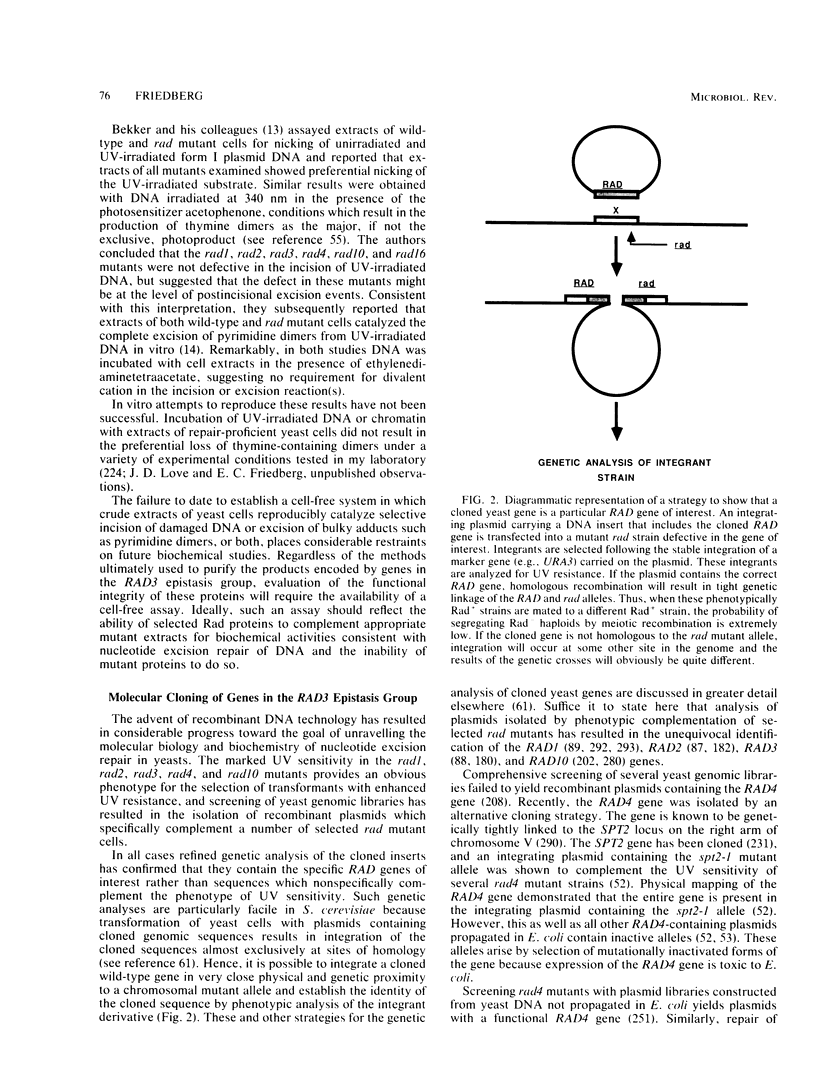
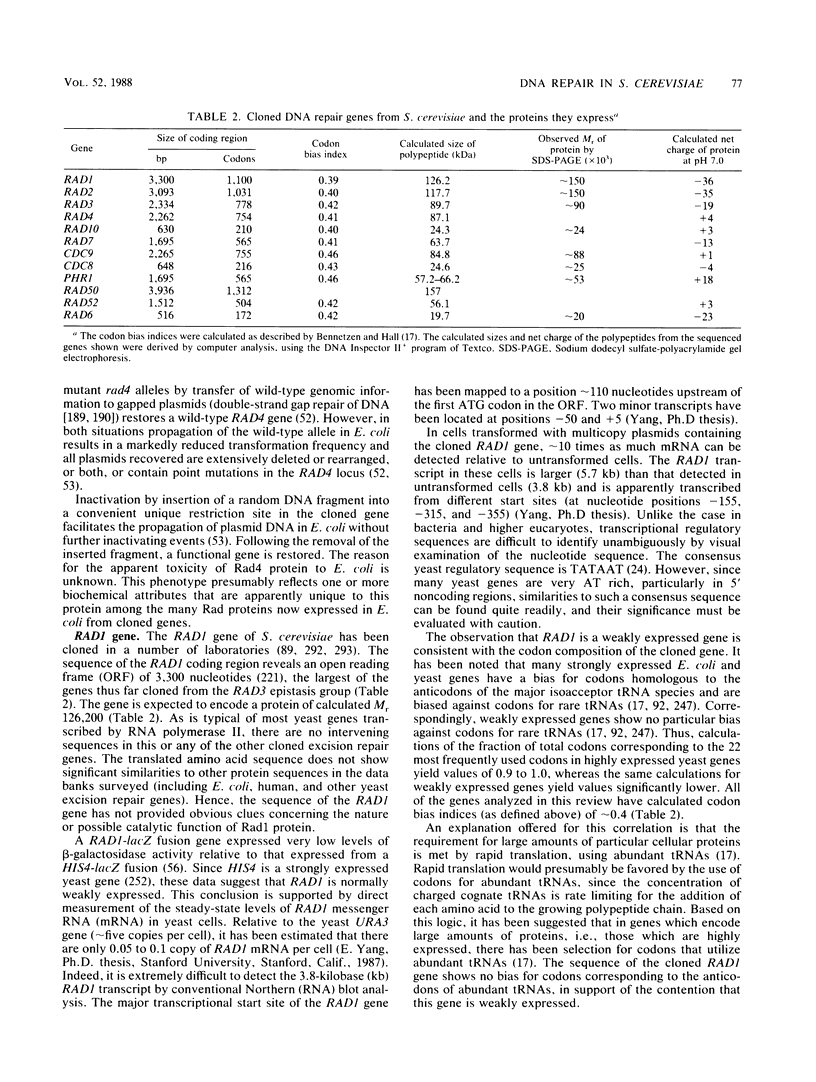
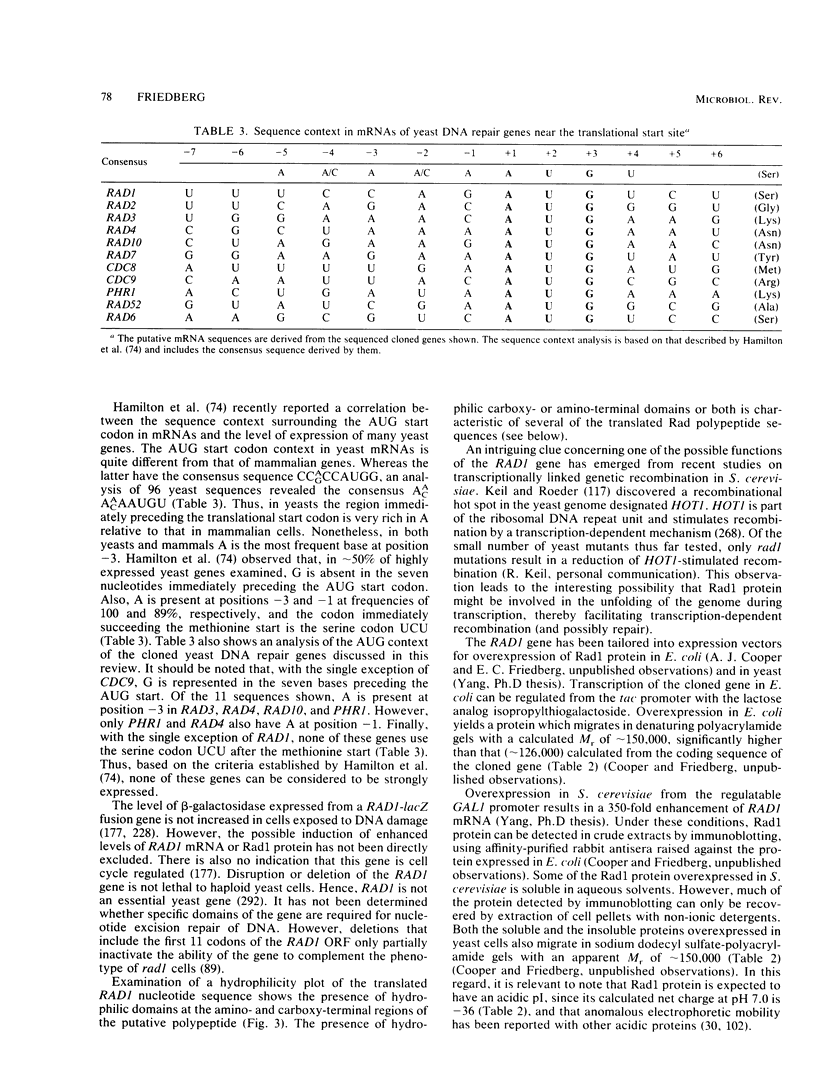
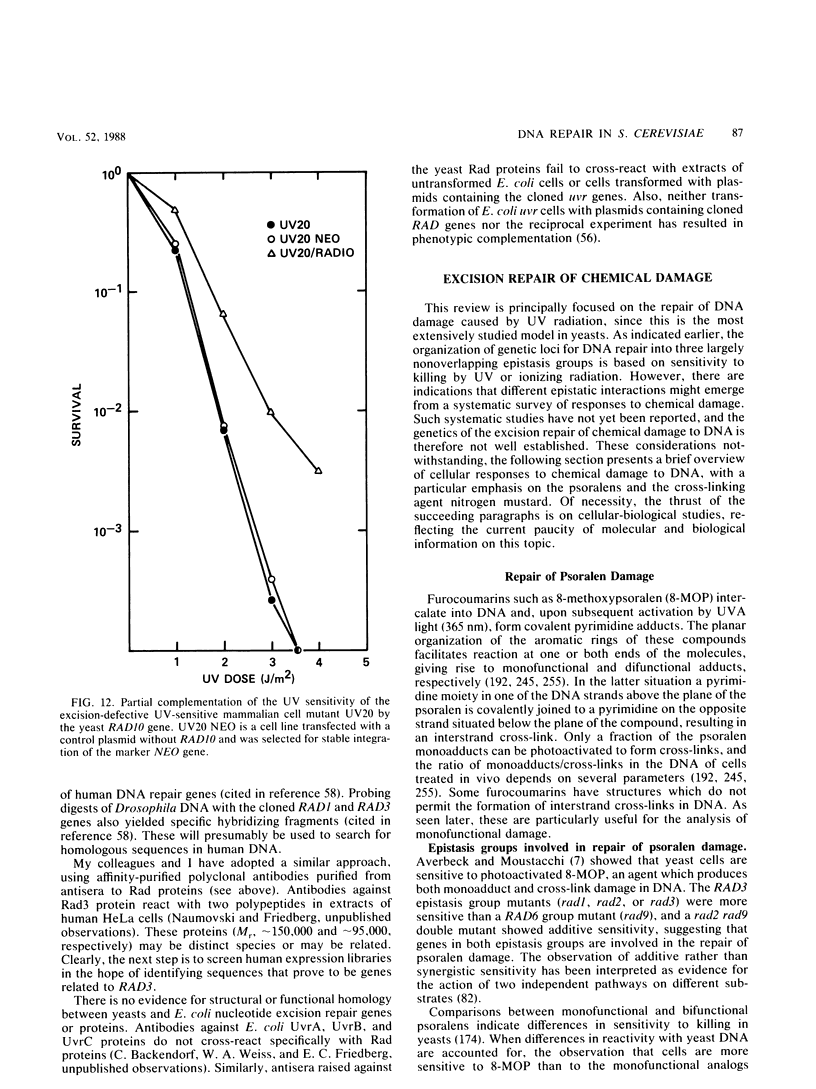
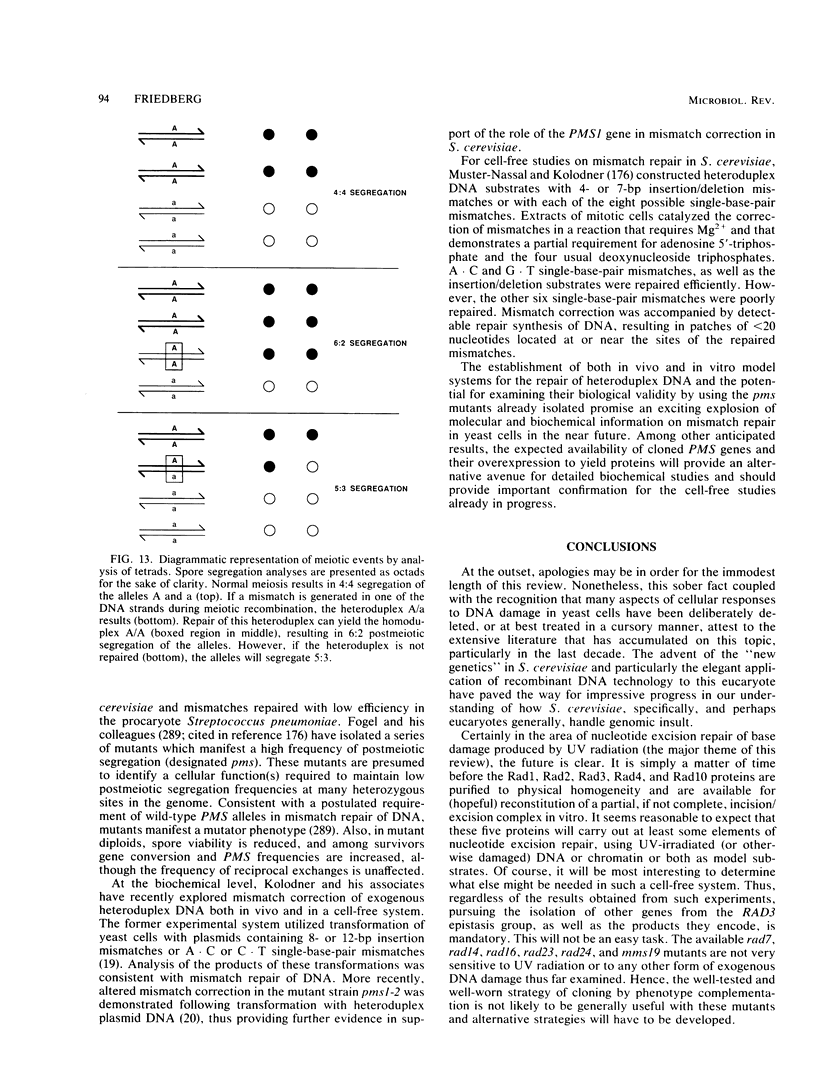
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Chanal M. C., Hoffmann-Berling H. DNA unwinding enzyme II of Escherichia coli. 1. Purification and characterization of the ATPase activity. Eur J Biochem. 1977 Sep 15;79(1):33–38. doi: 10.1111/j.1432-1033.1977.tb11780.x. [DOI] [PubMed] [Google Scholar]
- Adzuma K., Ogawa T., Ogawa H. Primary structure of the RAD52 gene in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2735–2744. doi: 10.1128/mcb.4.12.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. A., Nakashima Y., Coleman J. E. Chemical modifications of functional residues of fd gene 5 DNA-binding protein. Biochemistry. 1975 Mar 11;14(5):907–917. doi: 10.1021/bi00676a006. [DOI] [PubMed] [Google Scholar]
- Arikan E., Kulkarni M. S., Thomas D. C., Sancar A. Sequences of the E. coli uvrB gene and protein. Nucleic Acids Res. 1986 Mar 25;14(6):2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather A., Ahmed Z., Riazuddin S. Adaptive response of Micrococcus luteus to alkylating chemicals. Nucleic Acids Res. 1984 Feb 24;12(4):2111–2126. doi: 10.1093/nar/12.4.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck D., Laskowski W., Eckardt F., Lehmann-Brauns E. Four radiation sensitive mutants of Saccharomyces. Survival after UV- and x-ray-irradiation as well as UV-induced reversion rates from isoleucine-valine dependence to independence. Mol Gen Genet. 1970;107(2):117–127. doi: 10.1007/BF00333628. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E. 8-Methoxypsoralen plus 365 nm light effects and repair in yeast. Biochim Biophys Acta. 1975 Jul 23;395(4):393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E., Bisagni E. Biological effects and repair of damage photoinduced by a derivative of psoralen substituted at the 3,4 reaction site: photoreactivity of this compound and lethal effect in yeast. Biochim Biophys Acta. 1978 May 23;518(3):464–481. doi: 10.1016/0005-2787(78)90165-x. [DOI] [PubMed] [Google Scholar]
- Backendorf C., Spaink H., Barbeiro A. P., van de Putte P. Structure of the uvrB gene of Escherichia coli. Homology with other DNA repair enzymes and characterization of the uvrB5 mutation. Nucleic Acids Res. 1986 Apr 11;14(7):2877–2890. doi: 10.1093/nar/14.7.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Johnson A. L., Johnston L. H. An improved assay for DNA ligase reveals temperature-sensitive activity in cdc9 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):458–462. doi: 10.1007/BF00425731. [DOI] [PubMed] [Google Scholar]
- Barker D. G., Johnston L. H. Saccharomyces cerevisiae cdc9, a structural gene for yeast DNA ligase which complements Schizosaccharomyces pombe cdc17. Eur J Biochem. 1983 Aug 1;134(2):315–319. doi: 10.1111/j.1432-1033.1983.tb07568.x. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. The nucleotide sequence of the DNA ligase gene (CDC9) from Saccharomyces cerevisiae: a gene which is cell-cycle regulated and induced in response to DNA damage. Nucleic Acids Res. 1985 Dec 9;13(23):8323–8337. doi: 10.1093/nar/13.23.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker M. L., Kaboev O. K., Akhmedov A. T., Luchkina L. A. Quantitative characterization of pyrimidine dimer excision from UV-irradiated DNA (excision capacity) by cell-free extracts of the yeast Saccharomyces cerevisiae. FEBS Lett. 1984 Mar 26;168(2):245–248. doi: 10.1016/0014-5793(84)80255-0. [DOI] [PubMed] [Google Scholar]
- Bekker M. L., Kaboev O. K., Akhmedov A. T., Luchkina L. A. Ultraviolet-endonuclease activity in cell extracts of Saccharomyces cerevisiae mutants defective in excision of pyrimidine dimers. J Bacteriol. 1980 Apr;142(1):322–324. doi: 10.1128/jb.142.1.322-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker M. L., Kaboev O. K., Koval'tsova S. V. A new mutant of the yeast Saccharomyces cerevisiae defective in excision of UV-damaged sites in DNA. Mol Gen Genet. 1980 Feb;177(3):541–544. doi: 10.1007/BF00271495. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Birkenmeyer L. G., Hill J. C., Dumas L. B. Saccharomyces cerevisiae CDC8 gene and its product. Mol Cell Biol. 1984 Apr;4(4):583–590. doi: 10.1128/mcb.4.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Kolodner R. D. Repair of heteroduplex plasmid DNA after transformation into Saccharomyces cerevisiae. Mol Cell Biol. 1986 Oct;6(10):3401–3409. doi: 10.1128/mcb.6.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Williamson M. S., Fogel S., Kolodner R. D. The role of heteroduplex correction in gene conversion in Saccharomyces cerevisiae. Nature. 1987 Jul 23;328(6128):362–364. doi: 10.1038/328362a0. [DOI] [PubMed] [Google Scholar]
- Boatwright D. T., Madden J. J., Denson J., Werbin H. Yeast DNA photolyase: molecular weight, subunit structure, and reconstruction of active enzyme from its subunits. Biochemistry. 1975 Dec 16;14(25):5418–5421. doi: 10.1021/bi00696a006. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Interactions among genes controlling sensitivity to radiation and alkylation in yeast. Mol Gen Genet. 1973 Sep 12;125(3):197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Kinetics and genetic control of the incorporation of thymidine monophosphate in yeast DNA. Mol Gen Genet. 1972;117(1):39–44. doi: 10.1007/BF00268835. [DOI] [PubMed] [Google Scholar]
- Brendel M., Langjahr U. G. "Thymineless death" in a strain of Saccharomyces cerevisiae auxotrophic for deoxythymidine-5'-monophosphate. Mol Gen Genet. 1974;131(4):351–358. doi: 10.1007/BF00264865. [DOI] [PubMed] [Google Scholar]
- Brendel M., Ruhland A. Relationships between functionality and genetic toxicology of selected DNA-damaging agents. Mutat Res. 1984 Jan;133(1):51–85. doi: 10.1016/0165-1110(84)90003-4. [DOI] [PubMed] [Google Scholar]
- Bryant D. W., Haynes R. H. Endonuclease alpha from Saccharomyces cerevisiae shows increased activity on ultraviolet irradiated native DNA. Mol Gen Genet. 1978 Nov 29;167(2):139–145. doi: 10.1007/BF00266907. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet R., Cassier C., Moustacchi E. Genetic control of the bypass of mono-adducts and of the repair of cross-links photoinduced by 8-methoxypsoralen in yeast. Mutat Res. 1985 May;145(3):145–155. doi: 10.1016/0167-8817(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Chenevert J. M., Naumovski L., Schultz R. A., Friedberg E. C. Partial complementation of the UV sensitivity of E. coli and yeast excision repair mutants by the cloned denV gene of bacteriophage T4. Mol Gen Genet. 1986 Apr;203(1):163–171. doi: 10.1007/BF00330398. [DOI] [PubMed] [Google Scholar]
- Cole G. M., Schild D., Lovett S. T., Mortimer R. K. Regulation of RAD54- and RAD52-lacZ gene fusions in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1987 Mar;7(3):1078–1084. doi: 10.1128/mcb.7.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. J., Waters R. A complex pattern of sensitivity to simple monofunctional alkylating agents exists amongst the rad mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1987 Aug;209(1):142–148. doi: 10.1007/BF00329849. [DOI] [PubMed] [Google Scholar]
- Cox B. S., Parry J. M. The isolation, genetics and survival characteristics of ultraviolet light-sensitive mutants in yeast. Mutat Res. 1968 Jul-Aug;6(1):37–55. doi: 10.1016/0027-5107(68)90101-2. [DOI] [PubMed] [Google Scholar]
- Cox B., Game J. Repair systems in Saccharomyces. Mutat Res. 1974 Aug;26(4):257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- Domiński Z., Jachymczyk W. J. Repair of UV-irradiated plasmid DNA in a Saccharomyces cerevisiae rad3 mutant deficient in excision-repair of pyrimidine dimers. Mol Gen Genet. 1984;193(1):167–171. doi: 10.1007/BF00327432. [DOI] [PubMed] [Google Scholar]
- Echols H. Multiple DNA-protein interactions governing high-precision DNA transactions. Science. 1986 Sep 5;233(4768):1050–1056. doi: 10.1126/science.2943018. [DOI] [PubMed] [Google Scholar]
- Eckardt-Schupp F., Siede W., Game J. C. The RAD24 (= Rs1) gene product of Saccharomyces cerevisiae participates in two different pathways of DNA repair. Genetics. 1987 Jan;115(1):83–90. doi: 10.1093/genetics/115.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt F., Kowalskí S., Laskowski W. The effects of three rad genes on UV induced mutation rates in haploid and diploid Saccharomyces cells. Mol Gen Genet. 1975;136(3):261–272. doi: 10.1007/BF00334021. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Dekker R. H., Berends W. Photoreactivating enzyme from Streptomyces griseus-IV. On the nature of the chromophoric cofactor in Streptomyces griseus photoreactivating enzyme. Photochem Photobiol. 1981 Jan;33(1):65–72. doi: 10.1111/j.1751-1097.1981.tb04298.x. [DOI] [PubMed] [Google Scholar]
- Eker A. P. Photoreactivating enzyme from Streptomyces griseus--II. Evidence for the presence of an intrinsic chromophore. Photochem Photobiol. 1980 Nov;32(5):593–600. doi: 10.1111/j.1751-1097.1980.tb04027.x. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Identification and isolation of the gene encoding the small subunit of ribonucleotide reductase from Saccharomyces cerevisiae: DNA damage-inducible gene required for mitotic viability. Mol Cell Biol. 1987 Aug;7(8):2783–2793. doi: 10.1128/mcb.7.8.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Storey A., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of recD, the structural gene for the alpha subunit of Exonuclease V of Escherichia coli. Nucleic Acids Res. 1986 Nov 11;14(21):8583–8594. doi: 10.1093/nar/14.21.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Wilson R. E., Brown K., Hickson I. D., Tomkinson A. E., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli recC gene and of the thyA-recC intergenic region. Nucleic Acids Res. 1986 Jun 11;14(11):4437–4451. doi: 10.1093/nar/14.11.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleer R., Nicolet C. M., Pure G. A., Friedberg E. C. RAD4 gene of Saccharomyces cerevisiae: molecular cloning and partial characterization of a gene that is inactivated in Escherichia coli. Mol Cell Biol. 1987 Mar;7(3):1180–1192. doi: 10.1128/mcb.7.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleer R., Siede W., Friedberg E. C. Mutational inactivation of the Saccharomyces cerevisiae RAD4 gene in Escherichia coli. J Bacteriol. 1987 Nov;169(11):4884–4892. doi: 10.1128/jb.169.11.4884-4892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury F., Lahaye A. Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 1987 May;6(5):1441–1449. doi: 10.1002/j.1460-2075.1987.tb02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Backendorf C., Burke J., Collins A., Grossman L., Hoeijmakers J. H., Lehmann A. R., Seeberg E., van der Schans G. P., van Zeeland A. A. Molecular aspects of DNA repair. Mutat Res. 1987 Sep;184(2):67–86. doi: 10.1016/0167-8817(87)90063-0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Barbis D. P., Chenevert J. M., Fleer R., Kalainov D., Naumovski L., Nicolet C. M., Robinson G. W., Schultz R. A., Weiss W. A. Molecular approaches to the study of nucleotide excision repair in eukaryotes. Basic Life Sci. 1986;38:311–318. doi: 10.1007/978-1-4615-9462-8_33. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Fleer R., Naumovski L., Nicolet C. M., Robinson G. W., Weiss W. A., Yang E. Nucleotide excision repair genes from the yeast Saccharomyces cerevisiae. Basic Life Sci. 1986;39:231–242. doi: 10.1007/978-1-4684-5182-5_20. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Nucleotide excision repair of DNA in eukaryotes: comparisons between human cells and yeast. Cancer Surv. 1985;4(3):529–555. [PubMed] [Google Scholar]
- Fäth W. W., Brendel M. Specific DNA-labelling by exogenous thymidine-5'-monophosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1974;131(1):57–67. doi: 10.1007/BF00269387. [DOI] [PubMed] [Google Scholar]
- Game J. C., Cox B. S. Epistatic interactions between four rad loci in yeast. Mutat Res. 1972 Dec;16(4):353–362. doi: 10.1016/0027-5107(72)90203-5. [DOI] [PubMed] [Google Scholar]
- Game J. C., Cox B. S. Synergistic interactions between rad mutations in yeast. Mutat Res. 1973 Oct;20(1):35–44. doi: 10.1016/0027-5107(73)90095-x. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C., Lewis R. J., Hanawalt P. C. Processivity of T4 endonuclease V is sensitive to NaCl concentration. Biochemistry. 1986 Sep 23;25(19):5751–5755. doi: 10.1021/bi00367a060. [DOI] [PubMed] [Google Scholar]
- Golin J. E., Esposito M. S. Evidence for joint genic control of spontaneous mutation and genetic recombination during mitosis in Saccharomyces. Mol Gen Genet. 1977 Jan 18;150(2):127–135. doi: 10.1007/BF00695392. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Gruskin E. A., Lloyd R. S. The DNA scanning mechanism of T4 endonuclease V. Effect of NaCl concentration on processive nicking activity. J Biol Chem. 1986 Jul 25;261(21):9607–9613. [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971 Jul 14;59(1):183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings P. J., Quah S. K., von Borstel R. C. Spontaneous mutation by mutagenic repair of spontaneous lesions in DNA. Nature. 1976 Dec 23;264(5588):719–722. doi: 10.1038/264719a0. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Moustacchi E. Interactions between mutations for sensitivity to psoralen photoaddition (pso) and to radiation (rad) in Saccharomyces cerevisiae. J Bacteriol. 1981 Oct;148(1):248–256. doi: 10.1128/jb.148.1.248-256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques J. A., Moustacchi E. Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics. 1980 Jun;95(2):273–288. doi: 10.1093/genetics/95.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. R., Prakash L., Reynolds P., Prakash S. Isolation and characterization of the RAD2 gene of Saccharomyces cerevisiae. Gene. 1984 Oct;30(1-3):121–128. doi: 10.1016/0378-1119(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Higgins D. R., Prakash S., Reynolds P., Polakowska R., Weber S., Prakash L. Isolation and characterization of the RAD3 gene of Saccharomyces cerevisiae and inviability of rad3 deletion mutants. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5680–5684. doi: 10.1073/pnas.80.18.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. R., Prakash S., Reynolds P., Prakash L. Molecular cloning and characterization of the RAD1 gene of Saccharomyces cerevisiae. Gene. 1983 Dec;26(2-3):119–126. doi: 10.1016/0378-1119(83)90181-6. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. S. Induction of DNA double-strand breaks by X-rays in a radiosensitive strain of the yeast Saccharomyces cerevisiae. Mutat Res. 1975 Dec;30(3):327–334. [PubMed] [Google Scholar]
- Hoekema A., Kastelein R. A., Vasser M., de Boer H. A. Codon replacement in the PGK1 gene of Saccharomyces cerevisiae: experimental approach to study the role of biased codon usage in gene expression. Mol Cell Biol. 1987 Aug;7(8):2914–2924. doi: 10.1128/mcb.7.8.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M. F., Malone R. E. Expression of the Escherichia coli dam methylase in Saccharomyces cerevisiae: effect of in vivo adenine methylation on genetic recombination and mutation. Mol Cell Biol. 1985 Apr;5(4):610–618. doi: 10.1128/mcb.5.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M. F., Malone R. E. Hyper-mutation caused by the reml mutation in yeast is not dependent on error-prone or excision repair. Mutat Res. 1987 Jun;178(2):201–210. doi: 10.1016/0027-5107(87)90270-3. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Ikai K., Tano K., Ohnishi T., Nozu K. Repair of UV-irradiated plasmid DNA in excision repair deficient mutants of Saccharomyces cerevisiae. Photochem Photobiol. 1985 Aug;42(2):179–181. doi: 10.1111/j.1751-1097.1985.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Ishii S., Ihara M., Maekawa T., Nakamura Y., Uchida H., Imamoto F. The nucleotide sequence of the cloned nusA gene and its flanking region of Escherichia coli. Nucleic Acids Res. 1984 Apr 11;12(7):3333–3342. doi: 10.1093/nar/12.7.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Joe C. O., Werbin H. Evidence that deoxyribonucleic acid photolyase from baker's yeast is a flavoprotein. Biochemistry. 1980 Mar 18;19(6):1172–1176. doi: 10.1021/bi00547a021. [DOI] [PubMed] [Google Scholar]
- Jannsen S., Lochmann E. -R., Megnet R. Specific incorporation of exogenous thymidine monophosphate into DNA in Saccharomyces cerevisiae. FEBS Lett. 1970 Jun 1;8(3):113–115. doi: 10.1016/0014-5793(70)80239-3. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch S., McGrath J. P., Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987 Sep 10;329(6135):131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. L., Barker D. G., Johnston L. H. Induction of yeast DNA ligase genes in exponential and stationary phase cultures in response to DNA damaging agents. Curr Genet. 1986;11(2):107–112. doi: 10.1007/BF00378201. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Nasmyth K. A. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978 Aug 31;274(5674):891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- Johnston L. H. The DNA repair capability of cdc9, the Saccharomyces cerevisiae mutant defective in DNA ligase. Mol Gen Genet. 1979 Feb 16;170(1):89–92. doi: 10.1007/BF00268583. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., White J. H., Johnson A. L., Lucchini G., Plevani P. The yeast DNA polymerase I transcript is regulated in both the mitotic cell cycle and in meiosis and is also induced after DNA damage. Nucleic Acids Res. 1987 Jul 10;15(13):5017–5030. doi: 10.1093/nar/15.13.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Kassir Y., Kupiec M., Shalom A., Simchen G. Cloning and mapping of CDC40, a Saccharomyces cerevisiae gene with a role in DNA repair. Curr Genet. 1985;9(4):253–257. doi: 10.1007/BF00419952. [DOI] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Meiotic recombination and DNA synthesis in a new cell cycle mutant of Saccharomyces cerevisiae. Genetics. 1978 Sep;90(1):49–68. doi: 10.1093/genetics/90.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Ferguson J., Reed S. I. Temperature-sensitive lethal pseudorevertants of ste mutations in Saccharomyces cerevisiae. Genetics. 1987 Apr;115(4):627–636. doi: 10.1093/genetics/115.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil R. L., Roeder G. S. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell. 1984 Dec;39(2 Pt 1):377–386. doi: 10.1016/0092-8674(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Kiefer J. UV response of the temperature-conditional rad 54 mutant of the yeast Saccharomyces cerevisiae. Mutat Res. 1987 May;191(1):9–12. doi: 10.1016/0165-7992(87)90162-x. [DOI] [PubMed] [Google Scholar]
- Kimball R. F. Further studies on the induction of mutation in Haemophilus influenzae by N-methyl-N'-nitro-N-nitrosoguanidine: lack of an inducible error-free repair system and the effect of exposure medium. Mutat Res. 1980 Aug;72(3):361–372. doi: 10.1016/0027-5107(80)90111-6. [DOI] [PubMed] [Google Scholar]
- Kowalski S., Laskowski W. The effect of three rad genes on survival, inter- and intragenic mitotic recombination in Saccharomyces. I. UV irradiation without photoreactivation or liquid-holding post-treatment. Mol Gen Genet. 1975;136(1):75–86. doi: 10.1007/BF00275450. [DOI] [PubMed] [Google Scholar]
- Kumura K., Sekiguchi M., Steinum A. L., Seeberg E. Stimulation of the UvrABC enzyme-catalyzed repair reactions by the UvrD protein (DNA helicase II). Nucleic Acids Res. 1985 Mar 11;13(5):1483–1492. doi: 10.1093/nar/13.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. L., Campbell J. L. Cloning of Saccharomyces cerevisiae DNA replication genes: isolation of the CDC8 gene and two genes that compensate for the cdc8-1 mutation. Mol Cell Biol. 1983 Oct;3(10):1730–1737. doi: 10.1128/mcb.3.10.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec M., Simchen G. Cloning and mapping of the RAD50 gene of Saccharomyces cerevisiae. Mol Gen Genet. 1984;193(3):525–531. doi: 10.1007/BF00382094. [DOI] [PubMed] [Google Scholar]
- Kupiec M., Simchen G. DNA-repair characterization of cdc40-1, a cell-cycle mutant of Saccharomyces cerevisiae. Mutat Res. 1986 Aug;162(1):33–40. doi: 10.1016/0027-5107(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Kupiec M., Simchen G. Regulation of the RAD6 gene of Saccharomyces cerevisiae in the mitotic cell cycle and in meiosis. Mol Gen Genet. 1986 Jun;203(3):538–543. doi: 10.1007/BF00422083. [DOI] [PubMed] [Google Scholar]
- Kupiec M. The RAD50 gene of Saccharomyces cerevisiae is not essential for vegetative growth. Curr Genet. 1986;10(6):487–489. doi: 10.1007/BF00419878. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., Yasui A., Eker A. P. Expression of an Escherichia coli phr gene in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1985;199(3):396–400. doi: 10.1007/BF00330748. [DOI] [PubMed] [Google Scholar]
- Laskowski W., Lochmann E. R., Jannsen S., Fink E. Zur Isolierung einer strahlensensiblen Saccharomyces-Mutante. Empfindlichkeit des Koloniebildungsvermögens, der RNS- und Proteinsythese. Biophysik. 1968;4(3):233–242. doi: 10.1007/BF01191599. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Krauss B. R., Christensen R. B. New mutations affecting induced mutagenesis in yeast. Mutat Res. 1985 Jun-Jul;150(1-2):211–216. doi: 10.1016/0027-5107(85)90117-4. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W. Mutagenesis in Saccharomyces cerevisiae. Adv Genet. 1982;21:173–254. doi: 10.1016/s0065-2660(08)60299-0. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Nisson P. E., Christensen R. B. UV and chemical mutagenesis in rev7 mutants of yeast. Mol Gen Genet. 1985;200(1):86–91. doi: 10.1007/BF00383317. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics. 1971 May;68(1):21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemontt J. F. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. III. Genetic analysis and properties of mutants resitant to ultraviolet-induced forward mutation. Mutat Res. 1977 May;43(2):179–204. doi: 10.1016/0027-5107(77)90003-3. [DOI] [PubMed] [Google Scholar]
- Little J. G., Haynes R. H. Isolation and characterization of yeast mutants auxotrophic for 2'-deoxythymidine 5'-monophosphate. Mol Gen Genet. 1979 Jan 10;168(2):141–151. doi: 10.1007/BF00431440. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hahne S. Isolation of a condensed, intracellular form of the 2-micrometer DNA plasmid of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3727–3731. doi: 10.1073/pnas.76.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. S., Hanawalt P. C., Dodson M. L. Processive action of T4 endonuclease V on ultraviolet-irradiated DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5113–5127. doi: 10.1093/nar/8.21.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQuillan A. M., Herman A., Coberly J. S., Green G. A second photoreactivation-deficient mutation in Saccharomyces cerevisiae. Photochem Photobiol. 1981 Dec;34(6):673–677. [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Madura K., Prakash S. Nucleotide sequence, transcript mapping, and regulation of the RAD2 gene of Saccharomyces cerevisiae. J Bacteriol. 1986 Jun;166(3):914–923. doi: 10.1128/jb.166.3.914-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga J. A., McClanahan T. A., McEntee K. Transcriptional regulation of DNA damage responsive (DDR) genes in different rad mutant strains of Saccharomyces cerevisiae. Mol Gen Genet. 1986 Nov;205(2):276–284. doi: 10.1007/BF00430439. [DOI] [PubMed] [Google Scholar]
- Maga J. A., McEntee K. Response of S. cerevisiae to N-methyl-N'-nitro-N-nitrosoguanidine: mutagenesis, survival and DDR gene expression. Mol Gen Genet. 1985;200(2):313–321. doi: 10.1007/BF00425442. [DOI] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Henriques J. A., Chanet R., Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Hoekstra M. F. Relationships between a hyper-rec mutation (REM1) and other recombination and repair genes in yeast. Genetics. 1984 May;107(1):33–48. doi: 10.1093/genetics/107.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C., Buhler J. M., Treich I., Sentenac A. RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell. 1987 Feb 27;48(4):627–637. doi: 10.1016/0092-8674(87)90241-8. [DOI] [PubMed] [Google Scholar]
- McClanahan T., McEntee K. DNA damage and heat shock dually regulate genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Jan;6(1):90–96. doi: 10.1128/mcb.6.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClanahan T., McEntee K. Specific transcripts are elevated in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1984 Nov;4(11):2356–2363. doi: 10.1128/mcb.4.11.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready S. J., Boyce J. M., Cox B. S. Excision repair in the yeast, Saccharomyces cerevisiae. J Cell Sci Suppl. 1987;6:25–38. doi: 10.1242/jcs.1984.supplement_6.2. [DOI] [PubMed] [Google Scholar]
- McKee R. H., Lawrence C. W. Genetic analysis of gamma-ray mutagenesis in yeast. II. Allele-specific control of mutagenesis. Genetics. 1979 Oct;93(2):375–381. doi: 10.1093/genetics/93.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. L., Cardillo T. S., Sherman F. An extensive deletion causing overproduction of yeast iso-2-cytochrome c. Cell. 1981 Aug;25(2):409–419. doi: 10.1016/0092-8674(81)90059-3. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jurnak F. A., Wang A. H., Molineux I., Rich A. Structure at 2.3 A resolution of the gene 5 product of bacteriophage fd: a DNA unwinding protein. J Mol Biol. 1979 Nov 5;134(3):379–400. doi: 10.1016/0022-2836(79)90359-0. [DOI] [PubMed] [Google Scholar]
- Meechan P. J., Milam K. M., Cleaver J. E. Evaluation of homology between cloned Escherichia coli and yeast DNA photolyase genes and higher eukaryotic genomes. Mutat Res. 1986 Sep;166(2):143–147. doi: 10.1016/0167-8817(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Prakash L., Prakash S. Defective excision of pyrimidine dimers and interstrand DNA crosslinks in rad7 and rad23 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1982;188(2):235–239. doi: 10.1007/BF00332681. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Prakash L., Prakash S. Genetic control of excision of Saccharomyces cerevisiae interstrand DNA cross-links induced by psoralen plus near-UV light. Mol Cell Biol. 1982 Aug;2(8):939–948. doi: 10.1128/mcb.2.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Prakash S., Prakash L. Different effects of RAD genes of Saccharomyces cerevisiae on incisions of interstrand crosslinks and monoadducts in DNA induced by psoralen plus near UV light treatment. Photochem Photobiol. 1984 Mar;39(3):349–352. doi: 10.1111/j.1751-1097.1984.tb08189.x. [DOI] [PubMed] [Google Scholar]
- Morrison D. P., Hastings P. J. Characterization of the mutator mutation mut5-1. Mol Gen Genet. 1979 Aug;175(1):57–65. doi: 10.1007/BF00267856. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Contopoulou R., Schild D. Mitotic chromosome loss in a radiation-sensitive strain of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5778–5782. doi: 10.1073/pnas.78.9.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustacchi E. Cytoplasmic and nuclear genetic events induced by UV light in strains of Saccharomyces cerevisiae with different UV sensitivities. Mutat Res. 1969 Mar-Apr;7(2):171–185. doi: 10.1016/0027-5107(69)90029-3. [DOI] [PubMed] [Google Scholar]
- Muster-Nassal C., Kolodner R. Mismatch correction catalyzed by cell-free extracts of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7618–7622. doi: 10.1073/pnas.83.20.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal M. L., Higgins D. R., Prakash S. Expression of the RAD1 and RAD3 genes of Saccharomyces cerevisiae is not affected by DNA damage or during the cell division cycle. Mol Gen Genet. 1985;199(1):59–63. doi: 10.1007/BF00327510. [DOI] [PubMed] [Google Scholar]
- Nakai S., Matsumoto S. Two types of radiation-sensitive mutant in yeast. Mutat Res. 1967 Mar-Apr;4(2):129–136. doi: 10.1016/0027-5107(67)90064-4. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Chu G., Berg P., Friedberg E. C. RAD3 gene of Saccharomyces cerevisiae: nucleotide sequence of wild-type and mutant alleles, transcript mapping, and aspects of gene regulation. Mol Cell Biol. 1985 Jan;5(1):17–26. doi: 10.1128/mcb.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. A DNA repair gene required for the incision of damaged DNA is essential for viability in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4818–4821. doi: 10.1073/pnas.80.15.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Analysis of the essential and excision repair functions of the RAD3 gene of Saccharomyces cerevisiae by mutagenesis. Mol Cell Biol. 1986 Apr;6(4):1218–1227. doi: 10.1128/mcb.6.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Molecular cloning of eucaryotic genes required for excision repair of UV-irradiated DNA: isolation and partial characterization of the RAD3 gene of Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):323–331. doi: 10.1128/jb.152.1.323-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Saccharomyces cerevisiae RAD2 gene: isolation, subcloning, and partial characterization. Mol Cell Biol. 1984 Feb;4(2):290–295. doi: 10.1128/mcb.4.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. The RAD3 gene of Saccharomyces cerevisiae: isolation and characterization of a temperature-sensitive mutant in the essential function and of extragenic suppressors of this mutant. Mol Gen Genet. 1987 Oct;209(3):458–466. doi: 10.1007/BF00331150. [DOI] [PubMed] [Google Scholar]
- Nicolet C. M., Chenevert J. M., Friedberg E. C. The RAD2 gene of Saccharomyces cerevisiae: nucleotide sequence and transcript mapping. Gene. 1985;36(3):225–234. doi: 10.1016/0378-1119(85)90177-5. [DOI] [PubMed] [Google Scholar]
- Nicolet C. M., Friedberg E. C. Overexpression of the RAD2 gene of S. cerevisiae: identification and preliminary characterization of Rad2 protein. Yeast. 1987 Sep;3(3):149–160. doi: 10.1002/yea.320030303. [DOI] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- PATRICK M. H., HAYNES R. H. DARK RECOVERY PHENOMENA IN YEAST. II. CONDITIONS THAT MODIFY THE RECOVERY PROCESS. Radiat Res. 1964 Dec;23:564–579. [PubMed] [Google Scholar]
- Parry J. M., Parry E. M. The effects of UV-light post-treatments on the survival characteristics of 21 UV-sensitive mutants of Saccharomyces cerevisiae. Mutat Res. 1969 Nov-Dec;8(3):545–557. doi: 10.1016/0027-5107(69)90072-4. [DOI] [PubMed] [Google Scholar]
- Parsons B. J. Psoralen photochemistry. Photochem Photobiol. 1980 Dec;32(6):813–821. doi: 10.1111/j.1751-1097.1980.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Patrick M. H., Haynes R. H. Repair-induced changes in yeast radiosensitivity. J Bacteriol. 1968 Apr;95(4):1350–1354. doi: 10.1128/jb.95.4.1350-1354.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozzi G., Prakash S. RAD7 gene of Saccharomyces cerevisiae: transcripts, nucleotide sequence analysis, and functional relationship between the RAD7 and RAD23 gene products. Mol Cell Biol. 1986 May;6(5):1497–1507. doi: 10.1128/mcb.6.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L. Defective thymine dimer excision in radiation-sensitive mutants rad10 and rad16 of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Apr 29;152(3):125–128. doi: 10.1007/BF00268808. [DOI] [PubMed] [Google Scholar]
- Prakash L., Dumais D., Polakowska R., Perozzi G., Prakash S. Molecular cloning of the RAD10 gene of Saccharomyces cerevisiae. Gene. 1985;34(1):55–61. doi: 10.1016/0378-1119(85)90294-x. [DOI] [PubMed] [Google Scholar]
- Prakash L., Hinkle D., Prakash S. Decreased UV mutagenesis in cdc8, a DNA replication mutant of Saccharomyces cerevisiae. Mol Gen Genet. 1979;172(3):249–258. doi: 10.1007/BF00271724. [DOI] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 May;86(1):33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Three additional genes involved in pyrimidine dimer removal in Saccharomyces cerevisiae: RAD7, RAD14 and MMS19. Mol Gen Genet. 1979 Nov;176(3):351–359. doi: 10.1007/BF00333097. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in radiation-sensitive mutants rad3, rad4, rad6 and rad9 of Saccharomyces cerevisiae. Mutat Res. 1977 Oct;45(1):13–20. doi: 10.1016/0027-5107(77)90038-0. [DOI] [PubMed] [Google Scholar]
- Prakash L. The relation between repair of DNA and radiation and chemical mutagenesis in Saccharomyces cerevisiae. Mutat Res. 1976 Dec;41(2-3):241–248. doi: 10.1016/0027-5107(76)90097-x. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure G. A., Robinson G. W., Naumovski L., Friedberg E. C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J Mol Biol. 1985 May 5;183(1):31–42. doi: 10.1016/0022-2836(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Quah S. K., von Borstel R. C., Hastings P. J. The origin of spontaneous mutation in Saccharomyces cerevisiae. Genetics. 1980 Dec;96(4):819–839. doi: 10.1093/genetics/96.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPERT C. S. Photoreactivation of transforming DNA by an enzyme from bakers' yeast. J Gen Physiol. 1960 Jan;43:573–595. doi: 10.1085/jgp.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. P., Mathews C. K. Functional compartmentation of DNA precursors in T4 phage-infected bacteria. J Biol Chem. 1978 May 25;253(10):3461–3467. [PubMed] [Google Scholar]
- Resnick M. A. A photoreactivationless mutant of Saccharomyces cerevisiae. Photochem Photobiol. 1969 Apr;9(4):307–312. doi: 10.1111/j.1751-1097.1969.tb07294.x. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Chow T., Nitiss J., Game J. Changes in the chromosomal DNA of yeast during meiosis in repair mutants and the possible role of a deoxyribonuclease. Cold Spring Harb Symp Quant Biol. 1984;49:639–649. doi: 10.1101/sqb.1984.049.01.072. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics. 1969 Jul;62(3):519–531. doi: 10.1093/genetics/62.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Setlow J. K. Photoreactivation and gene dosage in yeast. J Bacteriol. 1972 Mar;109(3):1307–1309. doi: 10.1128/jb.109.3.1307-1309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Setlow J. K. Repair of pyrimidine dimer damage induced in yeast by ultraviolet light. J Bacteriol. 1972 Mar;109(3):979–986. doi: 10.1128/jb.109.3.979-986.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Sugino A., Nitiss J., Chow T. DNA polymerases, deoxyribonucleases, and recombination during meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2811–2817. doi: 10.1128/mcb.4.12.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Higgins D. R., Prakash L., Prakash S. The nucleotide sequence of the RAD3 gene of Saccharomyces cerevisiae: a potential adenine nucleotide binding amino acid sequence and a nonessential acidic carboxyl terminal region. Nucleic Acids Res. 1985 Apr 11;13(7):2357–2372. doi: 10.1093/nar/13.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Prakash L., Prakash S. Nucleotide sequence and functional analysis of the RAD1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Mar;7(3):1012–1020. doi: 10.1128/mcb.7.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P., Weber S., Prakash L. RAD6 gene of Saccharomyces cerevisiae encodes a protein containing a tract of 13 consecutive aspartates. Proc Natl Acad Sci U S A. 1985 Jan;82(1):168–172. doi: 10.1073/pnas.82.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanism of pyrimidine dimer excision in Saccharomyces cerevisiae. I. Studies with intact cells and cell-free systems. Basic Life Sci. 1980;15:121–139. doi: 10.1007/978-1-4684-3842-0_8. [DOI] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J., Love J. D., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: excision of dimers in cell extracts. J Bacteriol. 1981 Aug;147(2):705–708. doi: 10.1128/jb.147.2.705-708.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J. Removal of pyrimidine dimers from Saccharomyces cerevisiae nuclear DNA under nongrowth conditions as detected by a sensitive, enzymatic assay. Mutat Res. 1978 Apr;50(1):43–56. doi: 10.1016/0027-5107(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Robinson G. W., Nicolet C. M., Kalainov D., Friedberg E. C. A yeast excision-repair gene is inducible by DNA damaging agents. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1842–1846. doi: 10.1073/pnas.83.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodarte-Ramón U. S., Mortimer R. K. Radiation-induced recombination in Saccharomyces: isolation and genetic study of recombination-deficient mutants. Radiat Res. 1972 Jan;49(1):133–147. [PubMed] [Google Scholar]
- Roeder G. S., Beard C., Smith M., Keranen S. Isolation and characterization of the SPT2 gene, a negative regulator of Ty-controlled yeast gene expression. Mol Cell Biol. 1985 Jul;5(7):1543–1553. doi: 10.1128/mcb.5.7.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W., Murray A. W. Cloning regulated yeast genes from a pool of lacZ fusions. Methods Enzymol. 1983;101:253–269. doi: 10.1016/0076-6879(83)01019-8. [DOI] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W. Specific Saccharomyces cerevisiae genes are expressed in response to DNA-damaging agents. Mol Cell Biol. 1985 Jan;5(1):75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland A., Haase E., Siede W., Brendel M. Isolation of yeast mutants sensitive to the bifunctional alkylating agent nitrogen mustard. Mol Gen Genet. 1981;181(3):346–351. doi: 10.1007/BF00425609. [DOI] [PubMed] [Google Scholar]
- Ruhland A., Kircher M., Wilborn F., Brendel M. A yeast mutant specifically sensitive to bifunctional alkylation. Mutat Res. 1981 Nov;91(6):457–462. doi: 10.1016/0165-7992(81)90052-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol. 1984 Jan 15;172(2):223–227. doi: 10.1016/s0022-2836(84)80040-6. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G. B. Expression of a Saccharomyces cerevisiae photolyase gene in Escherichia coli. J Bacteriol. 1985 Feb;161(2):769–771. doi: 10.1128/jb.161.2.769-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G. B. Sequence of the Saccharomyces cerevisiae PHR1 gene and homology of the PHR1 photolyase to E. coli photolyase. Nucleic Acids Res. 1985 Nov 25;13(22):8231–8246. doi: 10.1093/nar/13.22.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Johnston J., Chang C., Mortimer R. K. Cloning and mapping of Saccharomyces cerevisiae photoreactivation gene PHR1. Mol Cell Biol. 1984 Sep;4(9):1864–1870. doi: 10.1128/mcb.4.9.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Tuohy T. M., Mosurski K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986 Jul 11;14(13):5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede W., Eckardt-Schupp F. A mismatch repair-based model can explain some features of u.v. mutagenesis in yeast. Mutagenesis. 1986 Nov;1(6):471–474. doi: 10.1093/mutage/1.6.471. [DOI] [PubMed] [Google Scholar]
- Siede W., Eckardt-Schupp F. DNA repair genes of Saccharomyces cerevisiae: complementing rad4 and rev2 mutations by plasmids which cannot be propagated in Escherichia coli. Curr Genet. 1986;11(3):205–210. doi: 10.1007/BF00420608. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Rose M., Botstein D., Fink G. R. Regulation of HIS4-lacZ fusions in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1212–1219. doi: 10.1128/mcb.2.10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Snow R. Mutants of yeast sensitive to ultraviolet light. J Bacteriol. 1967 Sep;94(3):571–575. doi: 10.1128/jb.94.3.571-575.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Ryu B. H., Sugino T., Naumovski L., Friedberg E. C. A new DNA-dependent ATPase which stimulates yeast DNA polymerase I and has DNA-unwinding activity. J Biol Chem. 1986 Sep 5;261(25):11744–11750. [PubMed] [Google Scholar]
- Sung P., Prakash L., Weber S., Prakash S. The RAD3 gene of Saccharomyces cerevisiae encodes a DNA-dependent ATPase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6045–6049. doi: 10.1073/pnas.84.17.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Collins C. C., Stewart S. A., Busch D. B., Weber C. A. Recent progress with the DNA repair mutants of Chinese hamster ovary cells. J Cell Sci Suppl. 1987;6:97–110. doi: 10.1242/jcs.1984.supplement_6.6. [DOI] [PubMed] [Google Scholar]
- Thøgersen H. C., Morris H. R., Rand K. N., Gait M. J. Location of the adenylylation site in T4 RNA ligase. Eur J Biochem. 1985 Mar 1;147(2):325–329. doi: 10.1111/j.1432-1033.1985.tb08753.x. [DOI] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B. S. The excision of pyrimidine dimers from DNA of ultraviolet irradiated yeast. Mol Gen Genet. 1971;113(4):359–362. doi: 10.1007/BF00272336. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Sancar A. Repair of N-methyl-N'-nitro-N-nitrosoguanidine-induced DNA damage by ABC excinuclease. J Bacteriol. 1987 Feb;169(2):540–545. doi: 10.1128/jb.169.2.540-545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Marsh L., Dodson L. A. Genetic analyses of DNA repair: inference and extrapolation. Annu Rev Genet. 1985;19:103–126. doi: 10.1146/annurev.ge.19.120185.000535. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The disappearance of ultraviolet-induced pyrimidine dimers from the nuclear DNA of exponential and stationary phase cells of Saccharomyces cerevisiae following various post-irradiation treatments. Biochim Biophys Acta. 1974 Jul 24;353(4):407–419. doi: 10.1016/0005-2787(74)90048-3. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The fate of ultraviolet-induced pyrimidine dimers in the mitochondrial DNA of Saccharomyces cerevisiae following various post-irradiation cell treatments. Biochim Biophys Acta. 1974 Oct 28;366(3):241–250. doi: 10.1016/0005-2787(74)90282-2. [DOI] [PubMed] [Google Scholar]
- Weiss B., Grossman L. Phosphodiesterases involved in DNA repair. Adv Enzymol Relat Areas Mol Biol. 1987;60:1–34. doi: 10.1002/9780470123065.ch1. [DOI] [PubMed] [Google Scholar]
- Weiss W. A., Edelman I., Culbertson M. R., Friedberg E. C. Physiological levels of normal tRNA(CAGGln) can effect partial suppression of amber mutations in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8031–8034. doi: 10.1073/pnas.84.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Molecular cloning and characterization of the yeast RAD10 gene and expression of RAD10 protein in E. coli. EMBO J. 1985 Jun;4(6):1575–1582. doi: 10.1002/j.1460-2075.1985.tb03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Normal yeast tRNA(CAGGln) can suppress amber codons and is encoded by an essential gene. J Mol Biol. 1986 Dec 20;192(4):725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- White C. I., Sedgwick S. G. Repair of UV-irradiated plasmid DNA in Saccharomyces cerevisiae. Inability to complement mutational defects in excision repair by in vitro treatment with Micrococcus luteus UV endonuclease. Mutat Res. 1987 Mar;183(2):161–167. doi: 10.1016/0167-8817(87)90058-7. [DOI] [PubMed] [Google Scholar]
- White C. I., Sedgwick S. G. The use of plasmid DNA to probe DNA repair functions in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1985;201(1):99–106. doi: 10.1007/BF00397993. [DOI] [PubMed] [Google Scholar]
- White J. H., Barker D. G., Nurse P., Johnston L. H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986 Jul;5(7):1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Green S. R., Barker D. G., Dumas L. B., Johnston L. H. The CDC8 transcript is cell cycle regulated in yeast and is expressed coordinately with CDC9 and CDC21 at a point preceding histone transcription. Exp Cell Res. 1987 Jul;171(1):223–231. doi: 10.1016/0014-4827(87)90265-5. [DOI] [PubMed] [Google Scholar]
- White J. H., Lusnak K., Fogel S. Mismatch-specific post-meiotic segregation frequency in yeast suggests a heteroduplex recombination intermediate. Nature. 1985 May 23;315(6017):350–352. doi: 10.1038/315350a0. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox D. R., Prakash L. Incision and postincision steps of pyrimidine dimer removal in excision-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1981 Nov;148(2):618–623. doi: 10.1128/jb.148.2.618-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M. S., Game J. C., Fogel S. Meiotic gene conversion mutants in Saccharomyces cerevisiae. I. Isolation and characterization of pms1-1 and pms1-2. Genetics. 1985 Aug;110(4):609–646. doi: 10.1093/genetics/110.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger U., Karwan A. Retardation of cell cycle progression in yeast cells recovering from DNA damage: a study at the single cell level. Mol Gen Genet. 1987 May;207(2-3):320–327. doi: 10.1007/BF00331596. [DOI] [PubMed] [Google Scholar]
- Yang E., Friedberg E. C. Molecular cloning and nucleotide sequence analysis of the Saccharomyces cerevisiae RAD1 gene. Mol Cell Biol. 1984 Oct;4(10):2161–2169. doi: 10.1128/mcb.4.10.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui A., Langeveld S. A. Homology between the photoreactivation genes of Saccharomyces cerevisiae and Escherichia coli. Gene. 1985;36(3):349–355. doi: 10.1016/0378-1119(85)90190-8. [DOI] [PubMed] [Google Scholar]
- Zakharov I. A., Kozina T. N., Fedorova I. V. Effets de mutations vers la sensibilité au rayonnement ultraviolet chez la levure. Mutat Res. 1970 Jan;9(1):31–39. doi: 10.1016/0027-5107(70)90068-0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- von Borstel R. C., Hastings P. J. Situation-dependent repair of DNA damage in yeast. Basic Life Sci. 1985;34:121–145. doi: 10.1007/978-1-4684-4976-1_11. [DOI] [PubMed] [Google Scholar]



