Abstract
Using patch-clamp techniques, we studied the plasticity of acid-sensing ion channels (ASIC) and transient receptor potential V1 (TRPV1) channel function in dorsal root ganglia (DRG) neurons retrogradely labeled from the bladder. Saline (control) or cyclophosphamide (CYP) was given intraperitoneally on days 1, 3, and 5. On day 6, lumbosacral (LS, L6–S2) or thoracolumbar (TL, T13–L2) DRG were removed and dissociated. Bladders and bladder DRG neurons from CYP-treated rats showed signs of inflammation (greater myeloperoxidase activity; lower intramuscular wall pH) and increased size (whole cell capacitance), respectively, compared with controls. Most bladder neurons (>90%) responded to protons and capsaicin. Protons produced multiphasic currents with distinct kinetics, whereas capsaicin always triggered a sustained response. The TRPV1 receptor antagonist A-425619 abolished capsaicin-triggered currents and raised the threshold of heat-activated currents. Prolonged exposure to an acidic environment (pH range: 7.2 to 6.6) inhibited proton-evoked currents, potentiated the capsaicin-evoked current, and reduced the threshold of heat-activated currents in LS and TL bladder neurons. CYP treatment reduced density but not kinetics of all current components triggered by pH 5. In contrast, CYP-treatment was associated with an increased current density in response to capsaicin in LS and TL bladder neurons. Correspondingly, heat triggered current at a significantly lower temperature in bladder neurons from CYP-treated rats compared with controls. These results reveal that cystitis differentially affects TRPV1- and ASIC-mediated currents in both bladder sensory pathways. Acidification of the bladder wall during inflammation may contribute to changes in nociceptive transmission mediated through the TRPV1 receptor, suggesting a role for TRPV1 in hypersensitivity associated with cystitis.
Keywords: bladder sensory neurons, ASIC, TRPV1 receptors, visceral hypersensitivity, cystitis, cyclophosphamide, capsaicin, proton, acid, pH, whole cell patch clamp
the prevalence of painful bladder syndrome/interstitial cystitis (PBS/IC) is estimated at ∼11% in women and 4.6% in men in managed care settings (Clemens et al. 2005, 2007). In addition to increased urinary frequency, urgency, and incontinence, PBS/IC patients also report significant discomfort and pain (Burkman 2004; Clemens et al. 2005, 2007; Nickel 2004), which often has a burning character (Ness et al. 2005). Although the etiology of PBS/IC remains unknown, the bladder of many PBS/IC patients shows evidence of inflammation, including edema, vasodilation, and infiltration of mast cells (Burkman 2004; Nickel 2004).
The bladder is innervated by the pelvic and hypogastric/lumbar splanchnic nerves with cell bodies in lumbosacral (LS, L6–S2) and thoracolumbar (TL, T13–L2) dorsal root ganglia (DRG), respectively. The relative roles of these pathways are not well established. The pelvic nerve plays a role in the sensation of bladder distension and micturition, because bilateral resection of the pelvic nerve abolishes bladder contractions induced by repetitive filling (Kontani and Hayashi 1997; Meen et al. 2001), whereas mechanical and chemical stimulation of the bladder also activate the hypogastric/lumbar splanchnic nerve, which may contribute to regulation of micturition and painful sensations after bladder irritation/damage (Meen et al. 2001; Mitsui et al. 2001; Moss et al. 1997; Xu and Gebhart 2008). Based on functional studies and differential expression of receptors/receptive fields, a growing body of evidence suggests that different nerves innervating a visceral organ mediate different functions (Brierley et al. 2004; Dang et al. 2005a; Lamb et al. 2003; Xu and Gebhart 2008). Consistent with this notion, bladder hypogastric/lumbar splanchnic afferents have been reported to respond more vigorously to chemical stimuli than do their pelvic counterparts (Moss et al. 1997).
Changes in sensory input from the bladder contribute to the development and maintenance of IC (Ness et al. 2005). The transient receptor potential V1 receptor (TRPV1) is considered important in temperature sensation (Caterina et al. 1997, 1998, 2000; Tominaga et al. 1998). Capsaicin, a TRPV1 agonist, produces a burning sensation when injected intradermally in humans and an inward current in sensory neurons, including bladder sensory neurons and urothelial cells, which play a role in sensory function (Birder 2006; Caterina et al. 1997, 1998, 2000; Charrua et al. 2009; Dang et al. 2005a; Tominaga et al. 1998); these effects of capsaicin are blunted by TRPV1 receptor antagonists. Furthermore, TRPV1 receptors play an important role in reflex bladder contractions in animals with an inflamed bladder and can be blunted by TRPV1 antagonists (Dinis et al. 2004). Intravesical administration of capsaicin and resiniferatoxin, a potent TRPV1 agonist, significantly reduces the expression of TRPV1 in the bladder and has been evaluated for the treatment of bladder hyperreflexia and PBS/IC (Avelino et al. 2002; Cruz et al. 1997; De Ridder et al. 1997; Matsuoka et al. 2012). Intradermal iontophoresis of acid, another activator of TRPV1 and other proton-gated channels, also produces pain that is attenuated by amiloride, which inhibits ASIC channels (Jones et al. 2004). Thus two different ligand-gated channels may contribute to nociceptive input during inflammation or ischemia, both of which are associated with tissue acidification.
Cystitis enhances excitability, increases P2X receptor-mediated currents, and induces expression of P2X3 receptors in bladder sensory neurons (Chen and Gebhart 2010; Dang et al. 2008; Yoshimura and de Groat, 1999). We hypothesized that changes in the sensitivity of bladder sensory neurons to endogenous acid-sensing ion channels (ASIC) and/or TRPV1 receptor agonists contribute to bladder dysfunction, discomfort, and pain. Using a well-established rat model of urinary bladder inflammation (Bon et al. 1997, 2003; Lanteri-Minet et al. 1995), we thus examined the effects of bladder inflammation on ASIC and TRPV1 currents in TL and LS bladder neurons.
MATERIALS AND METHODS
Male Sprague-Dawley rats (200–300 g; Harlan, Indianapolis, IN) were used throughout. Rats were housed under a 12:12-h light-dark cycle with free access to food and water. All experimental protocols were approved by the Animal Care and Use Committee of The University of Iowa.
Bladder Inflammation, Myeloperoxidase Activity, and pH Measurements
Systemic administration of cyclophosphamide (CYP), which is metabolized to the bladder irritant acrolein (Cox 1979), causes hemorrhagic cystitis in humans and produces a cystitis-like condition in rodents (Bon et al. 1997; Dang et al. 2008; Lanteri-Minet et al. 1995). Saline (control) or CYP (100 mg/kg) was administered systemically (intraperitoneally, ip) on days 1, 3, and 5. To assess inflammation, myeloperoxidase (MPO) activity in bladder and distal colon tissue was measured in a subgroup of rats on day 6 (n = 4/group). Rats were euthanized with pentobarbital (200 mg/kg ip), and the bladders and 1 cm of distal colon were removed rapidly, minced, homogenized in ice-cold 50 mM phosphate buffer (pH 6) containing 0.5% hexadecyltrimethylammonium bromide, centrifuged at 1,000 rpm for 5 min, and the supernatant retained. In the presence of hydrogen peroxide (30%) and o-dianisidine dihydrochloride (0.5%), the absorbance of the supernatant was determined with a U2001 photometric reader (Hitachi, Naperville, IL). To examine specificity of CYP-induced cystitis, we also measured MPO activity of descending colon (1 cm) from the same rats.
In a separate series of experiments, the effect of inflammation on bladder weight and tissue pH was assessed. On day 6, rats were anesthetized (pentobarbital, 50 mg/kg ip), the bladder was exposed by an abdominal incision, and pH was measured by inserting a needle electrode (20 gauge, Orion pH meter; Fisher Scientific, Corvallis, OR) into the bladder wall and advancing it stepwise into the bladder lumen (urine), allowing 1 min for stabilization at each step. The damaged urothelium made it more difficult to reliably determine the extent of penetration of the pH electrode into the bladder wall in CYP-treated rats. To confirm appropriate electrode placement during measurement of bladder wall pH, we withdrew the electrode following determination of bladder wall pH, applied ∼1 mg of Evans blue onto the penetration site, and gently compressed the bladder to rule out a small leak. We then removed the bladder and measured its wet weight after emptying the contents. Euthanasia was accomplished by exsanguination after removal of the bladder.
Bladder Neuron Labeling
Under pentobarbital anesthesia (50 mg/kg ip), the bladder was surgically exposed (lower abdominal incision ∼1 cm in length) and 1.1′-dioctadecyl-3,3,3,′3-tetramethyl indocarbocyanine methanesulfonate [DiI(18); 100 mg in 2 ml of DMSO; Molecular Probes, Eugene, OR] was injected into 6–8 sites within the wall of the bladder and around the trigone using a 30-gauge needle (∼6 μl per site). Any visible leakage of DiI from the injection site was removed with a cotton swab. The incision was closed with 4.0 silk suture, and rats were allowed to recover. Two-three weeks later, saline (control) or CYP (100 mg/kg) was administered systemically (ip) on days 1, 3, and 5 as described above.
Cell Dissociation and Plating
For patch-clamp experiments, rats were anesthetized on day 6 (pentobarbital, 50 mg/kg ip) and lumbosacral (LS, L6–S2) or thoracolumbar (TL, T13–L2) DRG were harvested for acute dissociation and whole cell recordings. The general protocols have been previously described (Dang et al. 2005a, 2005b). Briefly, after removal, ganglia were minced and incubated at 37°C, 5% CO2 for 60 min in serum-free, supplemented Neuro-A medium (B27 supplements, 5%; l-glutamine, 0.5 mM; penicillin-streptomycin mixture, 1%; all from Gibco, Invitrogen, Grand Island, NY) containing collagenase (type 4; 2 mg/ml), and trypsin (1 mg/ml; both from Worthington Biochemical, Lakewood, NJ). Tissue fragments were gently triturated to encourage cell dissociation. Cells were collected by 5 min of centrifugation at 150 g, washed 3 times with supplemented Neuro-A medium (without enzymes), and resuspended in supplemented, enzyme free Neuro-A medium. The cells were plated on poly-d-lysine-coated coverslips (Becton Dickinson Labware, Bedford, MA) and incubated at 37°C, 5% CO2 for 2–3 h before electrophysiological studies. Acutely dissociated neurons were round and devoid of any processes, thus reducing potential space-clamp errors. Only bladder sensory neurons (i.e., DiI-containing DRG neurons) were studied. All recordings were performed within 10 h after plating.
Solutions and Electrophysiological Recordings
Coverslips with cells were transferred to a recording chamber (1 ml) superfused continuously (2 ml/min) with external solution containing (in mM) 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with NaOH (310 mosM). Under low magnification (×50), bladder neurons were identified by DiI content using a rhodamine filter (bright orange/red color under UV light; excitation wavelength, 530–560 nm; and barrier filter, 573–648 nm). Fire-polished micropipettes with tip resistances of 1.5–2 MΩ were used for whole cell recordings. The uncompensated series resistance was generally about 7 MΩ or less. The pipette was filled with an internal solution consisting of (in mM) 130 KCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 HEPES, 4 Na2ATP, 0.5 Tris-GTP, and 0.5 GDP. The pH was adjusted to 7.2 using KOH (310 mosM). After the whole cell configuration was established, the voltage was clamped at −70 mV using an Axopatch 200B amplifier (Axon Instruments, Sunnyvale, CA), digitized at 1 kHz (Digidata 1350; Axon Instruments) and controlled by Clampex software (pClamp 9; Axon Instruments). Disruption of the neuron membrane during DRG dissociation and the use of patch-clamp recordings are unavoidable. We chose whole cell capacitance as a well-established surrogate marker of size to minimize additional confounders due to distortion in neuron shape. Cell capacitance was obtained by reading the value from the Axopatch 200B amplifier. Recordings began 2–3 min after whole cell configuration was established, to ensure stable recording conditions.
Drugs were applied using a fast-step SF-77B superfusion system (Warner Instruments, Hamden, CT) with a three-barrel pipette placed in close proximity (100 μm) to the cell, allowing complete solution exchange within 25 ms (Dang et al. 2005a). Agonists were applied for 4 s, whereas antagonists were superfused for 60 s before the application of agonists. A washout period of 2 min was allowed between agonist applications. Unless otherwise stated, drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and prepared fresh from stock solutions on the day of the experiment. All experiments were performed at room temperature (21–23°C). We used current increases above 20 pA as threshold to identify responses to agonists, because this level exceeded the baseline variability by a factor of 2.
Heat Application
After whole cell configuration was established and voltage was clamped at −70 mV, preheated external solution (∼80°C; same composition as above) was superfused for 30 s. We placed a temperature-sensitive microelectrode ∼100 μm in front of the recording cell (Physitemp, Clifton, NJ). Heat threshold (°C) was defined as the temperature when the line fitted to the heat-induced current intercepts the X-axis (time, s). Only one cell per coverslip was studied with a single application of heat to avoid complications that may result from repeated exposure to high temperatures.
Data Analyses
Data are means ± SE. Analyses were performed using the software Clampfit (Axon Instruments) and GraphPad Prism 4 (GraphPad Software, San Diego, CA). Sigmoidal concentration-response curves were generated using the following equation: Y = A/{1 + exp[−(log EC50 − X)/B]}, where X is the logarithm of concentration and Y is the response, which starts at 0 and goes to A with a sigmoidal shape. B is the Hill slope, Imax is the maximum current, EC50 is the concentration of agonist that evokes half-maximal response, and pH50 is the logarithm of EC50 of protons. To determine the kinetics of response onset, we measured the rise time, which is the time required for a signal to change from 10 to 90% of the peak amplitude. Desensitization kinetics were fitted with a standard exponential equation: Y = K0 + K1·exp(−t/τ), where Y is the current amplitude at time t, K0 is the amplitude of the nondesensitizing component, K0 + K1 is the peak current, and τ is the time constant. K0 and K1 represent the contribution to current amplitude from the fast and slow components of the current, respectively. For dichotomous variables, a χ2 test was employed. Unless otherwise indicated, results were evaluated using Wetch's t-test after logarithmic transformation. One-way ANOVA were employed to compare and confirm variables (e.g., pH50 values and desensitization constants of acid-evoked currents) in LS or TL neurons from saline-treated rats. Results were considered to be statistically significant when P < 0.05.
RESULTS
CYP Produces Bladder Damage and Inflammation (Cystitis)
Consistent with a previous study (Dang et al. 2008), the weight of bladders from CYP-treated rats was significantly greater than that from saline-treated rats (saline: 95 ± 4.8 g, n = 12; CYP: 273 ± 8.5 g, n = 12; P < 0.001). CYP treatment significantly and selectively increased bladder MPO activity relative to controls (saline: 0.36 ± 0.05 unit/g, n = 4; CYP: 1.0 ± 0.29 unit/g, n = 4; P < 0.001); colon MPO activity was not affected by systemic administration of CYP (saline: 0.63 ± 0.06 unit/g, n = 4; CYP: 0.56 ± 0.07 unit/g, n = 4; P > 0.05).
Cystitis Reduces pH of the Bladder Wall and Increases Size of Bladder Sensory Neurons
Although urine pH (36–37°C) from saline- and CYP-treated rats did not differ (saline: 6.3 ± 0.09, n = 12; CYP: 6.4 ± 0.07, n = 12; P > 0.05), pH of the bladder wall was significantly reduced in CYP-treated rats compared with controls (saline: 7.3 ± 0.03, n = 12; CYP: 7.0 ± 0.03, n = 12; P < 0.01). Based on whole cell capacitance, bladder inflammation significantly increased the size of bladder sensory neurons (saline LS: 28.6 ± 1.6 pF, n = 60; saline TL: 49.3 ± 1.2 pF, n = 53; CYP LS: 36.3 ± 1.2 pF, n = 62; CYP TL: 57.6 ± 2.3 pF, n = 57; P < 0.05).
Cystitis Alters Acid- and Capsaicin-Produced Currents in Bladder Neurons
We previously described the effects of acid and capsaicin on rat bladder sensory neurons (Dang et al. 2005a). In bladder neurons from saline-treated rats, we found in the present study that the proportions of responsive TL and LS neurons, peak current density, and kinetics in response to acid and capsaicin were similar to those we previously reported (Dang et al. 2005a), as summarized in Tables 1–4. Briefly, almost all LS and TL bladder neurons responded to acid (pH 5) with mixed current kinetics (a mixture of fast, intermediate, and/or sustained components), and all acid-sensitive bladder neurons responded to capsaicin (1 μM) with a sustained current only. On the basis of complete inhibition of acid-evoked currents by amiloride, an ASIC channel blocker, we concluded that transient currents triggered by acid were mediated by ASICs (Dang et al. 2005a). Capsaizepine and amiloride partially antagonized the sustained component of acid-triggered currents, suggesting that this component was mediated, in part, by TRPV1 receptors (Dang et al. 2005a). A-425619 (100 nM, 60 s), a competitive TRPV1 receptor antagonist (El Kouhen et al. 2005), abolished current triggered by capsaicin (100 nM), confirming involvement of TRPV1 (data not shown).
Table 1.
Number of bladder sensory neurons responding to protons and capsaicin and type of current produced
| Sal LS |
Sal TL |
CYP LS |
CYP TL |
|||||
|---|---|---|---|---|---|---|---|---|
| pH 5 | Capsaicin | pH 5 | Capsaicin | pH 5 | Capsaicin | pH 5 | Capsaicin | |
| No. of responders | 55/60(91.7) | 55/60(91.7) | 48/53(90.6) | 49/53(92.5) | 62/62(100) | 61/62(98.4) | 52/57(91.2) | 57/57(100) |
| Biphasic current with fast and intermediate components | 15/55(27.3) | 17/48(35.4) | 16/62(25.8) | 17/52(32.7) | ||||
| Slow current | 14/55(25.5) | 16/48(33.3) | 19/62(30.6) | 7/52*(13.5) | ||||
| Triphasic current with fast, intermediate, and sustained components | 26/55(47.2) | 15/48(31.3) | 27/62(43.5) | 28/52*(53.8) | ||||
Values are no. of lumbosacral (LS) and thoracolumbar (TL) bladder sensory neurons from saline (Sal; control)- and cyclophosphamide (CYP)-treated rats that responded to application of protons (pH 5) and capsaicin, with percentage of neurons (in parentheses) expressing biphasic, slow only, and triphasic currents in response to pH 5.
P < 0.05 vs. saline-treated counterparts.
Table 2.
Current density in response to protons or capsaicin in bladder sensory neurons
| Current Density, pA/pF |
||||
|---|---|---|---|---|
| Sal LS | Sal TL | CYP LS | CYP TL | |
| Fast | 55.4 ± 8.3 (41) | 58.2 ± 9.0 (32) | 33.6 ± 7.5* (43) | 32.2 ± 4.3* (45) |
| Intermediate | 64.4 ± 10.3 (41) | 54.8 ± 11.1 (32) | 41.9 ± 5.2* (43) | 32.9 ± 4.2* (45) |
| Slow | 55.8 ± 10.3 (14) | 64.5 ± 7.3 (16) | 29.6 ± 9.0* (19) | 36.5 ± 9.2* (7) |
| Sustained | 91.6 ± 12.9 (26) | 32.8 ± 7.1 (15) | 49.6 ± 9.4* (27) | 14.2 ± 3.9* (28) |
| Capsaicin | 142.4 ± 19.1 (55) | 89.6 ± 11.2 (49) | 258.2 ± 21.0* (61) | 254.6 ± 15.2* (57) |
Values are means ± SE of current density for various components triggered by application of pH 5 or capsaicin in LS and TL bladder sensory neurons taken from saline (control)- and CYP-treated rats (no. of neurons in parentheses). All comparisons were made between saline and CYP treatment.
P < 0.05 vs. saline-treated counterparts.
Table 3.
Imax and pH50 in response to protons in bladder sensory neurons
| Fast | Intermediate | Sustained | |
|---|---|---|---|
| Sal LS | |||
| Imax, pA/pF | 68.6 ± 12.7 (19) | 63.3 ± 10.0 (19) | 134.0 ± 12.0 (10) |
| pH50 | 5.6 ± 0.1 (19) | 5.4 ± 0.1 (19) | 4.5 ± 0.1 (10) |
| Sal TL | |||
| Imax, pA/pF | 62.3 ± 12.8 (11) | 46.9 ± 11 (11) | 93.8 ± 14 (10) |
| pH50 | 5.5 ± 0.2 (11) | 5.9 ± 0.3 (11) | 4.7 ± 0.1 (10) |
| CYP LS | |||
| Imax, pA/pF | 29.5 ± 8.4* (20) | 24.7 ± 7.3* (20) | 81.6 ± 16* (14) |
| pH50 | 5.7 ± 0.1 (20) | 5.5 ± 0.1 (20) | 4.7 ± 0.1 (14) |
| CYP TL | |||
| Imax, pA/pF | 38.9 ± 5.3* (18) | 22.3 ± 2.4* (18) | 57.1 ± 11.5* (8) |
| pH50 | 5.3 ± 0.1 (18) | 5.5 ± 0.1 (18) | 4.6 ± 0.1 (8) |
Values are means ± SE of peak current (Imax) and logarithm of EC50 for protons (pH50) for various proton-triggered components in LS and TL bladder sensory neurons from saline (control)- and CYP-treated rats (no of neurons in parentheses).
P < 0.05 vs. saline-treated counterparts.
Table 4.
Current kinetics in response to protons in bladder sensory neurons
| Sal LS | Sal TL | CYP LS | CYP TL | |
|---|---|---|---|---|
| Time to peak 10–90%, ms | ||||
| Fast | 111.2 ± 10.4 (41) | 136.9 ± 23.9 (32) | 90.8 ± 11.4 (43) | 115.6 ± 10.9 (45) |
| Intermediate | 639.4 ± 31.0 (41) | 630.9 ± 45.3 (32) | 586.0 ± 40.3 (43) | 715.3 ± 44.8 (45) |
| Slow | 914.0 ± 79.4 (14) | 1,002.0 ± 77.3 (16) | 812.5 ± 100.2 (19) | 1,049.0 ± 126.3 (7) |
| Desensitization constant, ms | ||||
| Fast | 225.1 ± 41.2 (41) | 252.9 ± 23.1 (32) | 180.5 ± 19.1 (43) | 233.5 ± 23.9 (45) |
| Intermediate | 661.5 ± 96.4 (41) | 815.9 ± 168.6 (32) | 704.5 ± 78.9 (43) | 934.0 ± 191.3 (45) |
| Slow | 3,433.0 ± 997 (14) | 4,022.0 ± 706 (32) | 3,033.0 ± 506 (19) | 3,349 ± 811 (7) |
Values are means ± SE of current kinetics for various components triggered by application of pH 5 in bladder sensory neurons from saline (control)- and CYP-treated rats (no. of neurons in parentheses).
P < 0.05 vs. saline-treated counterparts.
Biphasic, slow, and triphasic currents.
In bladder neurons from CYP-treated rats, acid (pH 5) produced currents in all 62 LS and 52 of 57 TL bladder neurons (not different from saline-treated controls; Table 1). Relative to saline treatment, CYP treatment increased the proportion of TL bladder neurons exhibiting acid-produced triphasic currents (CYP: 28/52, 53.8%; saline: 15/48, 31.3%; P < 0.05) and reduced the number of TL bladder neurons exhibiting acid-produced slow currents (CYP: 7/52, 13.5%; saline: 16/48, 33.3%; P < 0.05). Proportions of acid-produced biphasic, slow, and triphasic currents in LS bladder neurons were unaffected by CYP treatment (see examples in Fig. 1). See Table 1 for details.
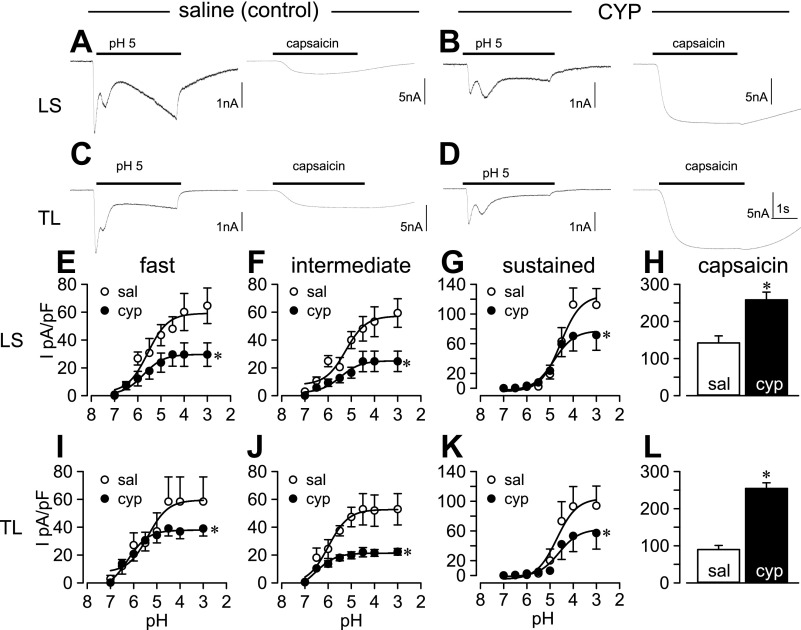
Fig. 1.
Effects of protons and capsaicin on bladder sensory neurons from saline (control)- and cyclophosphamide (CYP)-treated rats. Protons triggered predominantly a multiphasic current (A and C), whereas capsaicin always produced a sustained current in the same lumbosacral (LS) and thoracolumbar (TL) bladder neurons from saline-treated rats. The same current profiles were evident in LS and TL neurons from CYP-treated rats (B and D), although proton-evoked current was reduced and capsaicin-evoked current was increased in both LS and TL neurons from CYP-treated rats. Proton-evoked effects were concentration dependent. Concentration-response relationships for fast (E and I), intermediate (F and J), and sustained (G and K) current components are plotted as current density (I) against pH in LS and TL bladder neurons. Open and filled circles represent values from saline (Sal)- and CYP-treated rats, respectively. EC50 values were not different after CYP treatment, but maximal current (Imax) in response to acid was substantially reduced for all current components in both LS and TL neurons. In contrast, capsaicin (1 μM) triggered significantly greater current density in LS (H) and TL (L) bladder neurons from CYP-treated rats. *P < 0.05.
Current density Imax and pH50.
Acid (pH 5) produced significantly lower densities of fast, intermediate, slow, and sustained current components in both TL and LS bladder neurons from CYP-treated rats relative to their saline-treated counterparts (Table 2). Because we noted a reduction in current density in response to pH 5, we further examined Imax and pH50 values for fast, intermediate, and sustained currents triggered by acid application, the most prevalent components in bladder neurons. Whereas peak current densities (Imax) were significantly reduced in LS (e.g., Fig. 1, E–G, and Table 2) and TL (e.g., Fig. 1, I–K, and Table 2) bladder neurons from CYP-treated rats, bladder inflammation did not alter EC50 values for proton-triggered current components in these neurons (Table 3).
Current kinetics.
Bladder inflammation did not influence activation and inactivation kinetics of acid-evoked currents in bladder sensory neurons (Table 4). Because slowly activating and desensitizing current was identified in only a small number of bladder neurons, we did not systemically examine recovery kinetics. Based on single-exponential fittings, recovery time constants of transient currents in LS and TL bladder neurons from CYP-treated rats did not differ in comparison with neurons from saline-treated rats (e.g., Fig. 2).
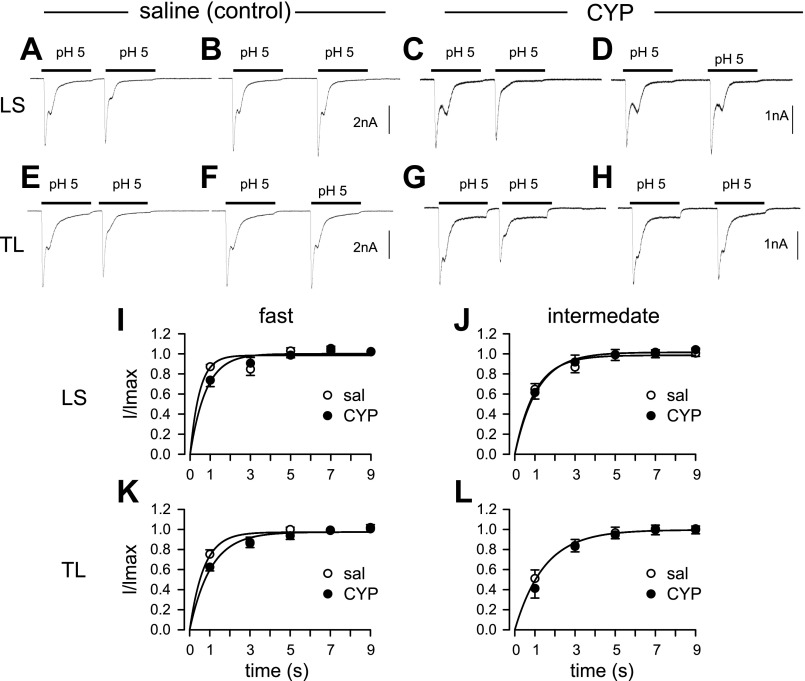
Fig. 2.
Recovery kinetics of transient currents in bladder neurons from saline (control)- and CYP-treated rats. To determine recovery kinetics, we systematically varied the interval between acid applications and measured the fraction of currents recovered from desensitization. Both fast and intermediate current components followed similar recoveries in saline- and CYP-treated LS and TL neurons. Partial recovery was evident at an interval of 1 s (A, C, E, and G), with nearly complete recovery at an interval of 3 s (B, D, F, and H). Peak current of the second application of pH 5 relative to the first (I/Imax) was fitted with a single-exponential function. Recovery time constants for fast (I) and intermediate (J) currents were 0.81 ± 0.14 and 1.41 ± 0.25 s (n = 15), respectively, for LS neurons from saline-treated rats, which did not differ from that for LS neurons from CYP-treated rats (0.89 ± 0.12 and 1.27 ± 0.17 s for fast and intermediate component, n = 12, respectively). Similarly, recovery time constants for fast (K) and intermediate (L) currents in TL neurons did not differ between groups (control: 0.77 ± 0.11 and 1.42 ± 0.17 s, n = 10; CYP: 0.73 ± 0.08 and 1.16 ± 0.09 s, n = 13, for fast and intermediate component, respectively).
Capsaicin current density is increased.
Virtually all bladder neurons responded to capsaicin (1 μM), and the proportion responding to capsaicin did not change after CYP-treatment (Table 1). In contrast to acid-evoked currents, capsaicin triggered significantly greater peak current density in both TL and LS bladder neurons (e.g., Fig. 1, H and L, and Table 2).
Protons inhibit responses to acid but increase responses to capsaicin.
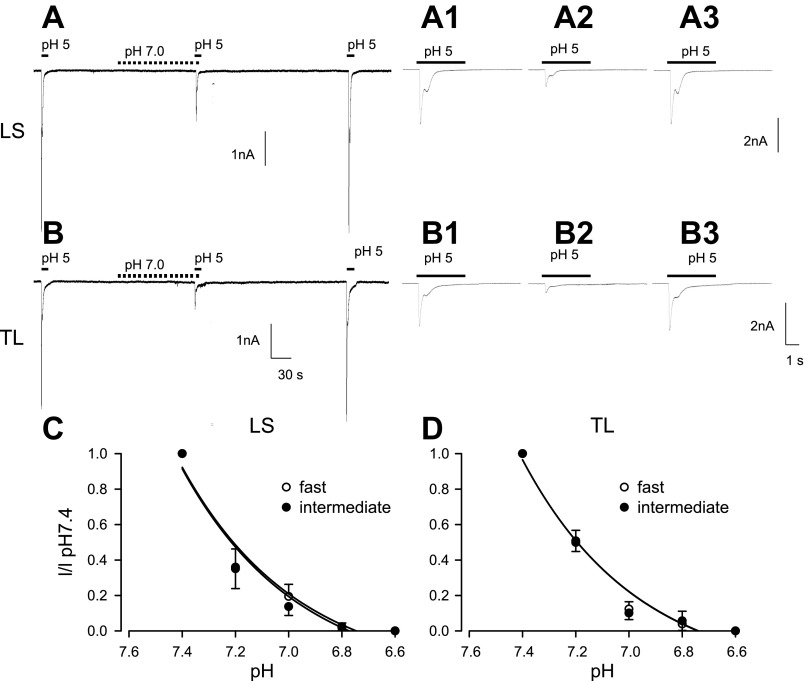
Because bladder wall pH is significantly decreased in this model of cystitis, we increased systematically the concentration of protons (pH range 7.4–6.6) by bathing bladder neurons for 60 s before application of acid (pH 5) or capsaicin (100 nM). In both LS and TL bladder neurons, prolonged exposure to pH 7.0 inhibited both rapidly and intermediately desensitizing current components triggered by acid (Fig. 3, A and B). As shown in Fig. 3, C and D, inhibition of acid-sensitive currents by extracellular acidosis was concentration dependent. A decrease from pH 7.4 to pH 7.2 depressed the response triggered by acid by more than one-half in both LS (Fig. 3C) and TL (Fig. 3D) bladder neurons.
Fig. 3.
Proton inhibition of currents triggered by pH 5 in LS (A) and TL (B) bladder neurons from saline-treated rats. A1–A3 and B1–B3 are expanded from the continuous traces in A and B, respectively. Application of pH 5 evoked biphasic currents in LS and TL neurons. In the presence of pH 7, both fast and intermediate components in both LS and TL neurons were depressed (A and A2, B and B2) and completely recovered by 2 min after exposure to pH 7.4 (A and A3, B and B3). This proton-proton inhibition showed concentration dependence in both LS (C) and TL (D) bladder neurons.
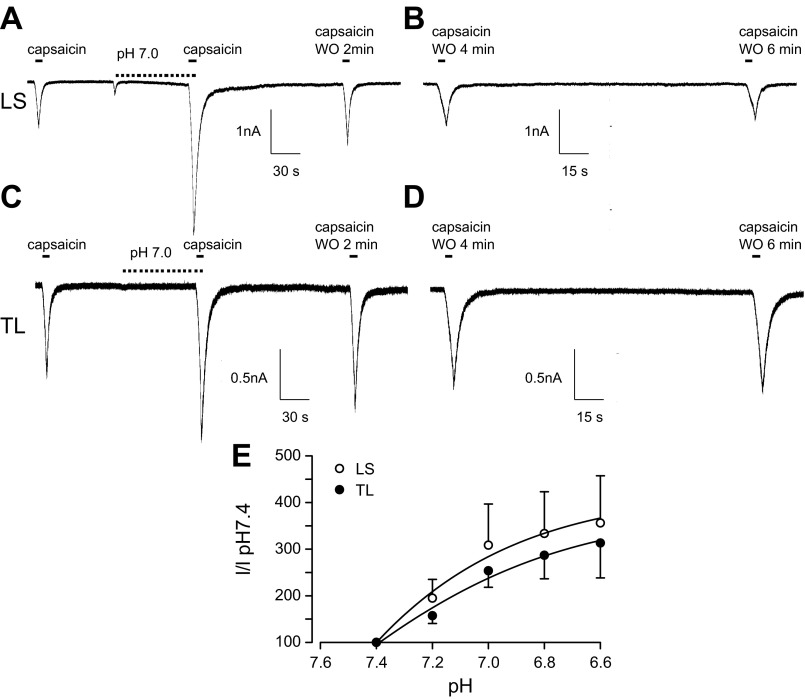
In contrast to acid-sensitive currents, lowering bath pH significantly increased the current density evoked by capsaicin in LS bladder neurons (195.0 ± 40, 308.6 ± 88, 333.4 ± 89, and 356.0 ± 101%, n = 6, for pH 7.2, 7.0, 6.8, and 6.6, respectively; Fig. 4, A, B, and E). Similarly, extracellular acidosis significantly increased current triggered by capsaicin in TL neurons (157.2 ± 17, 253.7 ± 35, 286.9 ± 50, and 313.2 ± 75%, n = 5, for pH 7.2, 7.0, 6.8, and 6.6, respectively; Fig. 4, C, D, and E).
Fig. 4.
Proton enhancement of current triggered by capsaicin in LS (A and B) and TL (C and D) bladder neurons from saline-treated rats. In both LS and TL neurons, capsaicin (100 nM) produced an inward current (A and C, first trace) that was potentiated in the presence of pH 7.0 (A and C, second trace) and remained enhanced after a 2-min washout (WO) following exposure to pH 7.4 (A and C, third trace). Capsaicin-triggered currents recovered 4–6 min after removal of pH 7.0 (B and D). Potentiation of capsaicin-evoked inward currents by protons showed strong pH dependency (E) in both LS and TL bladder neurons.
Cystitis and Acid Reduce Bladder Neuron Response Thresholds to Heat
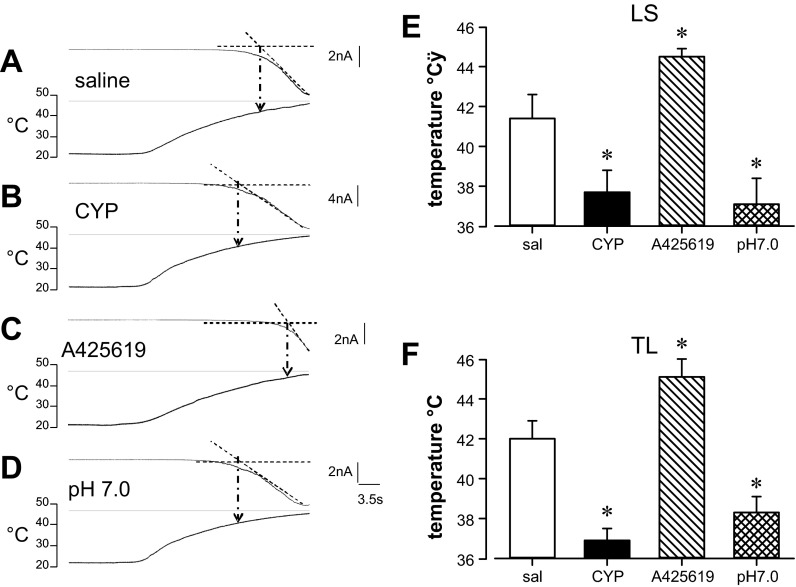
Considering the role of TRPV1 in thermal sensation, we examined the effect of increasing the temperature of the bath solution. We defined the heat activation threshold as the temperature at which X- and Y-axes intercept (Fig. 5A). Heat activated inward currents with a threshold of 41.4 ± 1.2°C (n = 11) and 42.0 ± 0.9°C (n = 11) in LS and TL neurons, respectively, from saline-treated rats. Relative to their saline-treated counterparts, CYP-treated rats had significantly reduced heat activation threshold for both groups of bladder neurons (CYP LS: 37.7 ± 1.1°C, n = 11; TL: 36.9 ± 0.6°C, n = 12; P < 0.01; Fig. 5B).
Fig. 5.
Activation threshold to heat and its modulation. Bath application of preheated solution triggered an inward current with an activation threshold defined as the temperature at which lines extrapolated from the X (time, s)- and Y (current, pA)-axes intercept. Extrapolation to the temperature ramp (bottom trace) yielded the activation threshold (°C). Examples are given in A–D; data are summarized in E and F for LS and TL neurons, respectively. In bladder neurons from saline-treated rats (A), heat triggered current at a threshold of ∼42°C. The heat-triggered current threshold was significantly reduced in bladder neurons from CYP-treated rats (B), significantly increased in the presence of A-425619, a selective TRPV1 antagonist (C), and significantly reduced by brief reduction of the external solution to pH 7.0 (D). See text for details. *P < 0.05.
To assess the role of TRPV1, we superfused A-425619 (300 nM) for 1 min, followed by administration of heated solution with A-425619. In the presence of A-425619, heat activation thresholds were significantly elevated to 44.5 ± 0.4°C (n = 6) and 45.1 ± 1.1°C (n = 6) for LS and TL bladder neurons, respectively (P < 0.05), suggesting that TRPV1 receptors mediate, in part, the current triggered by heat (Fig. 5C).
We further examined modulation of heat-activated threshold by lowering bath solution pH to pH 7.0 for 1 min, followed by application of preheated solutions (pH 7.0, 30 s). Heat triggered currents at significantly lower thresholds in pH 7.0 compared with pH 7.4 solution (LS: 37.1 ± 1.3°C, n = 5; TL: 38.3 ± 0.8°C, n = 6, P < 0.05; Fig. 5D). Effects of CYP treatment and other pharmacological manipulations are presented in Fig. 5, E and F.
DISCUSSION
We extended our prior investigations of ASIC and TRPV1 currents in identified bladder sensory neurons and in this study examined the relative contributions and changes of these currents after bladder inflammation. The key finding is a differential effect of cystitis on distinct current components. Whereas bladder inflammation suppressed proton-evoked currents, it increased peak current density in response to TRPV1 activation by capsaicin and lowered the activation threshold of TRPV1 to heat. Despite the attenuation of acid-induced current, acidification of the extracellular milieu, as seen during cystitis, enhanced responses to capsaicin, further supporting a role of TRPV1 rather than other acid-sensitive channels in peripheral sensitization during bladder inflammation.
CYP treatment produced a significant inflammation that was limited to the bladder, consistent with previous reports (Bon et al. 2003; Cox. 1979; Dang et al. 2008). Based on cell capacitance, bladder neurons from CYP-treated rats were increased in size, consistent with previous reports (Dang et al. 2008; Yoshimura and de Groat 1999). Although we did not address the mechanism(s) for the change in cell size, others have shown that neutralization of NGF prevents the increase in size of neurons innervating an inflamed hind paw (Nicholas et al. 1999). NGF increases during cystitis (Liu et al. 2009; Murray et al. 2004; Oddiah et al. 1998), suggesting that NGF may have contributed to the increase in cell size in the present study. The toxic CYP metabolite acrolein activates TRPA1 receptors, which also contribute to nociceptive responses. Both TRPV1 and TRPA1 play important roles in the development of neurogenic inflammation, which may indirectly or directly affect neuron structure (Bautista et al. 2006). As indicated above, we noted a significant decrease in bladder wall pH during inflammation, which may contribute to sensitization of afferent pathways. Similar effects have previously been shown by others (Cohen et al. 2009; Deval et al. 2010; Friese et al. 2007; Holzer 2011; Nagae et al. 2007), suggesting that in addition to changes in epithelial permeability, inflammation contributes to tissue acidosis. We recognize that other factors may contribute to the increased excitability of primary bladder afferents and bladder over activity (Arms et al. 2013; Dang et al. 2008,; Yoshimura and de Groat 1999). The toxic CYP metabolite acrolein directly affects TRPA1 channels through covalent modification of cysteine residues, thus potentially contributing to the development of neurogenic inflammation (Macpherson et al. 2007). Prior studies have demonstrated that TRPV1 and TRPA1 are coexpressed in rat bladder neurons (Streng et al. 2008). These TRP channels are also functionally linked, as has been demonstrated by cross-sensitization and desensitization (Andrade et al. 2006; Du et al. 2007; Ruparel et al. 2008). We did not directly address the role of TRPA1 in the present study, but it is unlikely that it affected acid-evoked currents, the focus of the present investigation. Because experiments were conducted after and not during induction of CYP-produced cystitis, the potential sensitizing effect of acrolein on coexpressed members of the TRP channels is similarly unlikely to skew results.
Bladder neurons exhibit multiple and complex current responses during exposure to protons, indicating that multiple subtypes of ASICs coexist and contribute to heteromultimeric channel complexes (Benson et al. 2002; Hesselager et al. 2004; Holzer 2011; Kobayashi et al. 2009; Mamet et al. 2002; Nagae et al. 2006, 2007; Voilley et al. 2001). Based on EC50 values, the transient proton-evoked currents share similar acid sensitivity. Relative to its potency in heterologous expression systems, acid was significantly less potent at ASICs in the native sensory neurons studied presently. The difference in potency may be due to the presence of accessory and/or regulatory protein(s) in bladder sensory neurons, which has previously been shown for TRPV1 receptors (Kim et al. 2006). Cystitis decreased peak amplitudes of acid-sensitive currents with relatively minor changes in current components, acid sensitivity, and kinetics of current components. Conflicting data obscure the role of ASICs in nociception. Whereas some have found that ASICs participate in nociceptive processing (Chen et al. 2011; Deval et al. 2008; Gautam et al. 2010; Mamet et al. 2003; Seo et al. 2010; Walder et al. 2010; Yen et al. 2009), others, on the basis of genetic manipulations, have reported evidence contradicting this notion (Kang et al. 2012; Mogil et al. 2005; Staniland and McMahon 2009; Yen et al. 2009). Our data fit into the latter group, arguing at least against a role of ASIC channels in peripheral sensitization during bladder inflammation. In contrast to the decrease in ASIC-mediated currents, responses to capsaicin and temperature were significantly enhanced during inflammation, suggesting a differential modulation of TRPV1 and ASIC channels.
Considering these findings and the importance of TRPV1 in nociception, we examined the modulation of this current during inflammatory conditions. Because capsaicin at ≥500 nM causes a long-lasting desensitization, which did not recover during the course of our experiment, we could not construct a dose-response relationship to determine Imax and EC50 values. Therefore, we focused on a single concentration of capsaicin well above the reported EC50 (Correll et al. 2004; Dray et al. 1990; Gunthorpe et al. 2000; McLatchie and Bevan 2001). Based on antagonism of capsaicin by A-425619, cystitis was associated with a significant increase in TRPV1-mediated peak current density, consistent with a previous study reporting similar augmentation of TRPV1-mediated current in a mixture of labeled/unlabeled LS neurons from cats with IC. It is unlikely that allosteric modulation of the channel contributes to the increased current in this study, because allosteric modulation of TRPV1 by ions has been reported to be transient, lasting only minutes after removal from the bath fluid (Caterina et al. 2000; McLatchie and Bevan 2001; Tominaga et al. 1998). In contrast, capsaicin activated TRPV1 receptors with increased current in bladder neurons from CYP-treated rats; these neurons were removed from the rats several hours previously and bathed in control pH (7.4), suggesting long-lasting alteration(s) had occurred. Although we did not directly determine channel expression or other mechanisms contributing to the observed changes, others have shown that inflammation increases the basal activity of PKC and/or the expression or functional properties of TRPV1 receptors in sensory neurons after tissue inflammation/damage (Amaya et al. 2004; Rashid et al. 2003; Zhu et al. 2011). As mentioned above, cystitis significantly increases levels of NGF in urine and receptor tyrosine kinase A (TrkA) in the mouse bladder (Murray et al. 2004; Oddiah et al. 1998). Furthermore, overexpression of NGF in the bladder triggers bladder hyperactivity and hypersensitivity (Boudes et al. 2011; Lamb et al. 2004; Schnegelsberg et al. 2010; Yoshimura et al. 2006). NGF has been shown to upregulate expression of TRPV1, which likely contributed to the effects observed in the present study (Mamet et al. 2002, 2003; Saban et al. 2011; Shimosato et al. 2005; Simonetti et al. 2006; Xue et al. 2007; Zhang et al. 2005).
Inflammation lowers tissue pH, as we confirmed in the present study, prompting us to determine the effect of acidosis on TRPV1-mediated current. Acute exposure to a slightly acidic environment significantly enhanced capsaicin- and heat-evoked currents, thereby potentially further enhancing excitability (see below). We did not directly examine the relative contribution of TRPV1 to the heat response in neurons from CYP-treated rats because we only applied heat once per neuron per coverslip to avoid complications with repeated exposure to heat. Although heat is a nonselective activator of TRPV1 channels, TRPV1 receptors play a role in temperature detection in rat bladder sensory neurons, because heat activation threshold was raised from 41 to 45°C in the presence of A-425619, consistent with previous reports demonstrating a key role of TRPV1 receptors in thermal sensation (Caterina et al. 1997). The present data suggest that enhanced function of TRPV1 may contribute, in part, to the lower heat activation threshold in bladder neurons from CYP-treated rats. Interestingly, the TRPV1 receptor antagonist A-425619 did not completely abolish current triggered by heat, suggesting that other heat modulated channels may be coexpressed in bladder neurons (Caterina et al. 1999; Hiura 2009; Smith et al. 2002; Tamura et al. 2005). Although other TRP channels may be affected by bladder inflammation, it is beyond the scope of this study and remains to be investigated.
Significance of Plasticity of TRPV1 Receptor Function and Its Modulation by Protons
Taken together, the present data demonstrate that cystitis differentially regulates the function of ASIC and TRPV1 channels, depressing ASIC-mediated currents while enhancing the TRPV1-mediated current. In addition to changes in channel expression or modulation of expressed channels, the proton concentration increases during inflammation, which further modulates channel function. Prolonged exposure to protons during tissue acidosis desensitizes the transient ASIC currents, suggesting that ASIC channels may participate in sensory detection under normal physiological conditions but do not significantly contribute to peripheral sensitization during inflammatory conditions such as cystitis. In contrast, bladder inflammation augmented TRPV1-mediated current and reduced the response threshold to heat; both effects can be enhanced by tissue acidification, suggesting that TRPV1 could be readily activated by normal body temperature and/or endogenous activators such as anandamide and protons. This study supports an important role of TRPV1 in the sensitization of bladder afferent pathways.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS035790.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.D., K.B., and G.F.G. conception and design of research; K.D. performed experiments; K.D. analyzed data; K.D., K.B., and G.F.G. interpreted results of experiments; K.D. prepared figures; K.D. drafted manuscript; K.D., K.B., and G.F.G. edited and revised manuscript; K.D., K.B., and G.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Abbott Laboratories for the gift of A-425619.
REFERENCES
- Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci 20: 2303–2309, 2004 [DOI] [PubMed] [Google Scholar]
- Andrade EL, Ferreira J, André E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol 72: 104–114, 2006 [DOI] [PubMed] [Google Scholar]
- Arms L, Girard BM, Malley SE, Vizzard M. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. Am J Physiol Renal Physiol. First published April 17, 2013; 10.1152/ajprenal.00139.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience 109: 787–798, 2002 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006 [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol 45: 221–226, 2006 [DOI] [PubMed] [Google Scholar]
- Bon K, Lanteri-Minet M, Menetrey D, Berkley KJ. Sex, time-of-day and estrous variations in behavioral and bladder histological consequences of cyclophosphamide-induced cystitis in rats. Pain 73: 423–429, 1997 [DOI] [PubMed] [Google Scholar]
- Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol 170: 1008–1012, 2003 [DOI] [PubMed] [Google Scholar]
- Boudes M, Uvin P, Kerselaers S, Vennekens R, Voets T, De Ridder D. Functional characterization of a chronic cyclophosphamide-induced overactive bladder model in mice. Neurourol Urodyn 30: 1659–1665, 2011 [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- Burkman RT. Chronic pelvic pain of bladder origin: epidemiology, pathogenesis and quality of life. J Reprod Med 49: 225–229, 2004 [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 289: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- Charrua A, Reguenga C, Cordeiro JM, Correiade-Sá P, Paule C, Nagy I, Cruz F, Avelino A. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J Urol 182: 2944–2950, 2009 [DOI] [PubMed] [Google Scholar]
- Chen WH, Hsieh CL, Huang CP, Lin TJ, Tzen JT, Ho TY, Lin YW. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J Biomed Sci 18: 82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naïve and bladder-inflamed mice. Pain 148: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM, Jr., McKinlay JB. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol 177: 1390–1394, 2007 [DOI] [PubMed] [Google Scholar]
- Clemens JQ, Meenan RT, O'Keeffe Rosetti MC, Brown SO, Gao SY, Calhoun EA. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol 174: 576–580, 2005 [DOI] [PubMed] [Google Scholar]
- Cohen A, Sagron R, Somech E, Segal-Hayoun Y, Zilberberg N. Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K(2P)2.1 channel. Mol Cell Neurosci 40: 382–389, 2009 [DOI] [PubMed] [Google Scholar]
- Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. Cloning and pharmacological characterization of mouse TRPV1. Neurosci Lett 370: 55–60, 2004 [DOI] [PubMed] [Google Scholar]
- Cox PJ. Cyclophosphamide cystitis–identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049, 1979 [DOI] [PubMed] [Google Scholar]
- Cruz F, Guimaraes M, Silva C, Reis M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet 350: 640–641, 1997 [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Lamb K, Gebhart GF. Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol 93: 3112–3119, 2005b [DOI] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Chandiramani V, Dasgupta P, Van Poppel H, Baert L, Fowler CJ. Intravesical capsaicin as a treatment for refractory detrusor hyperreflexia: a dual center study with long-term followup. J Urol 158: 2087–2092, 1997 [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 27: 3047–3055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128: 549–558, 2008 [DOI] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int 94: 153–157, 2004 [DOI] [PubMed] [Google Scholar]
- Dray A, Bettaney J, Forster P. Actions of capsaicin on peripheral nociceptors of the neonatal rat spinal cord-tail in vitro: dependence of extracellular ions and independence of second messengers. Br J Pharmacol 101: 727–733, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology 70: 826–831, 2007 [DOI] [PubMed] [Google Scholar]
- El Kouhen R, Surowy CS, Bianchi BR, Neelands TR, McDonald HA, Niforatos W, Gomtsyan A, Lee CH, Honore P, Sullivan JP, Jarvis MF, Faltynek CR. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J Pharmacol Exp Ther 314: 400–409, 2005 [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med 13: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- Gautam M, Benson CJ, Sluka KA. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience 170: 893–900, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J Physiol 525: 747–759, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- Hiura A. Is thermal nociception only sensed by the capsaicin receptor, TRPV1? Anat Sci Int 84: 122–128, 2009 [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid sensing by visceral afferent neurons. Acta Physiol (Oxf) 201: 63–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci 24: 10974–10979, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS One 7: e35225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, Park MY, Kim E, Kim M, Kim BM, Cho H, Shin Y, Oh U. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci 26: 2403–2412, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Yoshiyama M, Zakoji H, Takeda M, Araki I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int 104: 1746–1751, 2009 [DOI] [PubMed] [Google Scholar]
- Kontani H, Hayashi K. Urinary bladder response to hypogastric nerve stimulation after bilateral resection of the pelvic nerve or spinal cord injury in rats. Int J Urol 4: 394–400, 1997 [DOI] [PubMed] [Google Scholar]
- Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150–156, 2004 [DOI] [PubMed] [Google Scholar]
- Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res 105: 220–232, 1995 [DOI] [PubMed] [Google Scholar]
- Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int 104: 1476–1481, 2009 [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545, 2007 [DOI] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 278: 48907–48913, 2003 [DOI] [PubMed] [Google Scholar]
- Matsuoka PK, Haddad JM, Pacetta AM, Baracat EC. Intravesical treatment of painful bladder syndrome: a systematic review and meta-analysis. Int Urogynecol J 23: 1147–1153, 2012 [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Bevan S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br J Pharmacol 132: 899–908, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meen M, Coudore-Civiale MA, Eschalier A, Boucher M. Involvement of hypogastric and pelvic nerves for conveying cystitis induced nociception in conscious rats. J Urol 166: 318–322, 2001 [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J Neurophysiol 86: 2276–2284, 2001 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci 25: 9893–9901, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS. Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol Regul Integr Comp Physiol 272: R695–R703, 1997 [DOI] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172: 2434–2439, 2004 [DOI] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 39: 1107–1115, 2006 [DOI] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 25: 99–104, 2007 [DOI] [PubMed] [Google Scholar]
- Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol 173: 1983–1987, 2005 [DOI] [PubMed] [Google Scholar]
- Nicholas RS, Winter J, Wren P, Bergmann R, Woolf CJ. Peripheral inflammation increases the capsaicin sensitivity of dorsal root ganglion neurons in a nerve growth factor-dependent manner. Neuroscience 91: 1425–1433, 1999 [DOI] [PubMed] [Google Scholar]
- Nickel JC. Interstitial cystitis: a chronic pelvic pain syndrome. Med Clin North Am 88: 467–481, 2004 [DOI] [PubMed] [Google Scholar]
- Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9: 1455–1458, 1998 [DOI] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Bakoshi S, Ueda H. Increased expression of vanilloid receptor 1 on myelinated primary afferent neurons contributes to the antihyperalgesic effect of capsaicin cream in diabetic neuropathic pain in mice. J Pharmacol Exp Ther 306: 709–717, 2003 [DOI] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 135: 271–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban MR, Davis CA, Avelino A, Cruz F, Maier J, Bjorling DE, Sferra TJ, Hurst RE, Saban R. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC Physiol 11: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Roh DH, Yoon SY, Kang SY, Moon JY, Kim HW, Han HJ, Chung JM, Beitz AJ, Lee JH. Peripheral acid-sensing ion channels and P2X receptors contribute to mechanical allodynia in a rodent thrombus-induced ischemic pain model. J Pain 11: 718–727, 2010 [DOI] [PubMed] [Google Scholar]
- Shimosato G, Amaya F, Ueda M, Tanaka Y, Decosterd I, Tanaka M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain 119: 225–232, 2005 [DOI] [PubMed] [Google Scholar]
- Simonetti M, Fabbro A, D'Arco M, Zweyer M, Nistri A, Giniatullin R, Fabbretti E. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain 2: 11, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190, 2002 [DOI] [PubMed] [Google Scholar]
- Staniland AA, McMahon SB. Mice lacking acid-sensing ion channels (ASIC) 1 or 2, but not ASIC3, show increased pain behaviour in the formalin test. Eur J Pain 13: 554–563, 2009 [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordte SE, Bevan S, Andersson KE, Högestätt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–400, 2008 [DOI] [PubMed] [Google Scholar]
- Tamura S, Morikawa Y, Senba E. TRPV2, a capsaicin receptor homologue, is expressed predominantly in the neurotrophin-3-dependent subpopulation of primary sensory neurons. Neuroscience 130: 223–228, 2005 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain 11: 210–218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem 101: 212–222, 2007 [DOI] [PubMed] [Google Scholar]
- Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain 5: 1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24: 4211–4223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li C, Mehta K, Pasricha PJ. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology 141: 370–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]