Abstract
The fungal vacuole is an extremely complex organelle that is involved in a wide variety of functions. The vacuole not only carries out degradative processes, the role most often ascribed to it, but also is the primary storage site for certain small molecules and biosynthetic precursors such as basic amino acids and polyphosphate, plays a role in osmoregulation, and is involved in the precise homeostatic regulation of cytosolic ion and basic amino acid concentration and intracellular pH. These many functions necessitate an intricate interaction between the vacuole and the rest of the cell; the vacuole is part of both the secretory and endocytic pathways and is also directly accessible from the cytosol. Because of the various roles and properties of the vacuole, it has been possible to isolate mutants which are defective in various vacuolar functions including the storage and uptake of metabolites, regulation of pH, sorting and processing of vacuolar proteins, and vacuole biogenesis. These mutants show a remarkable degree of genetic overlap, suggesting that these functions are not individual, discrete properties of the vacuole but, rather, are closely interrelated.
Full text
PDF
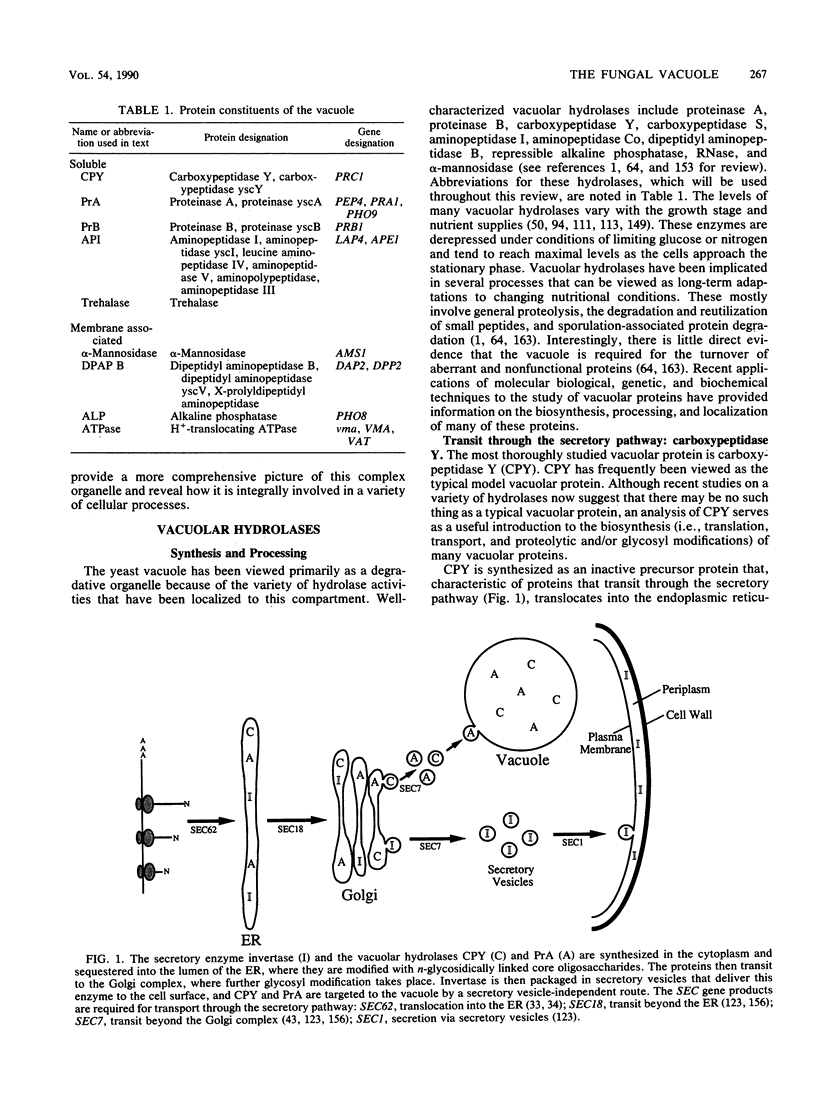











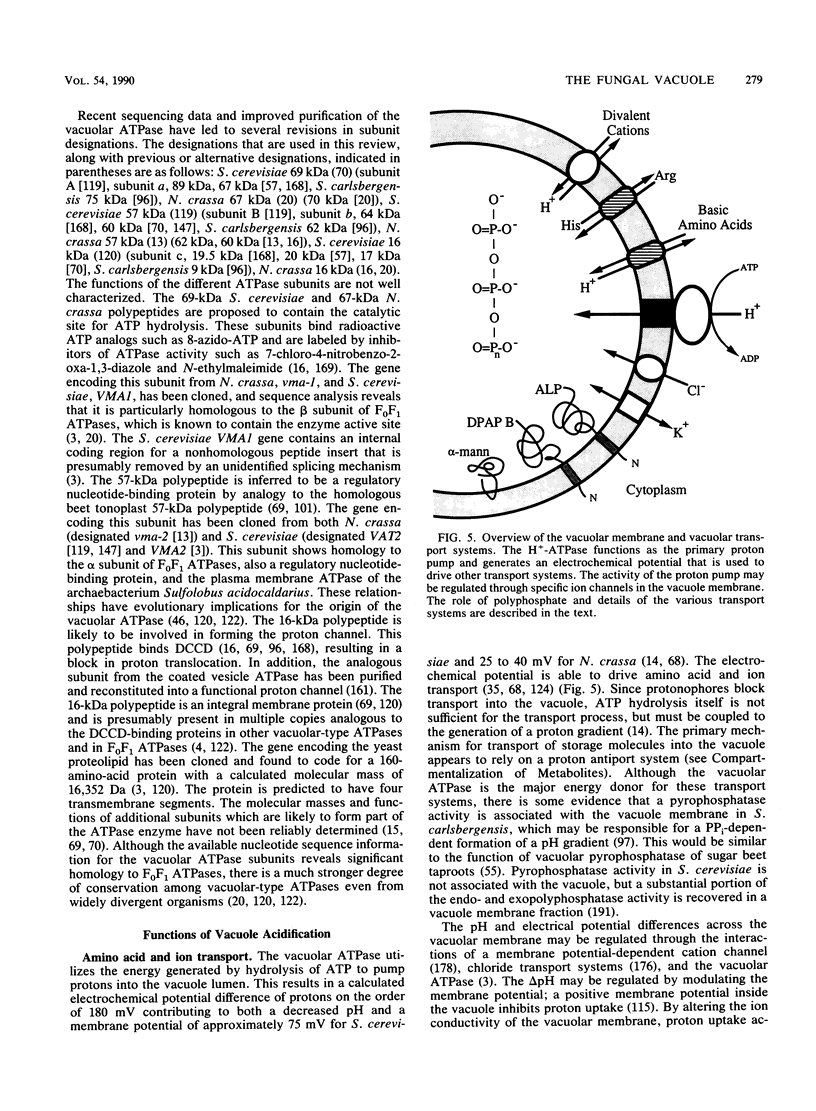













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast. 1985 Dec;1(2):139–157. doi: 10.1002/yea.320010203. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y., Umemoto N., Hirata R., Wada Y. Structure and function of the yeast vacuolar membrane proton ATPase. J Bioenerg Biomembr. 1989 Oct;21(5):589–603. doi: 10.1007/BF00808115. [DOI] [PubMed] [Google Scholar]
- Arai H., Terres G., Pink S., Forgac M. Topography and subunit stoichiometry of the coated vesicle proton pump. J Biol Chem. 1988 Jun 25;263(18):8796–8802. [PubMed] [Google Scholar]
- Aris J. P., Klionsky D. J., Simoni R. D. The Fo subunits of the Escherichia coli F1Fo-ATP synthase are sufficient to form a functional proton pore. J Biol Chem. 1985 Sep 15;260(20):11207–11215. [PubMed] [Google Scholar]
- Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L. M., Robinson J. S., Klionsky D. J., Emr S. D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988 Oct;107(4):1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H., Sigarlakie E. Localization of alkaline phosphatase in Saccharomyces cerevisiae by means of ultrathin frozen sections. J Ultrastruct Res. 1975 Feb;50(2):208–215. doi: 10.1016/s0022-5320(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Block M. R., Glick B. S., Rothman J. E., Balch W. E. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989 Jun 1;339(6223):397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Stevens T. H. Yeast carboxypeptidase Y can be translocated and glycosylated without its amino-terminal signal sequence. J Cell Biol. 1987 May;104(5):1183–1191. doi: 10.1083/jcb.104.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordallo C., Schwencke J., Suarez Rendueles M. Localization of the thermosensitive X-prolyl dipeptidyl aminopeptidase in the vacuolar membrane of Saccharomyces cerevisiae. FEBS Lett. 1984 Jul 23;173(1):199–203. doi: 10.1016/0014-5793(84)81046-7. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman B. J., Bowman E. J. H+-ATPases from mitochondria, plasma membranes, and vacuoles of fungal cells. J Membr Biol. 1986;94(2):83–97. doi: 10.1007/BF01871190. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Dschida W. J., Harris T., Bowman E. J. The vacuolar ATPase of Neurospora crassa contains an F1-like structure. J Biol Chem. 1989 Sep 15;264(26):15606–15612. [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol. 1982 Sep;151(3):1326–1337. doi: 10.1128/jb.151.3.1326-1337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J. Comparison of the vacuolar membrane ATPase of Neurospora crassa with the mitochondrial and plasma membrane ATPases. J Biol Chem. 1983 Dec 25;258(24):15238–15244. [PubMed] [Google Scholar]
- Bowman E. J., Mandala S., Taiz L., Bowman B. J. Structural studies of the vacuolar membrane ATPase from Neurospora crassa and comparison with the tonoplast membrane ATPase from Zea mays. Proc Natl Acad Sci U S A. 1986 Jan;83(1):48–52. doi: 10.1073/pnas.83.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Carpinelli G., Angiolella L., Maddaluno G., Podo F. 31P nuclear magnetic resonance study of growth and dimorphic transition in Candida albicans. J Gen Microbiol. 1983 May;129(5):1569–1575. doi: 10.1099/00221287-129-5-1569. [DOI] [PubMed] [Google Scholar]
- Chang Y. H., Smith J. A. Molecular cloning and sequencing of genomic DNA encoding aminopeptidase I from Saccharomyces cerevisiae. J Biol Chem. 1989 Apr 25;264(12):6979–6983. [PubMed] [Google Scholar]
- Chiang H. L., Dice J. F. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem. 1988 May 15;263(14):6797–6805. [PubMed] [Google Scholar]
- Chvatchko Y., Howald I., Riezman H. Two yeast mutants defective in endocytosis are defective in pheromone response. Cell. 1986 Aug 1;46(3):355–364. doi: 10.1016/0092-8674(86)90656-2. [DOI] [PubMed] [Google Scholar]
- Clark D. W., Tkacz J. S., Lampen J. O. Asparagine-linked carbohydrate does not determine the cellular location of yeast vacuolar nonspecific alkaline phosphatase. J Bacteriol. 1982 Nov;152(2):865–873. doi: 10.1128/jb.152.2.865-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C. L., Davis R. H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984 Apr 25;259(8):5152–5157. [PubMed] [Google Scholar]
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva R., García-Alvarez N., Suárez-Rendueles P. Yeast vacuolar aminopeptidase yscI. Isolation and regulation of the APE1 (LAP4) structural gene. FEBS Lett. 1989 Dec 18;259(1):125–129. doi: 10.1016/0014-5793(89)81510-8. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol Rev. 1986 Sep;50(3):280–313. doi: 10.1128/mr.50.3.280-313.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Kepes F., Böhni P. C. Genetic dissection of the early stages of protein secretion in yeast. Trends Genet. 1989 Mar;5(3):87–93. doi: 10.1016/0168-9525(89)90032-2. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drainas C., Weiss R. L. Energetics of vacuolar compartmentation of arginine in Neurospora crassa. J Bacteriol. 1982 May;150(2):770–778. doi: 10.1128/jb.150.2.770-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulić V., Riezman H. Characterization of the END1 gene required for vacuole biogenesis and gluconeogenic growth of budding yeast. EMBO J. 1989 May;8(5):1349–1359. doi: 10.1002/j.1460-2075.1989.tb03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Farley P. C., Shepherd M. G., Sullivan P. A. The cellular location of proteases in Candida albicans. J Gen Microbiol. 1986 Nov;132(11):3235–3238. doi: 10.1099/00221287-132-11-3235. [DOI] [PubMed] [Google Scholar]
- Farley P. C., Shepherd M. G., Sullivan P. A. The purification and properties of yeast proteinase B from Candida albicans. Biochem J. 1986 May 15;236(1):177–184. doi: 10.1042/bj2360177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A., Schekman R. Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 1989 Sep;8(9):2695–2702. doi: 10.1002/j.1460-2075.1989.tb08410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Röhm K. H. Subcellular localization and levels of aminopeptidases and dipeptidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Nov 10;527(1):31–41. doi: 10.1016/0005-2744(78)90253-x. [DOI] [PubMed] [Google Scholar]
- Futai M. Reconstitution of ATPase activity from the isolated alpha, beta, and gamma subunits of the coupling factor, F1, of Escherichia coli. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1231–1237. doi: 10.1016/0006-291x(77)91138-x. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N. J., Hussain M., Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987 Dec 7;926(3):205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Guthrie B. A., Wickner W. Yeast vacuoles fragment when microtubules are disrupted. J Cell Biol. 1988 Jul;107(1):115–120. doi: 10.1083/jcb.107.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. J., Switzer R. L., Hinze H., Holzer H. Effects of glucose and nitrogen source on the levels of proteinases, peptidases, and proteinase inhibitors in yeast. Biochim Biophys Acta. 1977 Jan 24;496(1):103–114. doi: 10.1016/0304-4165(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Harris S. D., Cotter D. A. Transport of yeast vacuolar trehalase to the vacuole. Can J Microbiol. 1988 Jul;34(7):835–838. doi: 10.1139/m88-143. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Cohen R. E., Zhang W. J., Ballou C. E. Carbohydrate chains on yeast carboxypeptidase Y are phosphorylated. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2244–2248. doi: 10.1073/pnas.78.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978 Apr 17;85(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A., Guern J., Flügge U. I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989 Oct;8(10):2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata R., Ohsumi Y., Anraku Y. Functional molecular masses of vacuolar membrane H+-ATPase from Saccharomyces cerevisiae as studied by radiation inactivation analysis. FEBS Lett. 1989 Feb 27;244(2):397–401. doi: 10.1016/0014-5793(89)80571-x. [DOI] [PubMed] [Google Scholar]
- Indge K. J. Polyphosphates of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):447–455. doi: 10.1099/00221287-51-3-447. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 A resolution. Nature. 1986 Jan 2;319(6048):33–38. doi: 10.1038/319033a0. [DOI] [PubMed] [Google Scholar]
- Jenness D. D., Spatrick P. Down regulation of the alpha-factor pheromone receptor in S. cerevisiae. Cell. 1986 Aug 1;46(3):345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Bankaitis V. A., Emr S. D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987 Mar 13;48(5):875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- Jones E. W., Zubenko G. S., Parker R. R. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Rothman J. H., Stevens T. H. Protein sorting in yeast: the role of the vacuolar proton-translocating ATPase. J Cell Sci Suppl. 1989;11:161–178. doi: 10.1242/jcs.1989.supplement_11.13. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Stevens T. H. Biochemical characterization of the yeast vacuolar H(+)-ATPase. J Biol Chem. 1989 Nov 15;264(32):19236–19244. [PubMed] [Google Scholar]
- Kaneko Y., Hayashi N., Toh-e A., Banno I., Oshima Y. Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase in Saccharomyces cerevisiae. Gene. 1987;58(1):137–148. doi: 10.1016/0378-1119(87)90036-9. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Tamai Y., Toh-e A., Oshima Y. Transcriptional and post-transcriptional control of PHO8 expression by PHO regulatory genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Jan;5(1):248–252. doi: 10.1128/mcb.5.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Toh-e A., Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasho V. N., Boyer P. D. Vacuolar ATPases, like F1,F0-ATPases, show a strong dependence of the reaction velocity on the binding of more than one ATP per enzyme. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8708–8711. doi: 10.1073/pnas.86.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F., Schellenberg M., Wiemken A. Localization of trehalase in vacuoles and of trehalose in the cytosol of yeast (Saccharomyces cerevisiae). Arch Microbiol. 1982 Jun;131(4):298–301. doi: 10.1007/BF00411175. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kitamoto K., Yoshizawa K., Ohsumi Y., Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988 Jun;170(6):2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto K., Yoshizawa K., Ohsumi Y., Anraku Y. Mutants of Saccharomyces cerevisiae with defective vacuolar function. J Bacteriol. 1988 Jun;170(6):2687–2691. doi: 10.1128/jb.170.6.2687-2691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Banta L. M., Emr S. D. Intracellular sorting and processing of a yeast vacuolar hydrolase: proteinase A propeptide contains vacuolar targeting information. Mol Cell Biol. 1988 May;8(5):2105–2116. doi: 10.1128/mcb.8.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Emr S. D. A new class of lysosomal/vacuolar protein sorting signals. J Biol Chem. 1990 Apr 5;265(10):5349–5352. [PubMed] [Google Scholar]
- Klionsky D. J., Emr S. D. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 1989 Aug;8(8):2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Simoni R. D. Assembly of a functional F1 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1985 Sep 15;260(20):11200–11206. [PubMed] [Google Scholar]
- Kominami E., Hoffschulte H., Holzer H. Purification and properties of proteinase B from yeast. Biochim Biophys Acta. 1981 Sep 15;661(1):124–135. doi: 10.1016/0005-2744(81)90091-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- Kuranda M. J., Robbins P. W. Cloning and heterologous expression of glycosidase genes from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 May;84(9):2585–2589. doi: 10.1073/pnas.84.9.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Kanamori K., Weiss R. L., Roberts J. D. Measurements of cytoplasmic and vacuolar pH in Neurospora using nitrogen-15 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Feb 15;22(4):899–903. doi: 10.1021/bi00273a029. [DOI] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of vacuolar arginine in Neurospora crassa. Mechanism and role of glutamine. J Biol Chem. 1984 Jul 25;259(14):8875–8879. [PubMed] [Google Scholar]
- Lehle L. Biosynthesis of the core region of yeast mannoproteins. Formation of a glucosylated dolichol-bound oligosaccharide precursor, its transfer to protein and subsequent modification. Eur J Biochem. 1980 Aug;109(2):589–601. doi: 10.1111/j.1432-1033.1980.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Lichko L. P., Okorokov L. A. Some properties of membrane-bound, solubilized and reconstituted into liposomes H+-ATPase of vacuoles of Saccharomyces carlsbergensis. FEBS Lett. 1984 Sep 3;174(2):233–237. doi: 10.1016/0014-5793(84)81164-3. [DOI] [PubMed] [Google Scholar]
- Lichko L. P., Okorokov L. A. What family of ATPases does the vacuolar H+-ATPase belong to? FEBS Lett. 1985 Aug 5;187(2):349–353. doi: 10.1016/0014-5793(85)81274-6. [DOI] [PubMed] [Google Scholar]
- Londesborough J., Varimo K. Characterization of two trehalases in baker's yeast. Biochem J. 1984 Apr 15;219(2):511–518. doi: 10.1042/bj2190511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarow M. Endocytosis in Saccharomyces cerevisiae: internalization of alpha-amylase and fluorescent dextran into cells. EMBO J. 1985 Jul;4(7):1861–1866. doi: 10.1002/j.1460-2075.1985.tb03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarow M. Endocytosis in Saccharomyces cerevisiae: internalization of enveloped viruses into spheroplasts. EMBO J. 1985 Jul;4(7):1855–1860. doi: 10.1002/j.1460-2075.1985.tb03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
- Mechler B., Hirsch H. H., Müller H., Wolf D. H. Biogenesis of the yeast lysosome (vacuole): biosynthesis and maturation of proteinase yscB. EMBO J. 1988 Jun;7(6):1705–1710. doi: 10.1002/j.1460-2075.1988.tb02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Müller H., Wolf D. H. Maturation of vacuolar (lysosomal) enzymes in yeast: proteinase yscA and proteinase yscB are catalysts of the processing and activation event of carboxypeptidase yscY. EMBO J. 1987 Jul;6(7):2157–2163. doi: 10.1002/j.1460-2075.1987.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Müller M., Müller H., Wolf D. H. In vivo biosynthesis of vacuolar proteinases in proteinase mutants of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Aug;107(3):770–778. doi: 10.1016/0006-291x(82)90590-3. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Metz G., Röhm K. H. Yeast aminopeptidase I. Chemical composition and catalytic properties. Biochim Biophys Acta. 1976 May 13;429(3):933–949. doi: 10.1016/0005-2744(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Mitchell J. K., Fonzi W. A., Wilkerson J., Opheim D. J. A particulate form of alkaline phosphatase in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1981 Feb 13;657(2):482–494. doi: 10.1016/0005-2744(81)90333-8. [DOI] [PubMed] [Google Scholar]
- Moehle C. M., Aynardi M. W., Kolodny M. R., Park F. J., Jones E. W. Protease B of Saccharomyces cerevisiae: isolation and regulation of the PRB1 structural gene. Genetics. 1987 Feb;115(2):255–263. doi: 10.1093/genetics/115.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Dixon C. K., Jones E. W. Processing pathway for protease B of Saccharomyces cerevisiae. J Cell Biol. 1989 Feb;108(2):309–325. doi: 10.1083/jcb.108.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Jones E. W. Consequences of growth media, gene copy number, and regulatory mutations on the expression of the PRB1 gene of Saccharomyces cerevisiae. Genetics. 1990 Jan;124(1):39–55. doi: 10.1093/genetics/124.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Tizard R., Lemmon S. K., Smart J., Jones E. W. Protease B of the lysosomelike vacuole of the yeast Saccharomyces cerevisiae is homologous to the subtilisin family of serine proteases. Mol Cell Biol. 1987 Dec;7(12):4390–4399. doi: 10.1128/mcb.7.12.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989 Feb 25;264(6):3577–3582. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. H+-translocating ATPase in Golgi apparatus. Characterization as vacuolar H+-ATPase and its subunit structures. J Biol Chem. 1989 Nov 5;264(31):18445–18450. [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. The vacuolar H+-ATPase, a proton pump controlled by a slip. Prog Clin Biol Res. 1988;273:387–394. [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Nelson H., Mandiyan S., Nelson N. A conserved gene encoding the 57-kDa subunit of the yeast vacuolar H+-ATPase. J Biol Chem. 1989 Jan 25;264(3):1775–1778. [PubMed] [Google Scholar]
- Nelson H., Nelson N. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci U S A. 1990 May;87(9):3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H., Nelson N. The progenitor of ATP synthases was closely related to the current vacuolar H+-ATPase. FEBS Lett. 1989 Apr 10;247(1):147–153. doi: 10.1016/0014-5793(89)81259-1. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983 May 10;258(9):5614–5617. [PubMed] [Google Scholar]
- Ohsumi Y., Kitamoto K., Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988 Jun;170(6):2676–2682. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Ohsumi Y., Anraku Y. Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. J Gen Microbiol. 1986 Apr;132(4):979–988. doi: 10.1099/00221287-132-4-979. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Kulakovskaya T. V., Kulaev I. S. Solubilization and partial purification of vacuolar ATPase of yeast Saccharomyces carlsbergensis. FEBS Lett. 1982 Aug 16;145(1):160–162. doi: 10.1016/0014-5793(82)81227-1. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Kulakovskaya T. V., Lichko L. P., Polorotova E. V. H+/ion antiport as the principal mechanism of transport systems in the vacuolar membrane of the yeast Saccharomyces carlsbergensis. FEBS Lett. 1985 Nov 18;192(2):303–306. doi: 10.1016/0014-5793(85)80130-7. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Lichko L. P., Kulaev I. S. Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergenis cells. J Bacteriol. 1980 Nov;144(2):661–665. doi: 10.1128/jb.144.2.661-665.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. R., Tkacz J. S., Lampen J. O. Glycoprotein nature of yeast alkaline phosphatase. Formation of active enzyme in the presence of tunicamycin. J Biol Chem. 1979 Dec 10;254(23):11943–11952. [PubMed] [Google Scholar]
- Opheim D. J. alpha-D-Mannosidase of Saccharomyces cerevisiae. Characterization and modulation of activity. Biochim Biophys Acta. 1978 May 11;524(1):121–130. doi: 10.1016/0005-2744(78)90110-9. [DOI] [PubMed] [Google Scholar]
- Paek Y. L., Weiss R. L. Identification of an arginine carrier in the vacuolar membrane of Neurospora crassa. J Biol Chem. 1989 May 5;264(13):7285–7290. [PubMed] [Google Scholar]
- Payne G. S., Baker D., van Tuinen E., Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988 May;106(5):1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston R. A., Murphy R. F., Jones E. W. Apparent endocytosis of fluorescein isothiocyanate-conjugated dextran by Saccharomyces cerevisiae reflects uptake of low molecular weight impurities, not dextran. J Cell Biol. 1987 Nov;105(5):1981–1987. doi: 10.1083/jcb.105.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston R. A., Murphy R. F., Jones E. W. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7027–7031. doi: 10.1073/pnas.86.18.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguzzi F., Lesuisse E., Crichton R. R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988 Apr 11;231(1):253–258. doi: 10.1016/0014-5793(88)80742-7. [DOI] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Pohlig G., Rothman J. H., Stevens T. H. Structure, biosynthesis, and localization of dipeptidyl aminopeptidase B, an integral membrane glycoprotein of the yeast vacuole. J Cell Biol. 1989 Apr;108(4):1363–1373. doi: 10.1083/jcb.108.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988 Nov;8(11):4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Howald I., Stevens T. H. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989 Jul;8(7):2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Hunter C. P., Valls L. A., Stevens T. H. Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3248–3252. doi: 10.1073/pnas.83.10.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Stevens T. H. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986 Dec 26;47(6):1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Yamashiro C. T., Kane P. M., Stevens T. H. Protein targeting to the yeast vacuole. Trends Biochem Sci. 1989 Aug;14(8):347–350. doi: 10.1016/0968-0004(89)90170-9. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Yamashiro C. T., Raymond C. K., Kane P. M., Stevens T. H. Acidification of the lysosome-like vacuole and the vacuolar H+-ATPase are deficient in two yeast mutants that fail to sort vacuolar proteins. J Cell Biol. 1989 Jul;109(1):93–100. doi: 10.1083/jcb.109.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Proteolytic activities in yeast. Biochim Biophys Acta. 1975 Mar 28;384(1):203–214. doi: 10.1016/0005-2744(75)90109-6. [DOI] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1984 Sep 25;259(18):11509–11511. [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Schwaiger H., Hasilik A., von Figura K., Wiemken A., Tanner W. Carbohydrate-free carboxypeptidase Y is transferred into the lysosome-like yeast vacuole. Biochem Biophys Res Commun. 1982 Feb 11;104(3):950–956. doi: 10.1016/0006-291x(82)91341-9. [DOI] [PubMed] [Google Scholar]
- Schwencke J., De Robichon-Szulmajster H. The transport of S-adenosyl-L-methionine in isolated yeast vacuoles and spheroplasts. Eur J Biochem. 1976 May 17;65(1):49–60. doi: 10.1111/j.1432-1033.1976.tb10388.x. [DOI] [PubMed] [Google Scholar]
- Severs N. J., Jordan E. G., Williamson D. H. Nuclear pore absence from areas of close association between nucleus and vacuole in synchronous yeast cultures. J Ultrastruct Res. 1976 Mar;54(3):374–387. doi: 10.1016/s0022-5320(76)80023-8. [DOI] [PubMed] [Google Scholar]
- Shih C. K., Wagner R., Feinstein S., Kanik-Ennulat C., Neff N. A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth. Mol Cell Biol. 1988 Aug;8(8):3094–3103. doi: 10.1128/mcb.8.8.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. H., Rothman J. H., Payne G. S., Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986 May;102(5):1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. Z., Xie X. S., Stone D. K. Isolation and reconstitution of the dicyclohexylcarbodiimide-sensitive proton pore of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1987 Oct 25;262(30):14790–14794. [PubMed] [Google Scholar]
- Suárez Rendueles P., Wolf D. H. Identification of the structural gene for dipeptidyl aminopeptidase yscV (DAP2) of Saccharomyces cerevisiae. J Bacteriol. 1987 Sep;169(9):4041–4048. doi: 10.1128/jb.169.9.4041-4048.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Teichert U., Mechler B., Müller H., Wolf D. H. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J Biol Chem. 1989 Sep 25;264(27):16037–16045. [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Nakamura H., Oshima Y. A gene controlling the synthesis of non specific alkaline phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1976 Mar 25;428(1):182–192. doi: 10.1016/0304-4165(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Trumbly R. J., Bradley G. Isolation and characterization of aminopeptidase mutants of Saccharomyces cerevisiae. J Bacteriol. 1983 Oct;156(1):36–48. doi: 10.1128/jb.156.1.36-48.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Characterization and function of catalytic subunit alpha of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. A study with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. J Biol Chem. 1988 Jan 5;263(1):45–51. [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y. Purification and properties of H+-translocating, Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1090–1095. [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Vaughn L. E., Davis R. H. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981 Sep;1(9):797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Kitamoto K., Kanbe T., Tanaka K., Anraku Y. The SLP1 gene of Saccharomyces cerevisiae is essential for vacuolar morphogenesis and function. Mol Cell Biol. 1990 May;10(5):2214–2223. doi: 10.1128/mcb.10.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Tanifuji M., Kasai M., Anraku Y. Vacuolar ion channel of the yeast, Saccharomyces cerevisiae. J Biol Chem. 1987 Dec 25;262(36):17260–17263. [PubMed] [Google Scholar]
- Weisman L. S., Bacallao R., Wickner W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol. 1987 Oct;105(4):1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Emr S. D., Wickner W. T. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988 Jul 29;241(4865):589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Matile P., Moor H. Vacuolar dynamics in synchronously budding yeast. Arch Mikrobiol. 1970;70(2):89–103. doi: 10.1007/BF00412200. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Schellenberg M. Does a cyclic AMP-dependent phosphorylation initiate the transfer of trehalase from the cytosol into the vacuoles in Saccharomyces cerevisiae? FEBS Lett. 1982 Dec 27;150(2):329–331. doi: 10.1016/0014-5793(82)80762-x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Rutherford A. V. The use of osmium-thiocarbohydrazide-osmium (OTO) and ferrocyanide-reduced osmium methods to enhance membrane contrast and preservation in cultured cells. J Histochem Cytochem. 1984 Apr;32(4):455–460. doi: 10.1177/32.4.6323574. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Wolf D. H., Weiser U. Studies on a carboxypeptidase Y mutant of yeast and evidence for a second carboxypeptidase Activity. Eur J Biochem. 1977 Mar 1;73(2):553–556. doi: 10.1111/j.1432-1033.1977.tb11350.x. [DOI] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T., Anraku Y. Nucleotide sequence of AMS1, the structure gene of vacuolar alpha-mannosidase of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989 Sep 15;163(2):908–915. doi: 10.1016/0006-291x(89)92308-5. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T., Ohsumi Y., Anraku Y. Solubilization and purification of alpha-mannosidase, a marker enzyme of vacuolar membranes in Saccharomyces cerevisiae. J Biol Chem. 1988 Apr 15;263(11):5158–5163. [PubMed] [Google Scholar]
- Zerez C. R., Weiss R. L., Franklin C., Bowman B. J. The properties of arginine transport in vacuolar membrane vesicles of Neurospora crassa. J Biol Chem. 1986 Jul 5;261(19):8877–8882. [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Genetic properties of mutations at the PEP4 locus in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):679–690. doi: 10.1093/genetics/102.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Mutations in PEP4 locus of Saccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc Natl Acad Sci U S A. 1983 Jan;80(2):510–514. doi: 10.1073/pnas.80.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]




