Abstract
Polysaccharide K (PSK) is a widely used mushroom extract that has shown anti-tumor and immunomodulatory effects in both preclinical and clinical studies. Therefore, it is important to understand the mechanism of actions of PSK. We recently reported that PSK can activate toll-like receptor 2 and enhances the function of NK cells. The current study was undertaken to study the effect of PSK on gamma delta (γδ) T cells, another important arm of the innate immunity. In vitro experiments using mouse splenocytes showed that γδ T cells produce IFN-γ after treatment with PSK and have up-regulated expression of CD25, CD69, and CD107a. To investigate whether the effect of PSK on γδ T cells is direct or indirect, purified γδ T cells were cultured either alone or together with bone marrow-derived DC in a co-culture or trans-well system and then stimulated with PSK. Results showed that direct cell-to-cell contact between γδ T cells and DC is required for optimal activation of γδ T cells. There was also reciprocal activation of DC by PSK-activated γδ T cells, as demonstrated by higher expression of costimulatory molecules and enhanced production of IL-12 by DC in the presence of γδ T cells. PSK can also co-stimulate γδ T cells with anti-TCR and anti-CD3 stimulation, in the absence of DC. Finally, in vivo treatment with PSK activates γδ T cells among the tumor infiltrating lymphocytes, and depleting γδ T cells during PSK treatment attenuated the anti-tumor effect of PSK. All together, these results demonstrated that γδ T cells are activated by PSK and contribute to the anti-tumor effect of PSK.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1436-4) contains supplementary material, which is available to authorized users.
Keywords: PSK, Polysaccharide, γδ T cells, TLR, Breast cancer, Innate immunity
Introduction
It is reported that complementary and alternative medicine (CAM) are widely pursued by cancer patients, ranging from 31 to 64 % in different countries [1, 2]. Medicinal mushrooms are among the commonly used natural products by cancer patients, especially in Asian countries [3, 4]. Polysaccharide K (PSK) is a hot water extract of Trametes versicolor and is a prescription drug in Japan [5]. It has shown anti-tumor efficacy in both preclinical and some clinical studies. For example, a meta-analysis of data from 1,094 patients has shown that PSK as an adjuvant to chemotherapy improved both overall and disease-free survival of patients with colorectal cancer [6]. Therefore, it is important to understand the mechanism of action of this product. Our recent study has shown that PSK activates toll-like receptor 2 (TLR2) and enhances the function of DC and NK cells [7]. The current study is undertaken to investigate the effect of PSK on gamma delta (γδ) T cells, another important arm of the innate immunity.
Gamma delta (γδ) T cells are a minor population of peripheral T cells and only account for 2–5 % of total T cells in the peripheral blood, yet they have been shown to play an important role in anti-tumor immunity [8]. They recognize their targets independent of major histocompatibility complex (MHC)-mediated antigen presentation and are considered as part of the innate immunity. γδ T cells can recognize stress-induced antigens on tumor cells such as MICA/B in human and Rae-1 in mice [9, 10]. Mice deficient for γδ T cells are more susceptible to the development of chemically induced cutaneous tumors and spontaneous prostate cancers [10, 11]. γδ T cells have been isolated from human tumors and have been shown to react in vitro to tumor cells but not healthy cells [8]. Activated γδ T cells can produce large amounts of IFN-γ, a cytokine that is critical to anti-tumor immune response [12]. The cytotoxicity of γδ T cells against a range of tumor cell lines has been demonstrated and appears to be greater than αβ T cells, so adoptive therapy using γδ T cells is being actively pursued [13–15]. In vivo activation of human γδ T cells using zoledronic acid followed by adoptive transfer of ex vivo expanded γδ T cells is an attractive strategy for cancer immunotherapy and is currently evaluated in both preclinical and clinical studies [8, 16–19]. Novel agents that can enhance γδ T cell function will be useful in cancer immunotherapy.
It has recently been shown that the expression of TLRs, especially TLR2, TLR3, and TLR4, can be detected on γδ T cells, and TLR agonists may modulate the function of γδ T cell, as summarized in a recent review by Wesch et al. [20]. Based on our recent discovery that PSK activates TLR2, we hypothesize that PSK may modulate the function of γδ T cells. Using neu-transgenic mice, a model of HER2+ breast cancer, the current study aims to investigate the effect of PSK on γδ T cells, the potential mechanism, and the role of γδ T cells in the anti-tumor effect of PSK. Results from this study not only help us understand the immunomodulatory and anticancer effects of PSK, but also reveal the potential of using natural products to modulate γδ T cell function for cancer immunotherapy.
Materials and methods
Animals
A colony of neu-transgenic mice [strain name, FVB/N-TgN (MMTVneu)-202Mul] was established in our animal facilities from breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained as previously described [21]. All of the procedures were performed in compliance with the University of Washington Institutional Animal Care and Use Committee guidelines.
Antibodies and other reagents
Fluorochrome-conjugated monoclonal antibodies against CD3, γδ TCR, CD25, CD69, IFN-γ, and CD107a were from eBiosciences (San Diego, CA). Phosphate-buffered saline (PBS), penicillin–streptomycin, and l-glutamine were obtained from Invitrogen Life Technologies (Grand Island, NY). PSK was purchased from Kureha Pharmaceuticals (Japan). PSK was dissolved in PBS at a stock concentration of 10 mg/ml. Aliquots of 100 μl were stored at −80 °C. The frozen aliquots were thawed immediately before use.
Measurement of PSK-induced IFN-γ production and γδ T cell activation in splenocytes
Total splenocytes from neu-transgenic mice were stimulated with PSK (25–400 μg/ml) for 24 h, and the culture supernatant was collected for IFN-γ measurement using an ELISA kit from eBiosciences. To determine the cellular source of PSK, intracellular staining of IFN-γ was performed using similar method as described before [7]. Brefeldin A (5 μg/ml, Sigma-Aldrich), a secretion inhibitor, was included during the last 16 h of the 48-h incubation in complete RPMI with or without PSK (100 μg/ml). At the end of the activation, the cells were first stained with fluorophore-conjugated antibodies to surface markers. After subsequent fixation and permeabilization, the cells were stained with anti-IFN-γ-PE. Samples were acquired on FACS Canto II. List mode files were analyzed using FlowJo (Treestar, OR). The expression of CD25 and CD69 on γδ T cells was measured after 24 or 48 h of stimulation with PSK.
CD107a degranulation assay
The degranulation of γδ T cells was measured by the expression of CD107a, lysosome-associated membrane protein-1 (LAMP-1). In brief, splenocytes were treated with PSK (100 μg/ml) or medium alone for 6 h. Anti-CD107a-PE antibody was added directly to the co-cultures. After 1-h incubation, brefeldin A was included to the culture and incubated for another 5 h. Cells were then stained with mAbs for CD3 and γδ TCR and analyzed on FACS Canto II.
Co-culture of γδ T cells with bone marrow-derived DC (BMDC) and PSK stimulation
Bone marrow cells were collected from neu-transgenic mice and filtered through a 0.7-μm cell strainer. ACK solution was used to lyse red blood cells, and remaining cells were added to a 6-well plate for 4 h. Non-attached cells were removed, and fresh culture medium (20 ng/ml IL-4 + 20 ng/ml GM-CSF) was added. On Day 3, non-attached cells were collected and replaced with fresh medium that was added back to the culture. On Day 7, cells were harvested. γδ T cells were MACS-enriched from splenocytes using the γδ T cell isolation kit (Miltenyi) and cultured in 96-well round-bottom plates coated with anti-mouse CD3ε (5 μg/ml) in complete RPMI medium plus rIL-2 (20 U/ml) for 3–4 days. Cells were then transferred to uncoated wells, and fresh medium was added (complete medium + rIL-2) for 3 more days. Both BMDCs and γδ T cells were harvested and added to 24-well plates at 105 cells/well at 1:1 ratio. For trans-well (0.4 μm pore size) inserts, γδ T cells were placed in the lower chamber and BMDCs were placed in the upper chamber. PSK (100 μg/ml) was added to the RPMI medium, and cells were incubated for 24 and 48 h. At the end of incubation, culture supernatant was harvested for analysis of IFN-γ by ELISA and the cells were harvested and stained for CD25-APC, CD69-PE-Cy7 to measure activation of γδ T cells. Intracellular staining of IFN-γ and CD107a expression was also performed similarly as described above, except that both brefeldin A (5 μg/ml) and monensin (5 μg/ml) were included as secretion inhibitors. To measure the activation of BMDC by PSK in the presence or absence of γδ T cells, BMDC culture alone or BMDC-γδ T cell co-culture was treated with PSK (100 μg/ml) for 24 or 48 h in 96-well flat-bottom plates. The expression of CD40 and CD86 on DC was measured by FACS at the end of the treatment. The secretion of IL-12p40 and IL-12p70 into culture supernatant was measured by ELISA using kits from eBiosciences.
Culture of purified γδ T cells and PSK co-stimulation with anti-γδ TCR or anti-CD3 mAb
MACS-purified γδ T cells were cultured in complete RPMI in 96-well plates at 2 × 105 cells/well. Some of the wells were coated with anti-γδ TCR mAb (clone UC7-13D5, 5 μg/ml) or anti-CD3 mAb (clone 145-2C11, 5 μg/ml). PSK (100 μg/ml) was added to the RPMI medium, and cells were incubated for 24 and 48 h. At the end of incubation, culture supernatant was harvested for IFN-γ ELISA.
Treatment of tumor-bearing mice with PSK and selective depletion of γδ T cells
Neu-transgenic mice received subcutaneous implant of 1 million MMC cells, a cell line derived from a syngeneic spontaneous breast cancer in these mice [22]. To measure the in vivo activation of γδ T cells, the mice with implanted MMC tumors (average size ≈600 mm3, n = 8 per group) received a single intratumoral injection of PSK (1 mg, dissolved in 100 μl of PBS) or control PBS on Day 1 and tumors were harvested 48 h after the PSK treatment. Tumor infiltrating lymphocytes (TIL) were isolated as previously described [21]. FACS analysis was performed to measure the activation status of γδ T cells in TIL and splenocytes after staining with anti-CD3, γδ TCR, and CD69 mAbs. To determine the role of γδ T cells in the anti-tumor effects of PSK, mice started PSK treatment (100 mg/kg, oral gavage, 3 times per week) when the tumors became palpable and the treatment continued for 4 weeks. Mice in the control group received oral gavage of PBS of the same volume. Mice in the γδ T cell depletion group received hamster anti-mouse anti-γδ TCR mAb (clone UC7-13D5, 200 μg, i.p., twice a week) during PSK treatment to deplete γδ T cells. Mice in the control group received a control hamster IgG during PSK treatment. Tumors were measured every other day with vernier calipers, and tumor volume was calculated as the product of length × width × height × 0.5236. In vivo data are presented as mean ± sem of each treatment group (n = 5 per group).
Statistical analysis
Statistical analysis was performed using GraphPad Software (San Diego, CA). Data were analyzed using the two-tailed Student’s t test or Mann–Whitney test. A value of p < 0.05 was considered statistically significant.
Results
PSK induces IFN-γ production and CD107a degranulation of γδ T cells in splenocytes
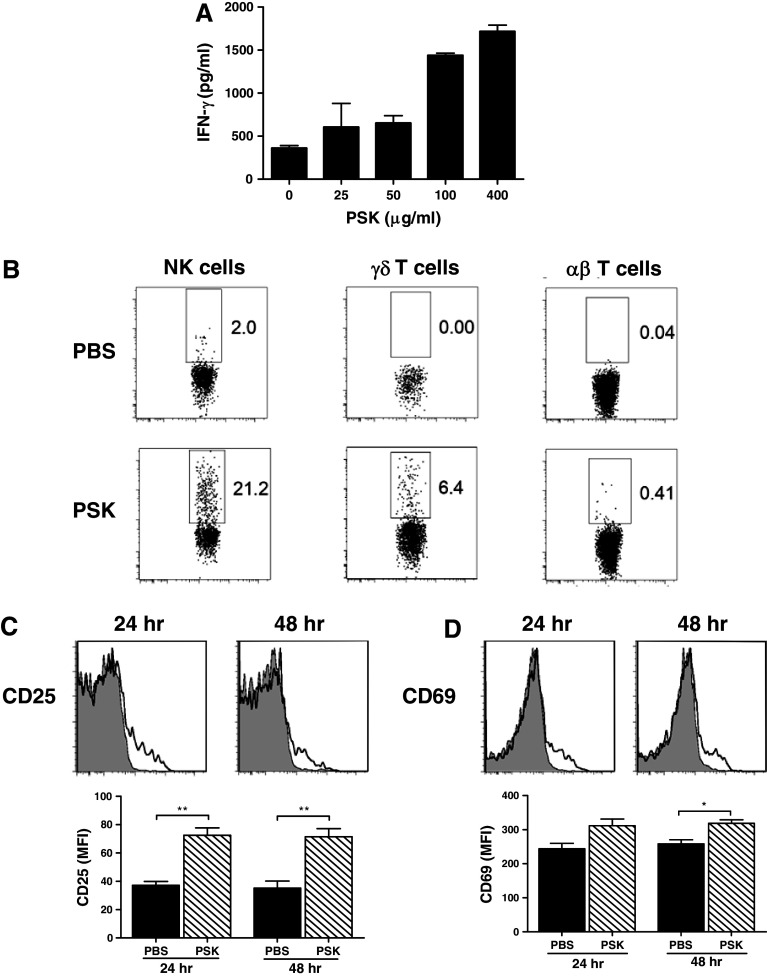
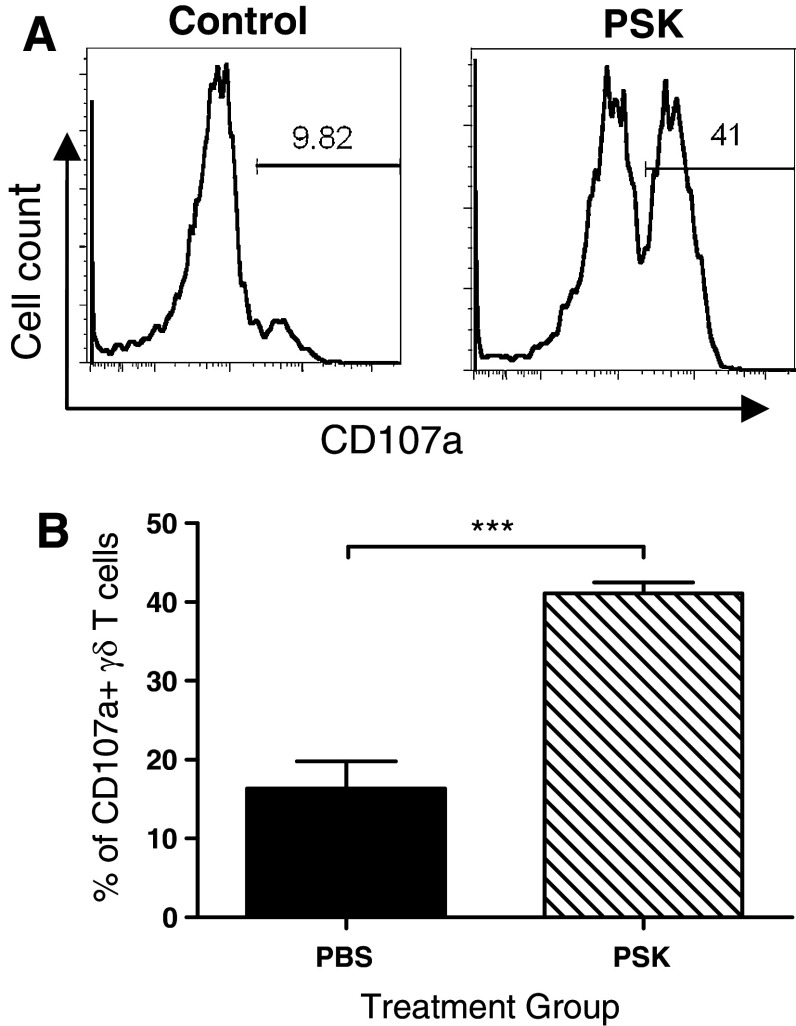
When total splenocytes from neu-transgenic mice were stimulated with PSK (25–400 μg/ml, 24 h), there was a dose-dependent induction of IFN-γ (Fig. 1a). Intracellular staining showed that NK cells and γδ T cells, but not αβ T cells, were the major producers of PSK-induced IFN-γ (Fig. 1b). The effect of PSK on NK cells has been previously reported by our group and others [7, 23, 24], so the current study focused on the effect of PSK on γδ T cells. The activation of γδ T cells was demonstrated by up-regulated expression of CD25 and CD69 after PSK treatment (Fig. 1c, d). Interestingly, the expression of CD25 and CD69 on αβ T cells was not significantly affected by PSK treatment (data not shown), suggesting γδ T cells might be more sensitive to PSK treatment than αβ T cells. The preferential activation of γδ T cells over αβ T cells was observed at different doses of PSK tested (10, 50, or 100 μg/ml, Supplemental Figure 1). PSK also induced CD107a expression in γδ T cells. As shown in Fig. 2a, b, the percentage of CD107a-positive γδ T cells among total γδ T cells are 16.3 ± 3.5 % in control group and 41.0 ± 1.3 % (p = 0.0002 from control) in splenocytes treated with PSK (100 μg/ml, 6 h) (Fig. 2b).
Fig. 1.
Gamma delta T cells are activated by PSK to produce IFN-γ. a Dose–response of PSK-stimulated IFN-γ production by splenocytes. Shown are mean ± SD of IFN-γ levels in triplicate culture wells treated with serial dilutions of PSK for 48 h, as determined by ELISA. Similar results were obtained from three independent experiments. b Representative FACS dot plots showing intracellular staining of IFN-γ in NK cells, γδ T cells, or αβ T cells in splenocytes from neu-transgenic mice. Splenocytes were treated with PSK (100 μg/ml) for 48 h. Similar results were obtained from three independent experiments. c, d Expression of CD25 and CD69 on γδ T cells in PSK-treated splenocytes. Shown are representative overlay histograms. Gray histogram: control; empty histogram: PSK-treated γδ T cells. The bar graphs summarize the results from three independent experiments. *p < 0.05, **p < 0.01 by two-tailed Student’s t test
Fig. 2.
PSK induces CD107a degranulation in γδ T cells. a Representative histograms showing the percentages of CD107a+ γδ T cells in control and PSK-treated splenocytes. b Summary graph showing the percentages of CD107a+ cells among total γδ T cells. The bars represent mean ± sem of results from three independent experiments. ***p < 0.001 by Student’s t test
Direct contact between γδ T cells and DC is required for optimal activation of γδ T cells by PSK
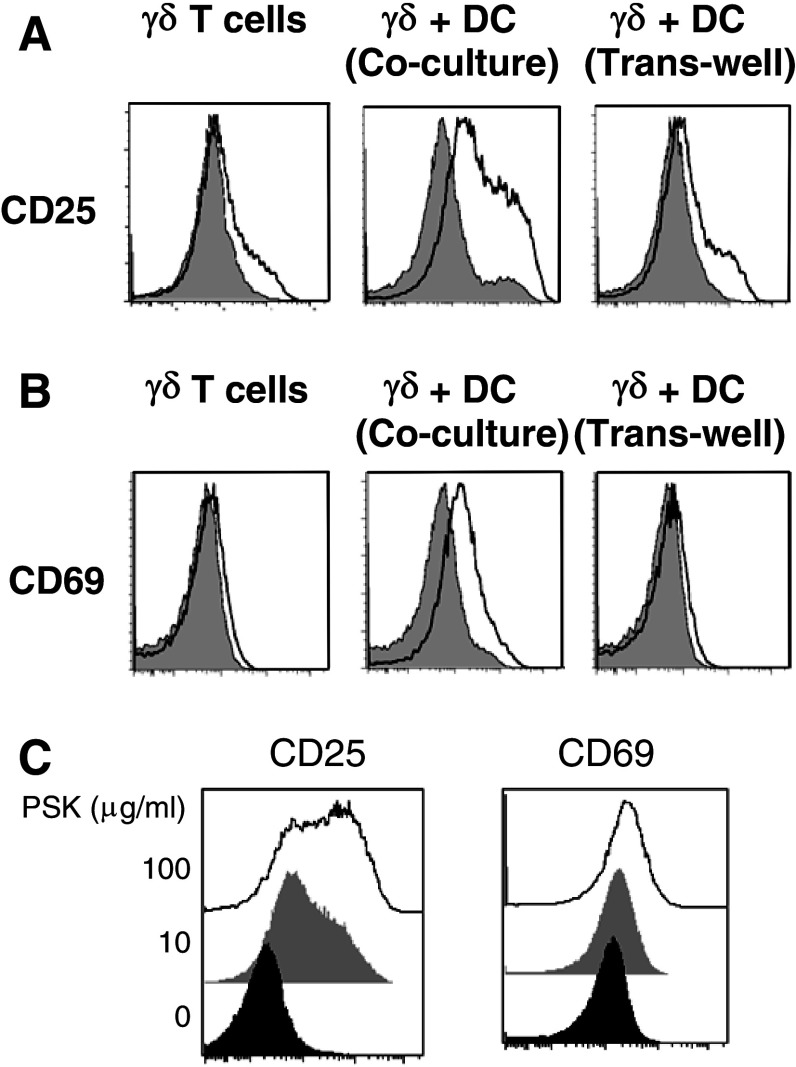
To investigate whether the effect of PSK on γδ T cells is direct or indirect via activation of DC, we used PSK to treat MACS-purified γδ T cells, co-culture of purified γδ T cells and BMDC, or trans-well culture of purified γδ T cells and BMDC. The activation of γδ T cells was measured after 24- or 48-h stimulation with PSK (100 μg/ml). As shown in Fig. 3a, b, PSK treatment of γδ T cells alone resulted in very low-level induction of CD25 and CD69. PSK treatment of γδ T cells and DC co-culture (in the same culture well), but not trans-well culture, resulted in optimal activation of γδ T cells (Fig. 3a, b), indicating that direct cell-to-cell contact between γδ T cells and DC is required for the activation of γδ T cells by PSK. In the γδ T cells alone and trans-well cultures, the low-level induction of CD25 and CD69 on γδ T cell culture is only observed after treatment with high dose of PSK (100 μg/ml). In the γδ-DC co-culture system, even low dose of PSK (10 μg/ml) resulted in significant induction of CD25 and CD69 (Fig. 3c).
Fig. 3.
Direct contact between DC and γδ T cells is required for optimal activation of γδ T cells by PSK. a Representative overlay histograms and summary graphs showing CD25 expression in PSK-treated purified γδ T cells, γδ + DC co-culture, and γδ + DC trans-well culture. b Representative overlay histograms and summary graphs showing CD69 expression in PSK-treated purified γδ T cells, γδ + DC co-culture, and γδ + DC trans-well culture. Gray histogram: control; empty histogram: PSK-treated γδ T cells. c Dose-dependent induction of CD25 and CD69 on γδ T cells in γδ + DC co-culture. Black histogram: control; gray histogram: low-dose PSK (10 μg/ml); white histogram: high-dose PSK (100 μg/ml)
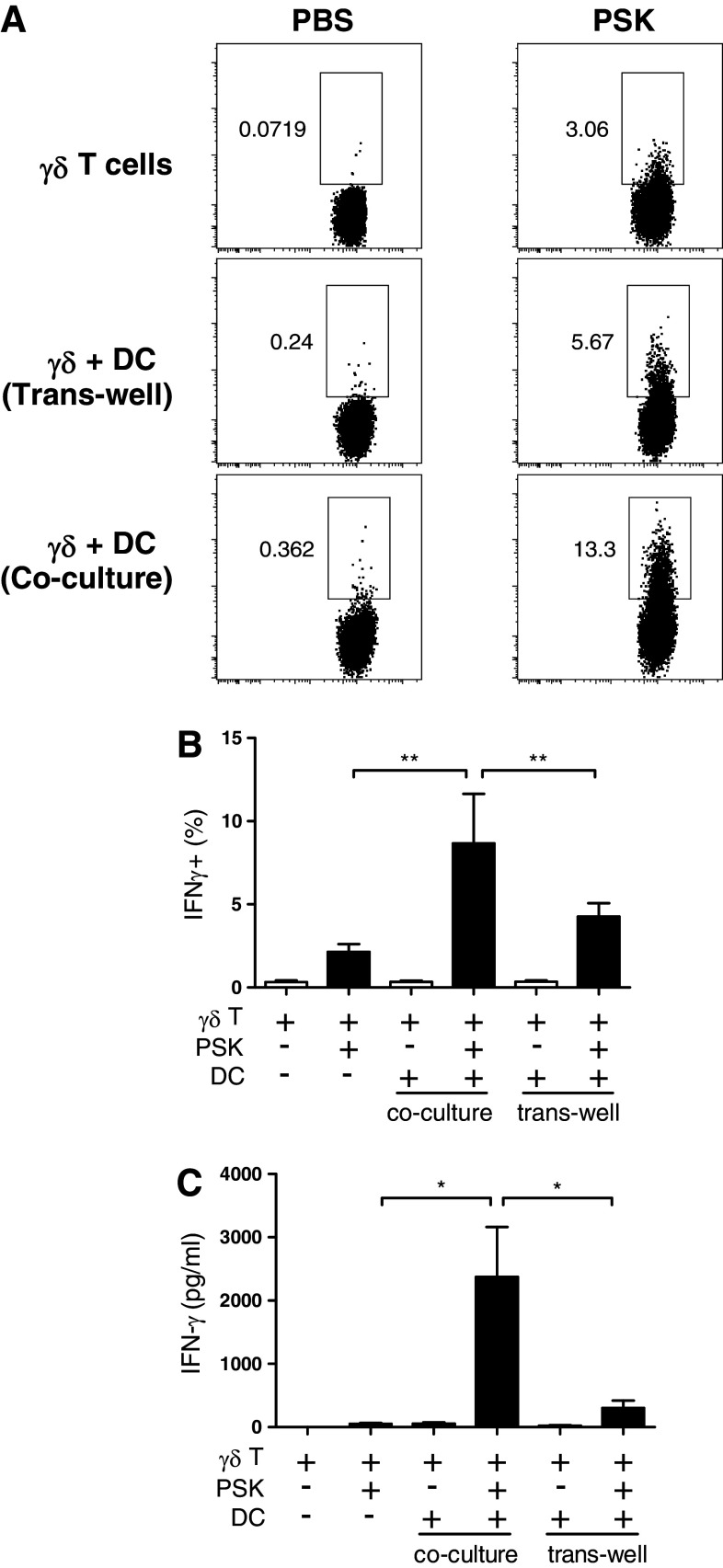
Measurement of IFN-γ production in γδ T cells cultured alone or in the presence of BMDC yielded results consistent with CD25 and CD69 expression, and the optimal induction of IFN-γ was only observed in the co-culture system (Fig. 4a). The percentages of IFN-γ+ γδ T cells in the co-culture were significantly higher than those in the γδ T cells alone or γδ-DC trans-well culture (Fig. 4b). ELISA measurement of IFN-γ level in culture supernatant confirmed that γδ + DC co-culture produced the highest level of IFN-γ in response to PSK. As shown in Fig. 4c, PSK (100 μg/ml, 24 h)–treated γδ + DC co-culture produced 2,372 ± 790 pg/ml IFN-γ, significantly higher than PSK-treated γδ T cells alone (48 ± 16 pg/ml) or PSK–treated γδ-DC trans-well culture (303 ± 114 pg/ml). PSK also induced CD107a expression in purified γδ T cells, and the effect is most significant when the γδ T cells were co-cultured with DC (Supplemental Figure 2).
Fig. 4.
Direct contact between DC and γδ T cells is required for PSK-induced IFN-γ production. a Representative dot plots showing the expression of IFN-γ in control PBS- and PSK-treated γδ T cells in different culture systems: γδ T cell alone, γδ + DC trans-well culture, or γδ + DC co-culture. b Bar graph summarizing the percentage of IFN-γ+ γδ T cells in PBS- and PSK-treated γδ T cells in different culture systems. The graph summarizes the results from three independent experiments. c Bar graph summarizing the level of IFN-γ in PBS- and PSK-treated γδ T cells in different culture systems. The graph summarizes the results from three independent experiments. *p < 0.05, **p < 0.01, by two-tailed Student’s t test
We have previously shown that PSK is a TLR2 agonist and the effect of PSK on DC activation is dependent on TLR2 [7]. To investigate the role of TLR2 in γδ T cell activation, we used side-by-side culture of γδ T cells and BMDC from wild-type C57/B6 mice and cells from TLR2−/− mice. Results showed that PSK-induced γδ T cell activation (as measured by IFN-γ release) is significantly decreased in cells from TLR2−/− mice (Supplemental Figure 3). Mismatched cultures of γδ T cells and BMDC from wild-type or TLR2−/− mice showed that the presence of TLR2 on DC is more important than TLR2 on γδ T cells in the activation effect of PSK.
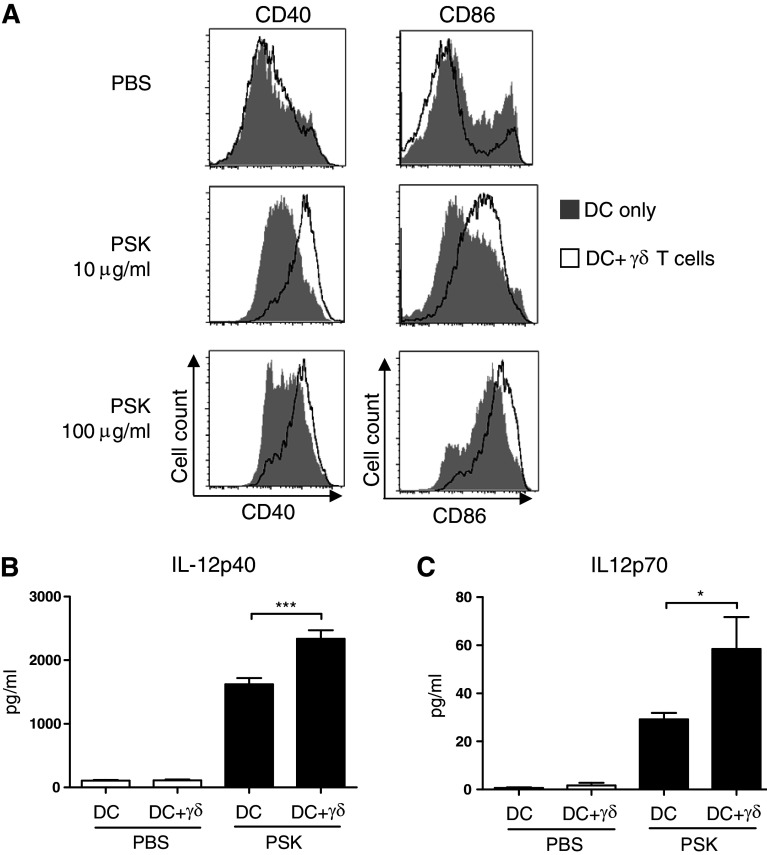
PSK-activated γδ T cells reciprocally activates DC
Activated γδ T cells produce large amounts of IFN-γ, a cytokine that has been shown to play a critical role in DC activation. Therefore, we hypothesize that PSK-activated γδ T cells may reciprocally activate DC. Comparison of DC activation by PSK in BMDC cultured alone or BMDC co-cultured with γδ T cell showed that PSK treatment (10 or 100 μg/ml, 24 h) leads to significantly higher expression of costimulatory molecules, CD40 and CD86, when BMDC is co-cultured with γδ T cells (Fig. 5a). Measurement of IL-12 in culture supernatant showed that in the presence of γδ T cells, PSK stimulation resulted in significantly higher production of both IL-12p40 and IL-12p70 from DC (Fig. 5b, c).
Fig. 5.
Reciprocal activation of DC in the BMDC-γδ T cell co-culture leads to increased expression of co-stimulatory molecules and enhanced production of IL-12. BMDC and BMDC-γδ T cell co-culture were treated with PSK for 24 h, and the cells were harvested for FACS analysis of DC activation markers. The culture supernatant was analyzed for IL-12 using ELISA. a Expression of CD40 and CD86 in BMDC treated with control RPMI medium or PSK (10 or 100 μg/ml). Shaded histograms: DC alone culture; empty histograms: DC + γδ T cell co-culture. b, c The levels of IL-12p40 and IL-12p70 in control untreated DC or DC + γδ T cell co-culture (white columns) and PSK-treated DC or DC + γδ T cell co-culture (black columns). *p < 0.05, by two-tailed Student’s t test
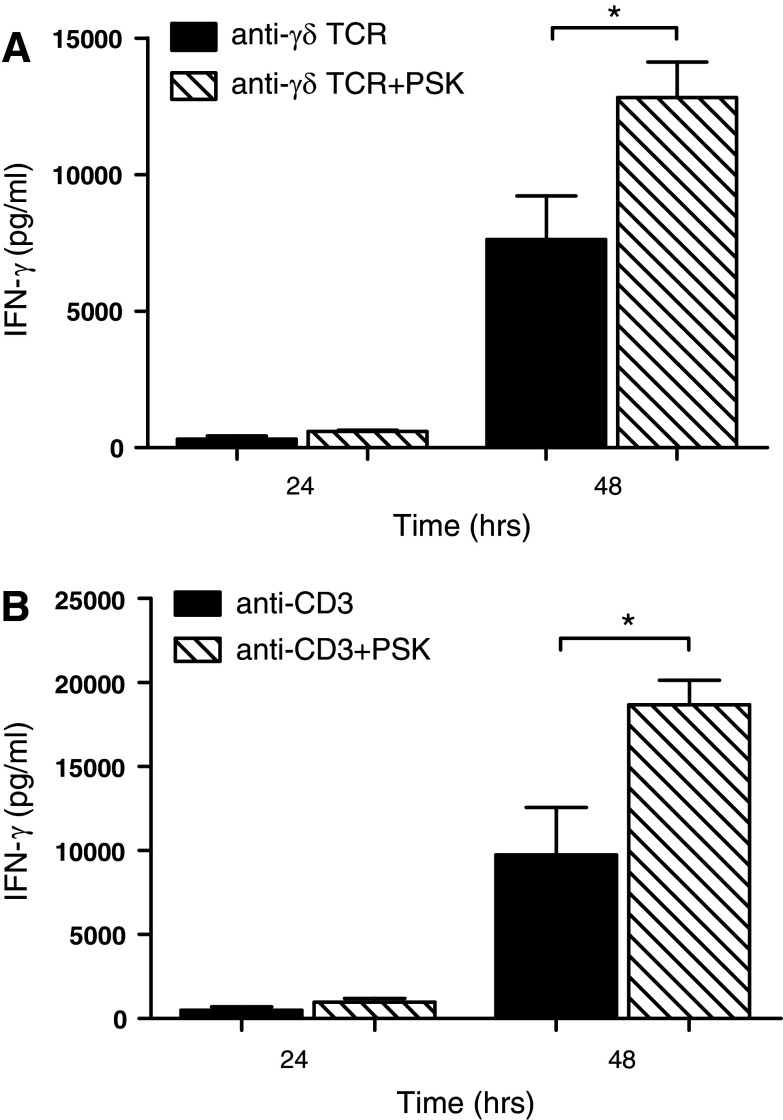
PSK co-activates purified γδ T cells with TCR cross-linking in the absence of DC
It has recently been shown that TLR agonists can activate γδ T cells directly by co-stimulating γδ T cells with TCR [25], so we questioned whether PSK and anti-γδ TCR mAb can activate γδ T cells in the absence of DC. MACS-purified γδ T cells were cultured on anti-γδ TCR mAb-coated plates in the presence or absence of PSK. As shown in Fig. 6a, the addition of PSK to anti-γδ TCR resulted in enhanced IFN-γ production (7634 ± 1591 pg/ml IFN-γ in γδ T cells stimulated with anti-γδ TCR alone for 48 h, 12,823 ± 1,293 pg/ml in γδ T cells stimulated with anti- γδ TCR and PSK for 48 h, p = 0.04). In a similar fashion, PSK augmented anti-CD3 mAb-induced IFN-γ production (Fig. 6b).
Fig. 6.
PSK co-stimulates purified γδ T cells with anti-γδ TCR mAb or anti-CD3 mAb to produce IFN-γ. a Purified γδ T cells were cultured on anti-γδ TCR mAb-coated plates in the presence or absence of PSK (100 μg/ml). The presence of PSK resulted in higher production of IFN-γ, as measured by ELISA. The bar graph summarizes results from three independent experiments. b Purified γδ T cells were cultured on anti-CD3 mAb-coated plates in the presence or absence of PSK (100 μg/ml). The presence of PSK resulted in higher production of IFN-γ, as measured by ELISA. The bar graph summarizes results from three independent experiments. *p < 0.05, by two-tailed Student’s t test
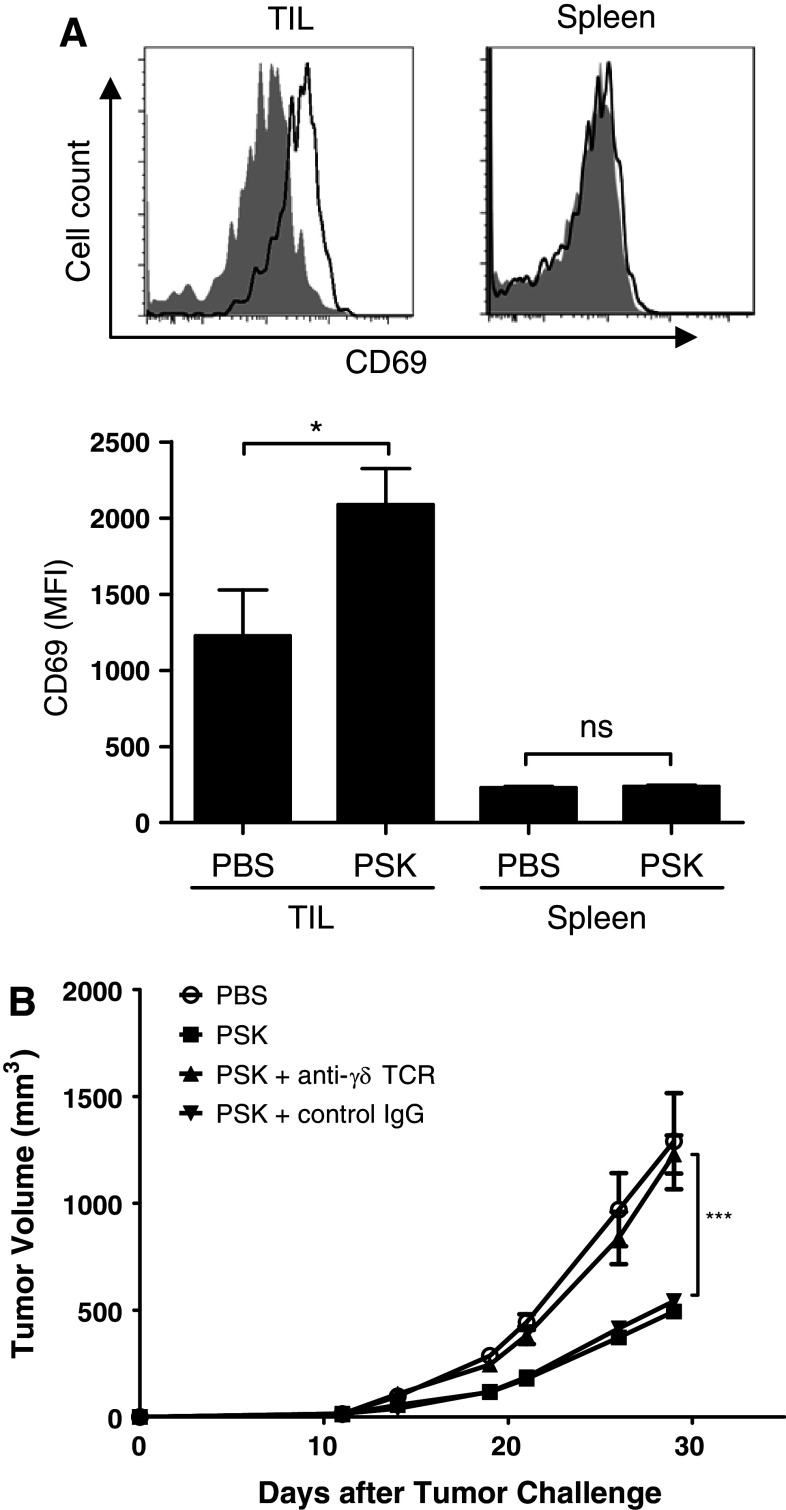
In vivo PSK treatment activates γδ T cells in TIL and γδ T cell contributes to the anti-tumor effect of PSK
Intratumoral injection of PSK resulted in activation of γδ T cells in TIL, but not in splenocytes. As shown in Fig. 7a, γδ T cells in TIL have significantly higher expression of CD69 after PSK treatment. The expression of CD69 on γδ T cells in splenocytes was much lower as compared to that in TIL and showed no difference between PBS and control PSK group (Fig. 7a). To determine the potential contribution of γδ T cells to the anti-tumor effect of PSK, mice were depleted of γδ T cells during PSK treatment using a mAb against γδ TCR. As shown in Fig. 7b, selective depletion of γδ T cells during PSK treatment significantly attenuated the anti-tumor effect of PSK (Fig. 7b), suggesting that γδ T cells contribute to the anti-tumor effect of PSK.
Fig. 7.
In vivo PSK treatment activates γδ T cells in TIL, and γδ T cell contributes to the anti-tumor effect of PSK. a Intratumoral injection of PSK results in CD69 upregulation on γδ T cells in TIL, but not in spleen. Shown are representative overlay histograms of CD69 expression on γδ T cells in TIL or spleen from control PBS- and PSK-treated mice. Shaded histogram PBS-treated mice (control); empty histogram: PSK-treated mice. The summary bar graph shows the mean fluorescence intensity (MFI) of CD69 expression in γδ T cells (mean ± sem) in TIL and spleen in PBS or PSK group (n = 8 mice per group). *p < 0.05, by two-tailed Student’s t test. b Depletion of γδ T cells during PSK treatment decreased the anti-tumor effect of PSK. Mice received anti-γδ TCR mAb or a control hamster IgG during PSK treatment. PSK by itself significantly inhibited tumor growth, and the effect is significantly attenuated when mice received anti-γδ TCR mAb. **p < 0.01 between PSK + γδ T cell depletion group (filled triangle) and PSK group (open square); ***p < 0.0001 between control PBS (open circle) and PSK group (open square). There was no difference between PSK + control IgG (filled inverted triangle) and PSK group (open square). N = 5 mice per group. Similar results were obtained from two independent experiments
Discussion
There has been a gap between the rapid progress in tumor immunology and our limited understanding of the mechanism of action of natural products. Our study was aimed to address the gap. The recent progress in our understanding of γδ T cell regulation indicates TLR agonists can activate γδ T cells in a direct or indirect manner [20]. Our study proves that PSK, a natural product with TLR2 agonist activity, can activate γδ T cells. To our knowledge, this is the first report on the effect of PSK on γδ T cells. These results provide novel insights into the mechanisms of the anti-tumor and immunostimulatory effects of PSK and also indicate the potential of using natural products to augment γδ T cell function.
The anti-tumor effects of PSK have been demonstrated in multiple clinical trials conducted in Japan. As an adjuvant to chemotherapy, it has shown beneficial effect on overall and progression-free survival, especially for patients with stomach and colorectal cancer [6, 26, 27]. Understanding the mechanism of action of this product is critically important. Our recent study has shown that PSK activates TLR2 and enhances the function of DC and NK cells [7]. In the current study, we demonstrated that γδ T cells are also activated by PSK. We have shown that γδ T cells can produce large amounts of IFN-γ in response to PSK stimulation (>2 ng/ml). IFN-γ plays an important role in promoting anti-tumor immune response and controlling tumor growth [28]. This cytokine also provides a secondary signal for DC activation. As expected, the presence of PSK-activated γδ T cells further contributes to DC activation by PSK leading to higher expression of costimulatory molecules and enhanced IL-12 production, which is important for priming of T cell and NK cell responses [29]. This complex interaction between PSK-activated DC, γδ T cells, αβ T cells, and NK cells might help explain that although our previous studies using selective depletion of CD4, CD8, and NK cells during PSK treatment suggest the anti-tumor effect of PSK is dependent on CD8 T cells and NK cells [7], the current study using selective depletion of γδ T cells suggests γδ T cells also contribute to the anti-tumor effect of PSK. Therefore, this information has improved our understanding of the multi-faceted immunomodulatory effects of PSK.
DC-dependent activation of γδ T cells by TLR agonists has previously been reported for TLR3 agonist poly (I:C), TLR4 agonist LPS, and TLR5 agonist flagellin [30, 31]. Devilder et al. [30] reported that poly(I:C), LPS, and flagellin activated human Vγ9 Vδ2 T cells through a process that strictly required the presence of either myeloid or plasmacytoid DC expressing the relevant TLR, and the response of γδ T cells to TLR agonists-treated DC requires type I IFN but not IL-12. Kunzmann et al. [31] also reported that poly (I:C)-mediated activation of γδ T cells is indirect and mediated via type I IFN derived from TLR3-expressing CD11c+ DCs. In our study, we observed that the optimal activation of mouse γδ T cells by PSK requires the cell-to-cell contact between DC and γδ T cells. At this point, we do not know what ligand and receptor are involved in PSK-induced γδ T cell activation by DC, we hypothesize that NKG2D ligand and receptor might be involved. NKG2D is an important activating receptor on γδ T cells [32–34]. The induction of NKG2D ligand expression by TLR agonists has been reported in other publications [35–37]. We also observed induction of MICA/B on human DC by PSK but did not observe the induction of mouse NKG2D ligands (Rae-1γ or Mult1) by PSK on mouse DC (data not shown), so the ligand(s) involved in PSK-stimulated DC and γδ T cell reciprocal activation remains to be identified. Unpublished data from our laboratory have shown that PSK does not induce IFN-α in PBMC, a cytokine that has previously been shown to mediate the DC-dependent activation of human γδ T cells [30, 31]. This might explain the lack of activation we observed in the trans-well culture of BMDC and murine γδ T cells.
In this study, we also observed DC-independent co-activation of γδ T cells with anti-CD3 and anti-TCR stimulation. This is consistent with previous reports that TLR1, 2, and 6 ligands have a direct co-stimulatory effect on human and mouse γδ T cells [20, 38, 39]. The publication by Deetz et al. [25] has reported activation of human γδ T cells by TLR2 agonist Pam3Cys. Hedges et al. [40] reported that bovine γδ T cells can respond directly to LPS and peptidoglycan. As for murine γδ T cells, Martin et al. showed that IL-17-producing γδ T cells but not other γδ T cell express TLR1 and TLR2 and could directly react to PAMP. Interestingly, our study suggests IFN-γ producing murine γδ T cells are also responsive to TLR2 agonist PSK. Whether the difference could be due to mouse strain or other experimental setting remains to be investigated. In addition to TLR2, mouse γδ T cells have been shown to respond to treatment with TLR4 agonist LPS and produce both IFN-γ and IL-17, which contributes to the development of experimental autoimmune encephalomyelitis (EAE) [41]. Our current finding on the effect of PSK not only adds to the published literature that TLR agonists can modulate γδ T cell function, but also highlights the potential of using natural products to enhance γδ T cell function.
Given the important role of γδ T cells in anti-tumor immunity, the potential of using natural products to enhance γδ T cell function is an area that needs more investigation. Indeed, several studies have shown promising results on the stimulatory effect of natural products on γδ T cells. A study by Kamath et al. [42] has shown that oral consumption of a tea component, L-theanine, enhanced γδ T cell proliferation and IFN-γ secretion. A recent study showed that polysaccharides isolated from Acai fruit induce innate immune response and activate γδ T cells in human, mouse, and bovine PBMC cultures [43]. Another study showed that polysaccharides derived from Yamoa (Funtumia elastic) can activate γδ T cells from different species and are more potent than LPS in activating human γδ T cells [44]. Most of these findings remain to be tested in clinical trials.
Although PSK and other mushroom products have been tested in multiple clinical trials in Asian countries, it has rarely been tested in Western countries. Two recent clinical trials in the United States have tested Grifola frondosa and T. versicolor in breast cancer patients [45, 46]. There is some preliminary evidence that these mushroom products could induce cytokines and modulate NK cell function in vivo [45, 46]. Results from our current study would encourage more studies on the effect of mushroom extracts on human γδ T cells. Indeed, some pilot in vitro experiments using human PBMC have shown that PSK-stimulated IFN-γ production in human γδ T cells (data not shown). However, given the difference in human and murine γδ T cells and the functional difference between different subsets of human γδ T cells, we cannot simply extrapolate the data from mouse to human. Further investigations are needed on the effect of PSK on human γδ T cells. Our group has recently obtained FDA approval to use PSK in clinical trial in cancer patients. Whether γδ T cell function may be improved in cancer patients after oral administration of PSK will be evaluated in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by NIH grants R01CA138547 (Hailing Lu), R01AT004314 (Mary L. Disis), U19AT006028 (Mary L. Disis), and a gift to the Tumor Vaccine Group.
Conflict of interest
The authors declare they have no conflict of interest.
Abbreviation
- CAM
Complementary and alternative medicine
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cells
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- γδ
Gamma delta
- IFN-γ
Interferon gamma
- IL
Interleukin
- mAb
Monoclonal antibody
- NK
Natural killer
- PAMP
Pathogen-associated molecular patterns
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PSK
Polysaccharide K
- TIL
Tumor infiltrating lymphocytes
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
References
- 1.Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer. 1998;83:777–782. doi: 10.1002/(SICI)1097-0142(19980815)83:4<777::AID-CNCR22>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: implications for research and clinical care. J Clin Oncol. 2005;23:2590–2592. doi: 10.1200/JCO.2005.11.922. [DOI] [PubMed] [Google Scholar]
- 3.Hyodo I, Amano N, Eguchi K, Narabayashi M, Imanishi J, Hirai M, Nakano T, Takashima S. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005;23:2645–2654. doi: 10.1200/JCO.2005.04.126. [DOI] [PubMed] [Google Scholar]
- 4.Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- 5.Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res. 2002;22:1737–1754. [PubMed] [Google Scholar]
- 6.Sakamoto J, Morita S, Oba K, Matsui T, Kobayashi M, Nakazato H, Ohashi Y. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol Immunother. 2006;55:404–411. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H, Yang Y, Gad E, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin Cancer Res. 2011;17:67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 9.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez RD, Xu S, Guo B, Negrin RS, Waller EK. CD2-mediated IL-12-dependent signals render human gamma delta-T cells resistant to mitogen-induced apoptosis, permitting the large-scale ex vivo expansion of functionally distinct lymphocytes: implications for the development of adoptive immunotherapy strategies. Blood. 2000;96:3827–3837. [PubMed] [Google Scholar]
- 16.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9 Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–1297. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma}delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck BH, Kim HG, Kim H, Samuel S, Liu Z, Shrestha R, Haines H, Zinn K, Lopez RD. Adoptively transferred ex vivo expanded gammadelta-T cells mediate in vivo antitumor activity in preclinical mouse models of breast cancer. Breast Cancer Res Treat. 2010;122:135–144. doi: 10.1007/s10549-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L. Harnessing gammadelta T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of gammadelta T cell responses by TLR ligands. Cell Mol Life Sci. 2011;68:2357–2370. doi: 10.1007/s00018-011-0699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Knutson KL, Gad E, Disis ML. The tumor antigen repertoire identified in tumor-bearing Neu transgenic mice predicts human tumor antigens. Cancer Res. 2006;66:9754–9761. doi: 10.1158/0008-5472.CAN-06-1083. [DOI] [PubMed] [Google Scholar]
- 22.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Yang Y, Gad E, Inatsuka C, Wenner CA, Disis ML, Standish LJ. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin Cancer Res. 2011;17:6742–6753. doi: 10.1158/1078-0432.CCR-11-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez E, Garcia-Lora A, Martinez M, Garrido F. Identification of the protein components of protein-bound polysaccharide (PSK) that interact with NKL cells. Cancer Immunol Immunother. 2005;54:395–399. doi: 10.1007/s00262-004-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2 Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oba K, Teramukai S, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol Immunother. 2007;56:905–911. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torisu M, Hayashi Y, Ishimitsu T, et al. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunol Immunother. 1990;31:261–268. doi: 10.1007/BF01740932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 30.Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 31.Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112:369–377. doi: 10.1111/j.1365-2567.2004.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185:55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- 33.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/S1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 34.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 35.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 36.Kloss M, Decker P, Baltz KM, Baessler T, Jung G, Rammensee HG, Steinle A, Krusch M, Salih HR. Interaction of monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA. J Immunol. 2008;181:6711–6719. doi: 10.4049/jimmunol.181.10.6711. [DOI] [PubMed] [Google Scholar]
- 37.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184:6901–6909. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 38.Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, Wesch D, Kabelitz D. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol. 2009;70:245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 39.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Hedges JF, Lubick KJ, Jutila MA. Gamma delta T cells respond directly to pathogen-associated molecular patterns. J Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc Natl Acad Sci USA. 2003;100:6009–6014. doi: 10.1073/pnas.1035603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holderness J, Schepetkin IA, Freedman B, Kirpotina LN, Quinn MT, Hedges JF, Jutila MA. Polysaccharides isolated from Acai fruit induce innate immune responses. PLoS ONE. 2011;6:e17301. doi: 10.1371/journal.pone.0017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graff JC, Kimmel EM, Freedman B, Schepetkin IA, Holderness J, Quinn MT, Jutila MA, Hedges JF. Polysaccharides derived from Yamoa (Funtumia elastica) prime gammadelta T cells in vitro and enhance innate immune responses in vivo. Int Immunopharmacol. 2009;9:1313–1322. doi: 10.1016/j.intimp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng G, Lin H, Seidman A, et al. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J Cancer Res Clin Oncol. 2009;135:1215–1221. doi: 10.1007/s00432-009-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torkelson CJ, Sweet E, Martzen MR, Sasagawa M, Wenner CA, Gay J, Putiri A, Standish LJ. Phase 1 clinical trial of trametes versicolor in women with breast cancer. ISRN Oncol. 2012;2012:251632. doi: 10.5402/2012/251632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.