Abstract
Background and Purpose
The natural response to disability in one limb is to learn new ways of using the other limb. This compensatory behavioral strategy after stroke has long been thought to contribute to persistent dysfunction in the paretic limb by encouraging its disuse. Our recent findings suggest that it goes beyond the encouragement of disuse to disrupt neural substrates of paretic limb functional improvements.
Methods
We overview recent findings from rodent models of chronic upper extremity impairments in which precise control and manipulation of forelimb experiences were used to understand bilateral and interhemispheric contributions to motor functional outcome.
Results
Skill learning with the less-affected (nonparetic) forelimb promotes neural plasticity in the contralesional motor cortex that subserves its function. At the same time, it exacerbates dysfunction and limits the efficacy of rehabilitative training in the paretic limb. The maladaptive effects of skill learning with the nonparetic forelimb are dependent on callosal connections and contralesional motor cortex, and linked with reduced neural activation of peri-infarct motor cortex during rehabilitative training.
Conclusions
These findings suggest that learning to rely on the nonparetic body side has the capacity to disrupt functionality in a region of the injured hemisphere that contributes to outcome of the paretic limb. Whether this effect generalizes across injury loci and functional modalities remains to be tested.
Keywords: learned nonuse, motor cortex, manual skill, motor rehabilitative training, experience-expectant plasticity
Functional impairment is a powerful incentive for behavioral change. Animals, including humans, with upper extremity impairments spontaneously learn to use the less-affected (“nonparetic”) hand in novel ways to perform daily activities.1-3 In intact brains, the acquisition of manual skills depends on practice-dependent synaptic structural and functional reorganization of motor cortex (MC)4, 5. After stroke, this skill acquisition overlaps with ongoing degenerative and regenerative responses to the injury, many of which are also neural activity-dependent6, 7 and sensitive to behavioral manipulations.8-10 When they converge on the same circuits, ischemia-induced and experience-driven remodeling responses interact.3 Learning to rely on the nonparetic hand is a particularly prevalent, and profound, form of poststroke behavioral compensation, but compensatory strategies can be found across different impairment modalities, body sides, and injury loci.11-13 Their development is among the most reliable consequences of brain injury survival. The implication is that understanding the brain's typical adaptation to stroke will require understanding its interactions with compensatory behavioral changes.
Relying on the better functioning limb after stroke encourages disuse of the affected (“paretic”) limb (i.e., learned-nonuse).14 Our recent findings indicate that it can also more directly disrupt the paretic limb's functionality. After unilateral ischemic MC damage in rats, a relatively subtle variation in behavioral experience—learning a single new motor skill with the nonparetic limb—reduces spontaneous recovery and limits functional improvements resulting from subsequent rehabilitative training of the paretic limb, but without affecting infarct size or cell loss.15-17 This is found in rats trained to perform a unimanual reach-to-grasp task, first with the nonparetic limb (“nonparetic limb training”, NPT) for the first few weeks after the infarcts and subsequently with the paretic limb, as rehabilitative training (Fig. 1). Deleterious NPT effects are found when the reaching skill is novel to either limb at the time of the infarct or was established in the to-be-paretic limb before the infarct.15, 16, 18 Learning a skill with one hand does not normally result in such notable decrements in the other. For example, in intact rats (sham-operates), training one limb in skilled reaching has no detrimental effect on the other limb.16 Bilateral skill training and unskilled use of the nonparetic limb are not deleterious for the paretic limb.16 It is specifically deleterious to learn new way of using the better functioning limb on its own or in a dominant manner.
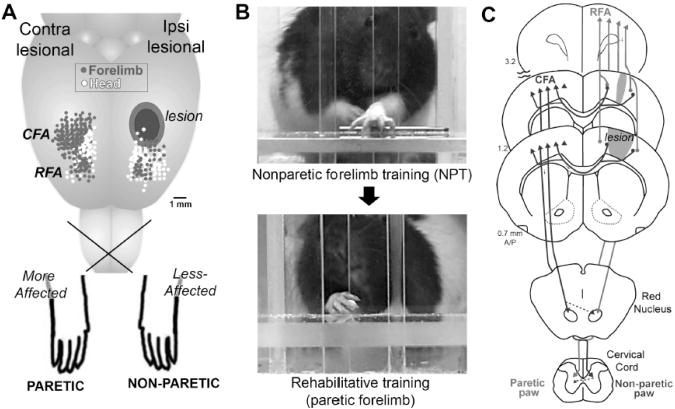
Fig. 1.
A, A rodent model of chronic upper extremity impairments resulting from focal ischemic damage to motor cortex (MC). Dots indicate movement representations revealed with intracortical microstimulation mapping (n=9 maps overlaid). B, Skilled reach training is used as a tool to investigate neural and behavioral effects of compensatory skill learning with the nonparetic forelimb. This training worsens function in the paretic limb and reduces the efficacy of subsequent rehabilitative training. C, Converging projections of ipsi and contralesional MC that contribute to forelimb movement. We hypothesize that learning with the nonparetic limb drives reorganizational patterns in converging projection areas that interfere with later change by experiences of the paretic limb. CFA, caudal forelimb area of primary MC, RFA, rostral forelimb area of premotor/supplementary MC.
The loss of rehabilitative training efficacy that results from unimanual skill learning with the nonparetic limb is linked with reduced neuronal activation of peri-infarct MC. This can be detected in the neuronal expression of ΔFosB, a transcription factor that is cumulatively and persistently expressed with repeated neuronal activation. As result of prior NPT, this expression is greatly dampened over a rehabilitative training period, even though the paretic limb's training activity is not reduced.16. Thus, NPT diminishes the neural responsiveness of peri-infarct MC to the paretic limb's activity. Activity-dependent neural reorganization in this same region is well-established to contribute to functional improvements in the paretic limb.19 These findings suggest that the behavioral manifestations of learned non-use can reflect, in part, the nonparetic limb's functional disruption of a peri-infarct region that could otherwise mediate better function in the paretic limb.
While peri-infarct MC is a neuroanatomical substrate for the maladaptive effects of skill learning with the nonparetic limb, interhemispheric connections are a route. The MC of either hemisphere is heavily interconnected via callosal projections, such that damage in one MC partially denervates the other. The callosal projections of contralesional MC also have a propensity to sprout into peri-infarct MC.20, 21 Layer V pyramidal neurons are the origin of most of these projections. If these connections are absent at the time of skill learning with the nonparetic limb (as a result of callosal transections), there are no deleterious effects of NPT.18 Furthermore, training one limb after bilateral MC injury has no negative impact on the other.18 Thus, the interhemispheric projections of contralesional MC mediate the maladaptive effects of skill learning with the nonparetic limb.
In intact animals, skill training of one forelimb results in dendritic growth and synaptogenesis in the contra-to-training MC4, 5. As a result of the convergence with reactive plasticity instigated by denervation of callosal projections to layer V, skill training of the nonparetic forelimb after unilateral MC infarcts results in an exaggerated growth response in contralesional MC, particularly in the basilar dendrites of layer V pyramidal neurons.3 Not only do these contralesional growth responses have no known benefit for the paretic limb, they result from the same skill training that worsens its function. Callosal transections block deleterious NPT effects on the paretic limb but not its promotion of contralesional dendritic growth, indicating that any contribution of the growth responses to paretic limb dysfunction is mediated by callosal projections. We postulate that skill learning with the nonparetic limb drives changes in interhemispheric projections that interfere with more functionally relevant (for the paretic side) reorganization.
The involvement of interhemispheric connections in the disruptive effects of skill learning with the nonparetic limb makes it seem likely that these experiences can contribute to clinical observations of abnormal interhemispheric activity after stroke.22 It also leads to the prediction that their influence will vary with injury loci and size. For example, if the MC of the injured hemisphere is too devastated to contribute to functional improvements in the paretic limb, or if the involvement of contra MC in regenerative plasticity is negligible, there is potentially no harm in compensatory skill learning with the nonparetic limb.
A more general implication of these findings is that different types of behavioral experience have the capacity to interfere with one another in driving reorganization of injured CNS. The results are reminiscent of “experience-expectant” plasticity, a brain developmental process in which experiences present during early sensitive periods sculpt circuitry patterns using mechanisms of activity-dependent synaptic competition (e.g., resulting in ocular dominance columns), after which time the connectional patterns are relatively resistant to later change.23 We speculate that a similar form of competitive circuitry remodeling occurs in converging projection areas of the injured and intact MC (Fig. 1C) and that it underlies the maladaptive effects of learning with the nonparetic limb. Ischemic cortical injury reveals an impressive capacity for reactive axonal sprouting of these projections.20, 21, 24 The neural activity-dependence of these responses6, 7, 10 should make them inherently sensitive to experiences that act on the same circuits. These responses are also time-dependent25, which may create an opportunity for the nonparetic limb to dominate reorganizational patterns in a manner that later becomes difficult to reverse.
Conclusion
Learning to rely on the less-affected upper extremity promotes neural changes that subserve its function, while impacting the injured hemisphere in a manner that interferes with functional improvements in the affected limb. The general implication is that, simply by adopting a natural strategy for resuming everyday activities, some stroke survivors could inadvertently squelch the potential for better functionality in the paretic side. We postulate that experience-driven interhemispheric competition in circuitry remodeling underlies the disruptive effects of learning with the nonparetic limb. We also expect that these effects will vary with injury locus and size, because of the pathway specificity of experience-driven and post-ischemic neural plasticity. A better understanding of the neural mechanisms of this phenomenon and its generalization across injuries and functional modalities could be useful for therapeutic decisions on when to promote vs. discourage compensation with less-affected modalities and, ultimately, for understanding how to optimize function bilaterally after stroke.
Acknowledgments
We thank Dr. JE Hsu for figure help.
Sources of Funding: NS056839
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gentile AM, Green S, Nieburgs A, Schmelzer W, Stein DG. Disruption and recovery of locomotor and manipulatory behavior following cortical lesions in rats. Behav Biol. 1978;22:417–455. doi: 10.1016/s0091-6773(78)92547-6. [DOI] [PubMed] [Google Scholar]
- 2.Lang CE, Wagner JM, Edwards DF, Dromerick AW. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J Neurol Phys Ther. 2007;31:56–63. doi: 10.1097/NPT.0b013e31806748bd. [DOI] [PubMed] [Google Scholar]
- 3.Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol. 2011;53:466–475. doi: 10.1002/dev.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: Motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- 5.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones TA, Adkins DL. Behavioral influences on neuronal events after stroke. In: Cramer SC, Nudo RJ, editors. Brain repair after stroke. Cambridge, UK: Cambridge University Press; 2010. pp. 23–34. [Google Scholar]
- 9.Kerr AL, Cheng SY, Jones TA. Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord. 2011;44:538–548. doi: 10.1016/j.jcomdis.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, et al. A role for ephrin-a5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109:E2230–E2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazzaniga MS. Interhemispheric cuing systems remaining after section of neocortical commissures in monkeys. Exp Neurol. 1966;16:28–35. doi: 10.1016/0014-4886(66)90083-5. [DOI] [PubMed] [Google Scholar]
- 12.Kitago T, Liang J, Huang VS, Hayes S, Simon P, Tenteromano L, et al. Improvement after constraint-induced movement therapy: Recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2012;27:99–109. doi: 10.1177/1545968312452631. [DOI] [PubMed] [Google Scholar]
- 13.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 14.Wolf SL. Revisiting constraint-induced movement therapy: Are we too smitten with the mitten? Is all nonuse “Learned”? And other quandaries. Phys Ther. 2007;87:1212–1223. doi: 10.2522/ptj.20060355. [DOI] [PubMed] [Google Scholar]
- 15.Allred RP, Maldonado MA, Hsu JE, Jones TA. Training the ‘less-affected’ forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurol Neurosci. 2005;23:297–302. [PubMed] [Google Scholar]
- 16.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2008;210:172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allred RP, Jones TA. Experience--a double edged sword for restorative neural plasticity after brain damage. Future Neurol. 2008;3:189–198. doi: 10.2217/14796708.3.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: Experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–295. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 23.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- 24.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]