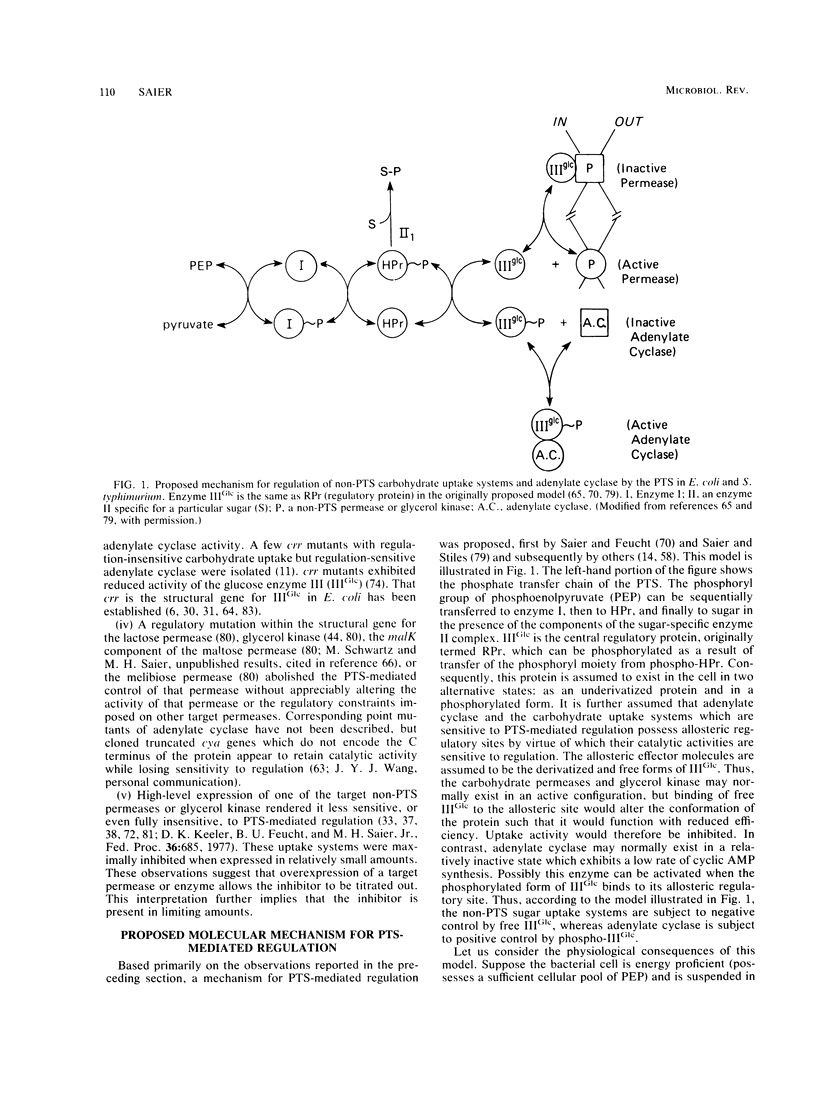
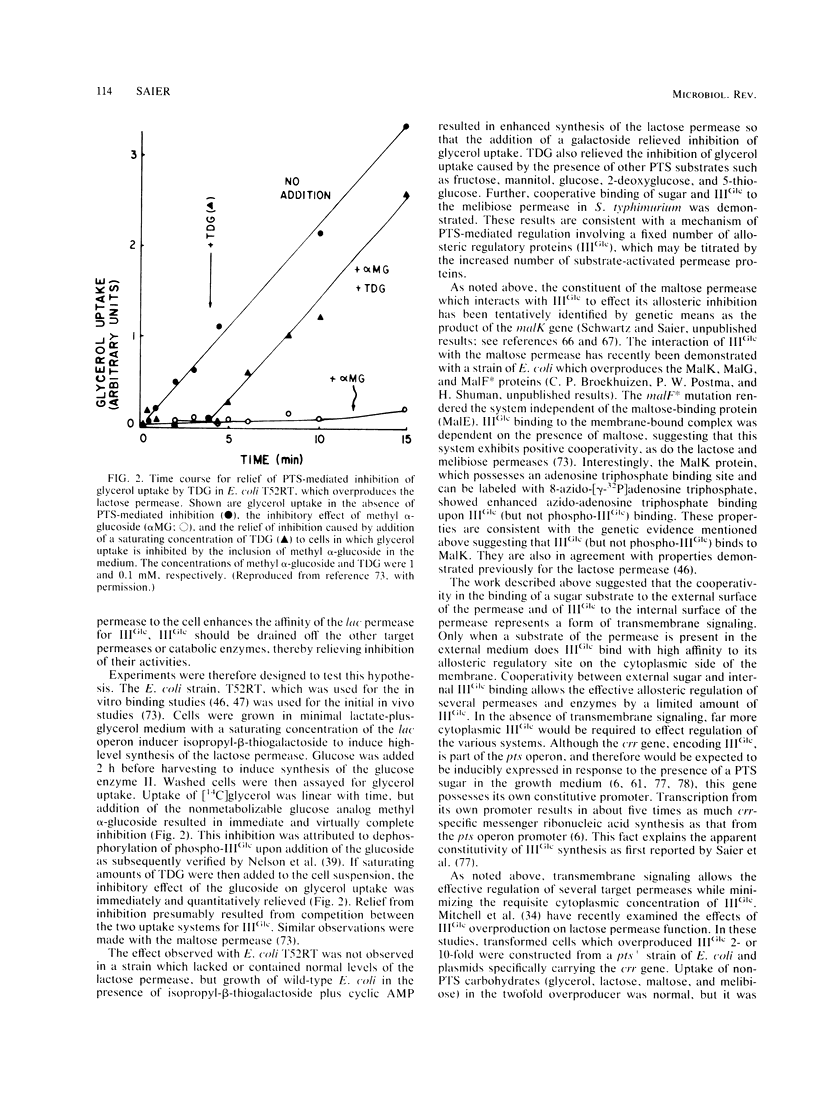
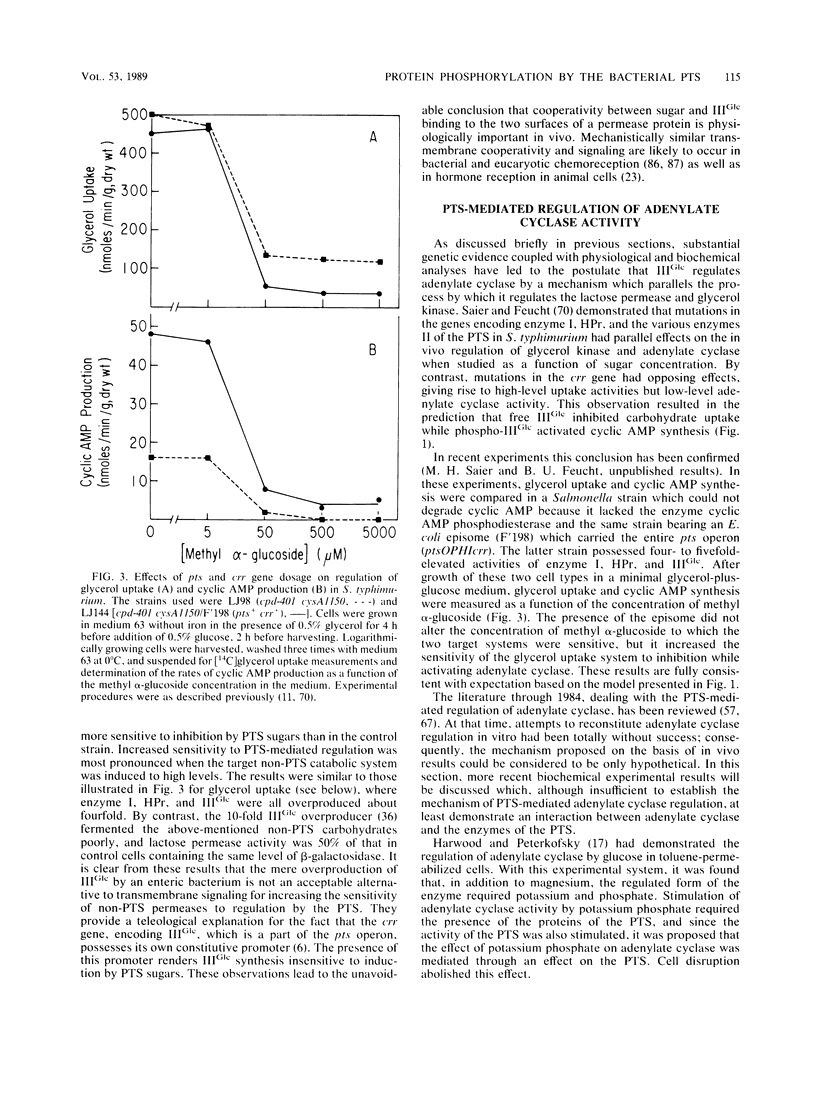
Abstract
The bacterial phosphotransferase system (PTS) functions in a variety of regulatory capacities. One of the best characterized of these is the process by which the PTS regulates inducer uptake and catabolite repression. Early genetic and physiological evidence supported a mechanism whereby the phosphorylation state of an enzyme of the PTS, the enzyme III specific for glucose (IIIGlc), allosterically inhibits the activities of a number of permeases and catabolic enzymes, the lactose, galactose, melibiose, and maltose permeases, as well as glycerol kinase. Extensive biochemical evidence now supports this model. Evidence is also available showing that substrate binding to those target proteins enhances their affinities for IIIGlc. In the case of the lactose permease, this positively cooperative interaction represents a well documented example of transmembrane signaling, demonstrated both in vivo and in vitro. Although the PTS-mediated regulation of cyclic AMP synthesis (catabolite repression) is not as well defined from a mechanistic standpoint, a model involving allosteric activation of adenylate cyclase by phospho-IIIGlc, together with the evidence supporting it, is presented. These regulatory mechanisms may prove to be operative in gram-positive as well as gram-negative bacteria, but the former organisms may have introduced variations on the theme by covalently attaching IIIGlc-like moieties to some of the target permeases and catabolic enzymes. It appears likely that the general process of PTS-catalyzed protein phosphorylation-dephosphorylation will prove to be important to the regulation of numerous bacterial physiological processes, including chemotaxis, intermediary metabolism, gene transcription, and virulence.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymerich S., Steinmetz M. Cloning and preliminary characterization of the sacS locus from Bacillus subtilis which controls the regulation of the exoenzyme levansucrase. Mol Gen Genet. 1987 Jun;208(1-2):114–120. doi: 10.1007/BF00330431. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation and characterization of membrane vesicles from mutant and wild type Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14565–14575. [PubMed] [Google Scholar]
- Castro L., Feucht B. U., Morse M. L., Saier M. H., Jr Regulation of carbohydrate permeases and adenylate cyclase in Escherichia coli. Studies with mutant strains in which enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system is thermolabile. J Biol Chem. 1976 Sep 25;251(18):5522–5527. [PubMed] [Google Scholar]
- Daniels G. A., Drews G., Saier M. H., Jr Properties of a Tn5 insertion mutant defective in the structural gene (fruA) of the fructose-specific phosphotransferase system of Rhodobacter capsulatus and cloning of the fru regulon. J Bacteriol. 1988 Apr;170(4):1698–1703. doi: 10.1128/jb.170.4.1698-1703.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J Bacteriol. 1988 Sep;170(9):3827–3837. doi: 10.1128/jb.170.9.3827-3837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Sauerwald H. Stimulation of dihydroxyacetone and glycerol kinase activity in Streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J Bacteriol. 1986 Jun;166(3):829–836. doi: 10.1128/jb.166.3.829-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Schmidt M. R., Saier M. H., Jr Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):239–244. doi: 10.1002/jcb.1982.240180211. [DOI] [PubMed] [Google Scholar]
- FAITH W. T., Jr, GIORGIO N. A., Jr, MALLETTE M. F. MECHANISM OF GLUCOSE INHIBITION OF BETA-GALACTOSIDASE BIOSYNTHESIS IN RESTING CULTURES OF ESCHERICHIA COLI. Arch Biochem Biophys. 1964 Dec;108:430–439. doi: 10.1016/0003-9861(64)90424-2. [DOI] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. E., Peterkofsky A. The mechanism of sugar-dependent repression of synthesis of catabolic enzymes in Escherichia coli. J Supramol Struct. 1977;6(4):495–502. doi: 10.1002/jss.400060404. [DOI] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Steinmetz M. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: cloning of the region containing the ptsH and ptsI genes and evidence for a crr-like gene. J Bacteriol. 1987 May;169(5):2287–2290. doi: 10.1128/jb.169.5.2287-2290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Gazdar C., Prasad C., Peterkofsky A., Curtis S. J., Epstein W. Involvement of the glucose enzymes II of the sugar phosphotransferase system in the regulation of adenylate cyclase by glucose in Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2462–2468. [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Armstrong J. B. Dierect measurement of the binding of labeled sugars to the lactose permease M protein. J Biol Chem. 1974 Jan 10;249(1):33–37. [PubMed] [Google Scholar]
- Levitzki A. Transmembrane signalling to adenylate cyclase in mammalian cells and in Saccharomyces cerevisiae. Trends Biochem Sci. 1988 Aug;13(8):298–301. doi: 10.1016/0968-0004(88)90122-3. [DOI] [PubMed] [Google Scholar]
- Liberman E., Reddy P., Gazdar C., Peterkofsky A. The Escherichia coli adenylate cyclase complex. Stimulation by potassium and phosphate. J Biol Chem. 1985 Apr 10;260(7):4075–4081. [PubMed] [Google Scholar]
- Liberman E., Saffen D., Roseman S., Peterkofsky A. Inhibition of E. coli adenylate cyclase activity by inorganic orthophosphate is dependent on IIIglc of the phosphoenolpyruvate:glycose phosphotransferase system. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1138–1144. doi: 10.1016/s0006-291x(86)80162-0. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Matsuoka M., McFadden B. A. Isolation, hyperexpression, and sequencing of the aceA gene encoding isocitrate lyase in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4528–4536. doi: 10.1128/jb.170.10.4528-4536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Meadow N. D., Rosenberg J. M., Pinkert H. M., Roseman S. Sugar transport by the bacterial phosphotransferase system. Evidence that crr is the structural gene for the Salmonella typhimurium glucose-specific phosphocarrier protein IIIGlc. J Biol Chem. 1982 Dec 10;257(23):14538–14542. [PubMed] [Google Scholar]
- Meadow N. D., Saffen D. W., Dottin R. P., Roseman S. Molecular cloning of the crr gene and evidence that it is the structural gene for IIIGlc, a phosphocarrier protein of the bacterial phosphotransferase system. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2528–2532. doi: 10.1073/pnas.79.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko T. P., Mitchell W. J., Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J Biol Chem. 1987 Nov 25;262(33):16261–16266. [PubMed] [Google Scholar]
- Mitchell W. J., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Regulation of other transport systems (lactose and melibiose). J Biol Chem. 1982 Dec 10;257(23):14553–14564. [PubMed] [Google Scholar]
- Mitchell W. J., Saffen D. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. In vivo regulation of lactose transport in Escherichia coli by IIIGlc, a protein of the phosphoenolpyruvate:glycose phosphotransferase system. J Biol Chem. 1987 Nov 25;262(33):16254–16260. [PubMed] [Google Scholar]
- Nelson S. O., Lengeler J., Postma P. W. Role of IIIGlc of the phosphoenolpyruvate-glucose phosphotransferase system in inducer exclusion in Escherichia coli. J Bacteriol. 1984 Oct;160(1):360–364. doi: 10.1128/jb.160.1.360-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Postma P. W. Interactions in vivo between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and the glycerol and maltose uptake systems of Salmonella typhimurium. Eur J Biochem. 1984 Feb 15;139(1):29–34. doi: 10.1111/j.1432-1033.1984.tb07971.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Schuitema A. R., Postma P. W. The phosphoenolpyruvate:glucose phosphotransferase system of Salmonella typhimurium. The phosphorylated form of IIIGlc. Eur J Biochem. 1986 Jan 15;154(2):337–341. doi: 10.1111/j.1432-1033.1986.tb09402.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Newman M. J., Wilson T. H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10583–10586. [PubMed] [Google Scholar]
- Niaudet B., Gay P., Dedonder R. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol Gen Genet. 1975;136(4):337–349. doi: 10.1007/BF00341718. [DOI] [PubMed] [Google Scholar]
- Novotny M. J., Frederickson W. L., Waygood E. B., Saier M. H., Jr Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1985 May;162(2):810–816. doi: 10.1128/jb.162.2.810-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Hess J. F., Simon M. I. Mutants defective in bacterial chemotaxis show modified protein phosphorylation. Cell. 1988 Apr 8;53(1):89–96. doi: 10.1016/0092-8674(88)90490-4. [DOI] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Mechanism of regulation of the lactose permease by the phosphotransferase system in Escherichia coli: evidence for protein-protein interaction. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):269–273. [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. The Escherichia coli adenylate cyclase complex: activation by phosphoenolpyruvate. J Supramol Struct. 1978;9(2):219–230. doi: 10.1002/jss.400090207. [DOI] [PubMed] [Google Scholar]
- Pettigrew D. W., Ma D. P., Conrad C. A., Johnson J. R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988 Jan 5;263(1):135–139. [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989 Jan;171(1):244–253. doi: 10.1128/jb.171.1.244-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Epstein W., Schuitema A. R., Nelson S. O. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984 Apr;158(1):351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Meadow N., Roseman S., Peterkofsky A. Reconstitution of regulatory properties of adenylate cyclase in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8300–8304. doi: 10.1073/pnas.82.24.8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Stuiver I., Saier M. H., Jr Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J Bacteriol. 1984 Jul;159(1):243–250. doi: 10.1128/jb.159.1.243-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Hoyt J. C., Reeves H. C. Evidence of histidine phosphorylation in isocitrate lyase from Escherichia coli. J Biol Chem. 1988 Feb 15;263(5):2477–2482. [PubMed] [Google Scholar]
- Roy A., Danchin A., Joseph E., Ullmann A. Two functional domains in adenylate cyclase of Escherichia coli. J Mol Biol. 1983 Mar 25;165(1):197–202. doi: 10.1016/s0022-2836(83)80251-4. [DOI] [PubMed] [Google Scholar]
- Saffen D. W., Presper K. A., Doering T. L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J Biol Chem. 1987 Nov 25;262(33):16241–16253. [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Feucht B. U., Novotny M. J. Evidence for the functional association of enzyme I and HPr of the phosphoenolpyruvate-sugar phosphotransferase system with the membrane in sealed vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):231–238. doi: 10.1002/jcb.1982.240180210. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Keeler D. K., Feucht B. U. Physiological desensitization of carbohydrate permeases and adenylate cyclase to regulation by the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Involvement of adenosine cyclic 3',5'-phosphate and inducer. J Biol Chem. 1982 Mar 10;257(5):2509–2517. [PubMed] [Google Scholar]
- Saier M. H., Jr, Novotny M. J., Comeau-Fuhrman D., Osumi T., Desai J. D. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1983 Sep;155(3):1351–1357. doi: 10.1128/jb.155.3.1351-1357.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D., Roseman S. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J Biol Chem. 1970 Nov 10;245(21):5870–5873. [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Schnetz K., Rak B. Regulation of the bgl operon of Escherichia coli by transcriptional antitermination. EMBO J. 1988 Oct;7(10):3271–3277. doi: 10.1002/j.1460-2075.1988.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Mutation in the crp gene of Salmonella typhimurium which interferes with inducer exclusion. J Bacteriol. 1980 Feb;141(2):751–757. doi: 10.1128/jb.141.2.751-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Levinthal M., Kundig F. D., Kundig W., Anderson B., Hartman P. E., Roseman S. Genetic evidence for the role of a bacterial phosphotransferase system in sugar transport. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1963–1970. doi: 10.1073/pnas.58.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Smith J. M., Rowsell E. H., Shioi J., Taylor B. L. Identification of a site of ATP requirement for signal processing in bacterial chemotaxis. J Bacteriol. 1988 Jun;170(6):2698–2704. doi: 10.1128/jb.170.6.2698-2704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Ullmann A. Catabolite repression 1985. Biochimie. 1985 Jan;67(1):29–34. doi: 10.1016/s0300-9084(85)80227-3. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]
- Wylie D., Stock A., Wong C. Y., Stock J. Sensory transduction in bacterial chemotaxis involves phosphotransfer between Che proteins. Biochem Biophys Res Commun. 1988 Mar 15;151(2):891–896. doi: 10.1016/s0006-291x(88)80365-6. [DOI] [PubMed] [Google Scholar]


