Abstract
Recent progress in studies on the bacterial chromosome is summarized. Although the greatest amount of information comes from studies on Escherichia coli, reports on studies of many other bacteria are also included. A compilation of the sizes of chromosomal DNAs as determined by pulsed-field electrophoresis is given, as well as a discussion of factors that affect gene dosage, including redundancy of chromosomes on the one hand and inactivation of chromosomes on the other hand. The distinction between a large plasmid and a second chromosome is discussed. Recent information on repeated sequences and chromosomal rearrangements is presented. The growing understanding of limitations on the rearrangements that can be tolerated by bacteria and those that cannot is summarized, and the sensitive region flanking the terminator loci is described. Sources and types of genetic variation in bacteria are listed, from simple single nucleotide mutations to intragenic and intergenic recombinations. A model depicting the dynamics of the evolution and genetic activity of the bacterial chromosome is described which entails acquisition by recombination of clonal segments within the chromosome. The model is consistent with the existence of only a few genetic types of E. coli worldwide. Finally, there is a summary of recent reports on lateral genetic exchange across great taxonomic distances, yet another source of genetic variation and innovation.
Full text
PDF



































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S. Multipartite genetic control elements: communication by DNA loop. Annu Rev Genet. 1989;23:227–250. doi: 10.1146/annurev.ge.23.120189.001303. [DOI] [PubMed] [Google Scholar]
- Akiyama M., Maki H., Sekiguchi M., Horiuchi T. A specific role of MutT protein: to prevent dG.dA mispairing in DNA replication. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3949–3952. doi: 10.1073/pnas.86.11.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allardet-Servent A., Bourg G., Ramuz M., Pages M., Bellis M., Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988 Oct;170(10):4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allewell N. Why does DNA bend? Trends Biochem Sci. 1988 Jun;13(6):193–195. doi: 10.1016/0968-0004(88)90079-5. [DOI] [PubMed] [Google Scholar]
- Amikam D., Razin S., Glaser G. Ribosomal RNA genes in Mycoplasma. Nucleic Acids Res. 1982 Jul 24;10(14):4215–4222. doi: 10.1093/nar/10.14.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Andersson S. G., Kurland C. G. Codon preferences in free-living microorganisms. Microbiol Rev. 1990 Jun;54(2):198–210. doi: 10.1128/mr.54.2.198-210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfalvi Z., Kondorosi E., Kondorosi A. Rhizobium meliloti carries two megaplasmids. Plasmid. 1985 Mar;13(2):129–138. doi: 10.1016/0147-619x(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Comparative nucleotide sequence analysis of growth-rate-regulated gnd alleles from natural isolates of Escherichia coli and from Salmonella typhimurium LT-2. J Bacteriol. 1988 Jan;170(1):372–379. doi: 10.1128/jb.170.1.372-379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautsch W. Rapid physical mapping of the Mycoplasma mobile genome by two-dimensional field inversion gel electrophoresis techniques. Nucleic Acids Res. 1988 Dec 23;16(24):11461–11467. doi: 10.1093/nar/16.24.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis H. A., Bibb M. J. Organisation of the ribosomal RNA genes in Streptomyces coelicolor A3(2). Mol Gen Genet. 1988 Feb;211(2):191–196. doi: 10.1007/BF00330593. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Morrison S. G., van der Ley P., Swanson J. Expression and phase variation of gonococcal P.II genes in Escherichia coli involves ribosomal frameshifting and slipped-strand mispairing. Mol Microbiol. 1989 Jun;3(6):777–786. doi: 10.1111/j.1365-2958.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Beltran P., Musser J. M., Helmuth R., Farmer J. J., 3rd, Frerichs W. M., Wachsmuth I. K., Ferris K., McWhorter A. C., Wells J. G., Cravioto A. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7753–7757. doi: 10.1073/pnas.85.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovier H., Kafri O., Sela S. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem Biophys Res Commun. 1986 May 14;136(3):1136–1141. doi: 10.1016/0006-291x(86)90452-3. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Bernardi G. Codon usage and genome composition. J Mol Evol. 1985;22(4):363–365. doi: 10.1007/BF02115693. [DOI] [PubMed] [Google Scholar]
- Berry J. O., Atherly A. G. Induced plasmid-genome rearrangements in Rhizobium japonicum. J Bacteriol. 1984 Jan;157(1):218–224. doi: 10.1128/jb.157.1.218-224.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuve A., Boesten B., Crasnier M., Danchin A., O'Gara F. Rhizobium meliloti adenylate cyclase is related to eucaryotic adenylate and guanylate cyclases. J Bacteriol. 1990 May;172(5):2614–2621. doi: 10.1128/jb.172.5.2614-2621.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A., Häusler A., Hütter R. Genome rearrangement and genetic instability in Streptomyces spp. J Bacteriol. 1990 Aug;172(8):4138–4142. doi: 10.1128/jb.172.8.4138-4142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A., Häusler A., Vögtli M., Krek W., Hütter R. Extremely large chromosomal deletions are intimately involved in genetic instability and genomic rearrangements in Streptomyces glaucescens. Mol Gen Genet. 1989 Jun;217(2-3):447–458. doi: 10.1007/BF02464916. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R. P., Vielmetter W. Complete maps of IS1, IS2, IS3, IS4, IS5, IS30 and IS150 locations in Escherichia coli K12. Mol Gen Genet. 1989 Dec;220(1):147–153. doi: 10.1007/BF00260869. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R. P., Vielmetter W. Cosmid-derived map of E. coli strain BHB2600 in comparison to the map of strain W3110. Nucleic Acids Res. 1989 Jul 11;17(13):5057–5069. doi: 10.1093/nar/17.13.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenson L. E. Dynamic adhesion and separation of cells in vitro. I. Mathematical analysis of the experimental system. J Cell Physiol. 1967 Aug;70(1):7–22. doi: 10.1002/jcp.1040700103. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Bouché J. P. Physical map of a 470 x 10(3) base-pair region flanking the terminus of DNA replication in the Escherichia coli K12 genome. J Mol Biol. 1982 Jan 5;154(1):1–20. doi: 10.1016/0022-2836(82)90413-2. [DOI] [PubMed] [Google Scholar]
- Bouma J. E., Lenski R. E. Evolution of a bacteria/plasmid association. Nature. 1988 Sep 22;335(6188):351–352. doi: 10.1038/335351a0. [DOI] [PubMed] [Google Scholar]
- Brewer B. J. When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell. 1988 Jun 3;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Arthur M., Courvalin P. Evidence for natural gene transfer from gram-positive cocci to Escherichia coli. J Bacteriol. 1988 Apr;170(4):1739–1745. doi: 10.1128/jb.170.4.1739-1745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. P., Chiang S. J., Tuan J. S., Katz L. Site-specific integration in Saccharopolyspora erythraea and multisite integration in Streptomyces lividans of actinomycete plasmid pSE101. J Bacteriol. 1988 May;170(5):2287–2295. doi: 10.1128/jb.170.5.2287-2295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Burgin A. B., Parodos K., Lane D. J., Pace N. R. The excision of intervening sequences from Salmonella 23S ribosomal RNA. Cell. 1990 Feb 9;60(3):405–414. doi: 10.1016/0092-8674(90)90592-3. [DOI] [PubMed] [Google Scholar]
- Burkardt B., Schillik D., Pühler A. Physical characterization of Rhizobium meliloti megaplasmids. Plasmid. 1987 Jan;17(1):13–25. doi: 10.1016/0147-619x(87)90004-7. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Campbell A. Evolutionary significance of accessory DNA elements in bacteria. Annu Rev Microbiol. 1981;35:55–83. doi: 10.1146/annurev.mi.35.100181.000415. [DOI] [PubMed] [Google Scholar]
- Canard B., Cole S. T. Genome organization of the anaerobic pathogen Clostridium perfringens. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6676–6680. doi: 10.1073/pnas.86.17.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casse F., Pascal M. C., Chippaux M. Comparison between the chromosomal maps of Escherichia coli and Salmonella typhimurium. Length of the inverted segment in the trp region. Mol Gen Genet. 1973 Aug 17;124(3):253–257. doi: 10.1007/BF00293096. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Mocca L. F., Frasch C. E., Frøholm L. O., Zollinger W. D., Selander R. K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987 Jun;169(6):2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Keseler I. M., Shimkets L. J. Genome size of Myxococcus xanthus determined by pulsed-field gel electrophoresis. J Bacteriol. 1990 Aug;172(8):4206–4213. doi: 10.1128/jb.172.8.4206-4213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Finch L. R. Novel arrangement of rRNA genes in Mycoplasma gallisepticum: separation of the 16S gene of one set from the 23S and 5S genes. J Bacteriol. 1989 May;171(5):2876–2878. doi: 10.1128/jb.171.5.2876-2878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Walsh G. P. Conservation of genomic sequences among isolates of Mycobacterium leprae. J Bacteriol. 1989 Sep;171(9):4844–4851. doi: 10.1128/jb.171.9.4844-4851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks B. G., Pyle L. E., Finch L. R. A physical map of the genome of Ureaplasma urealyticum 960T with ribosomal RNA loci. Nucleic Acids Res. 1989 Aug 25;17(16):6713–6719. doi: 10.1093/nar/17.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Prevalence of novel repeat sequences in and around the P1 operon in the genome of Mycoplasma pneumoniae. Gene. 1990 Mar 1;87(1):91–96. doi: 10.1016/0378-1119(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Contreras A, Casadesús J. Tn10 mutagenesis in Azotobacter vinelandii. Mol Gen Genet. 1987 Sep;209(2):276–282. doi: 10.1007/BF00329654. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Ghosal D., Saedler H. Tn951: a new transposon carrying a lactose operon. Mol Gen Genet. 1978 Apr 6;160(2):215–224. doi: 10.1007/BF00267484. [DOI] [PubMed] [Google Scholar]
- Cullum J., Altenbuchner J., Flett F., Piendl W. DNA amplification and genetic instability in Streptomyces. Biotechnol Genet Eng Rev. 1986;4:59–78. doi: 10.1080/02648725.1986.10647823. [DOI] [PubMed] [Google Scholar]
- Daniels D. L. The complete AvrII restriction map of the Escherichia coli genome and comparisons of several laboratory strains. Nucleic Acids Res. 1990 May 11;18(9):2649–2651. doi: 10.1093/nar/18.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D. Transcriptional bias: a non-Lamarckian mechanism for substrate-induced mutations. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5005–5009. doi: 10.1073/pnas.86.13.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Demuyter P., Leblond P., Decaris B., Simonet J. M. Characterization of two families of spontaneously amplifiable units of DNA in Streptomyces ambofaciens. J Gen Microbiol. 1988 Jul;134(7):2001–2007. doi: 10.1099/00221287-134-7-2001. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Molecular biology of archaebacteria. J Bacteriol. 1986 Nov;168(2):471–478. doi: 10.1128/jb.168.2.471-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon N. E., Kornberg A. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):424–428. doi: 10.1073/pnas.81.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Higgins C. F. Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol. 1987 Aug;169(8):3840–3843. doi: 10.1128/jb.169.8.3840-3843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs D. M., Roth J. R. A novel P22 prophage in Salmonella typhimurium. Genetics. 1987 Nov;117(3):367–380. doi: 10.1093/genetics/117.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose R. F., Dykhuizen D. E., Hartl D. L. Genetic exchange among natural isolates of bacteria: recombination within the phoA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7036–7040. doi: 10.1073/pnas.85.18.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger M., Bjornsti M. A., Uetz T., Hobot J. A., Kellenberger E. Intracellular location of the histonelike protein HU in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4757–4768. doi: 10.1128/jb.170.10.4757-4768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Sweet D. S., Vaughn V., Friedman D. I. Integration host factor is required for the DNA inversion that controls phase variation in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6506–6510. doi: 10.1073/pnas.84.18.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Ely T. W., Gerardot C. J., Dingwall A. Circularity of the Caulobacter crescentus chromosome determined by pulsed-field gel electrophoresis. J Bacteriol. 1990 Mar;172(3):1262–1266. doi: 10.1128/jb.172.3.1262-1266.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds D., Dower N., Stahl M. M., Stahl F. W. Orientation-dependent recombination hotspot activity in bacteriophage lambda. J Mol Biol. 1979 Jul 15;131(4):681–695. doi: 10.1016/0022-2836(79)90197-9. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feulner G., Gray J. A., Kirschman J. A., Lehner A. F., Sadosky A. B., Vlazny D. A., Zhang J., Zhao S., Hill C. W. Structure of the rhsA locus from Escherichia coli K-12 and comparison of rhsA with other members of the rhs multigene family. J Bacteriol. 1990 Jan;172(1):446–456. doi: 10.1128/jb.172.1.446-456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa N., Bossi L. Transcription induces gyration of the DNA template in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9416–9420. doi: 10.1073/pnas.85.24.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Rosteck P. R., Jr, Hershberger C. L. A 2.2-kilobase repeated DNA segment is associated with DNA amplification in Streptomyces fradiae. J Bacteriol. 1985 Jan;161(1):199–206. doi: 10.1128/jb.161.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. DNA dynamic flexibility and protein recognition: differential stimulation by bacterial histone-like protein HU. Cell. 1988 Aug 26;54(5):713–721. doi: 10.1016/s0092-8674(88)80016-3. [DOI] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François V., Louarn J., Patte J., Rebollo J. E., Louarn J. M. Constraints in chromosomal inversions in Escherichia coli are not explained by replication pausing at inverted terminator-like sequences. Mol Microbiol. 1990 Apr;4(4):537–542. doi: 10.1111/j.1365-2958.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Frutos R., Pages M., Bellis M., Roizes G., Bergoin M. Pulsed-field gel electrophoresis determination of the genome size of obligate intracellular bacteria belonging to the genera Chlamydia, Rickettsiella, and Porochlamydia. J Bacteriol. 1989 Aug;171(8):4511–4513. doi: 10.1128/jb.171.8.4511-4513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Pseudomonas putida: conservation among three bacteria, Bacillus subtilis, Escherichia coli and P. putida. Mol Gen Genet. 1989 Feb;215(3):381–387. doi: 10.1007/BF00427033. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Mifuchi I. Unique organization of Leptospira interrogans rRNA genes. J Bacteriol. 1989 Nov;171(11):5763–5767. doi: 10.1128/jb.171.11.5763-5767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks K. A., Marrs C. F., Stevens S. P., Green M. R. Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. J Bacteriol. 1990 Jan;172(1):310–316. doi: 10.1128/jb.172.1.310-316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J Biol Chem. 1987 Jul 25;262(21):10327–10334. [PubMed] [Google Scholar]
- Furukawa K., Chakrabarty A. M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982 Sep;44(3):619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Hayase N., Taira K., Tomizuka N. Molecular relationship of chromosomal genes encoding biphenyl/polychlorinated biphenyl catabolism: some soil bacteria possess a highly conserved bph operon. J Bacteriol. 1989 Oct;171(10):5467–5472. doi: 10.1128/jb.171.10.5467-5472.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo-Viola D. Enzyme polymorphism, prodigiosin production, and plasmid fingerprints in clinical and naturally occurring isolates of Serratia marcescens. J Clin Microbiol. 1989 May;27(5):860–868. doi: 10.1128/jcm.27.5.860-868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Gilson E., Perrin D., Hofnung M. DNA polymerase I and a protein complex bind specifically to E. coli palindromic unit highly repetitive DNA: implications for bacterial chromosome organization. Nucleic Acids Res. 1990 Jul 11;18(13):3941–3952. doi: 10.1093/nar/18.13.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Perrin D., Saurin W., Hofnung M. Species specificity of bacterial palindromic units. J Mol Evol. 1987;25(4):371–373. doi: 10.1007/BF02603122. [DOI] [PubMed] [Google Scholar]
- Giroux S., Beaudet J., Cedergren R. Highly repetitive tRNA(Pro)-tRNA(His) gene cluster from Photobacterium phosphoreum. J Bacteriol. 1988 Dec;170(12):5601–5606. doi: 10.1128/jb.170.12.5601-5606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin D., Slater J. H. The influence of the growth environment on the stability of a drug resistance plasmid in Escherichia coli K12. J Gen Microbiol. 1979 Mar;111(1):201–210. doi: 10.1099/00221287-111-1-201. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Carrasco C. D., Mulligan M. E., Schneider G. J., Haselkorn R. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Nov;170(11):5034–5041. doi: 10.1128/jb.170.11.5034-5041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Goursot R., Goze A., Niaudet B., Ehrlich S. D. Plasmids from Staphylococcus aureus replicate in yeast Saccharomyces cerevisiae. Nature. 1982 Jul 29;298(5873):488–490. doi: 10.1038/298488a0. [DOI] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Bacterial gene regulation from distant DNA sites. Cell. 1989 Apr 21;57(2):193–195. doi: 10.1016/0092-8674(89)90955-0. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Thornton S., Trust T. J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990 Apr;172(4):1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Trust T. J. Genomic rearrangements associated with antigenic variation in Campylobacter coli. J Bacteriol. 1988 Jan;170(1):316–319. doi: 10.1128/jb.170.1.316-319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen N., Gabor M. H., Hotchkiss R. D., Hirschbein L. Isolation and characterization of the nucleoid of non-complementing diploids from protoplast fusion in Bacillus subtilis. Mol Gen Genet. 1982;185(1):69–74. doi: 10.1007/BF00333792. [DOI] [PubMed] [Google Scholar]
- Guillen N., Sanchez-Rivas C., Hirschbein L. Absence of functional RNA encoded by a silent chromosome in non-complementing diploids obtained from protoplast fusion in Bacillus subtilis. Mol Gen Genet. 1983;191(1):81–85. doi: 10.1007/BF00330893. [DOI] [PubMed] [Google Scholar]
- Guillén N., Amar M., Hirschbein L. Stabilized non-complementing diploids (Ncd) from fused protoplast products of B. subtilis. EMBO J. 1985 May;4(5):1333–1338. doi: 10.1002/j.1460-2075.1985.tb03781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiso N., Ullmann A. Expression and regulation of lactose genes carried by plasmids. J Bacteriol. 1976 Aug;127(2):691–697. doi: 10.1128/jb.127.2.691-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Hatfield G. W. Nonrandom utilization of codon pairs in Escherichia coli. Proc Natl Acad Sci U S A. 1989 May;86(10):3699–3703. doi: 10.1073/pnas.86.10.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Meyer T. F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986 Jan 17;44(1):107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988 Dec;120(4):887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics. 1990 Sep;126(1):5–16. doi: 10.1093/genetics/126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Intramolecular DNA triplexes in supercoiled plasmids. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6292–6296. doi: 10.1073/pnas.85.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman L., Riley M. Conservation and variation of nucleotide sequences in Escherichia coli strains isolated from nature. J Bacteriol. 1980 Nov;144(2):560–568. doi: 10.1128/jb.144.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E. The population genetics of Escherichia coli. Annu Rev Genet. 1984;18:31–68. doi: 10.1146/annurev.ge.18.120184.000335. [DOI] [PubMed] [Google Scholar]
- Hartmann R. K., Erdmann V. A. Thermus thermophilus 16S rRNA is transcribed from an isolated transcription unit. J Bacteriol. 1989 Jun;171(6):2933–2941. doi: 10.1128/jb.171.6.2933-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. K., Toschka H. Y., Ulbrich N., Erdmann V. A. Genomic organization of rDNA in Pseudomonas aeruginosa. FEBS Lett. 1986 Jan 20;195(1-2):187–193. doi: 10.1016/0014-5793(86)80158-2. [DOI] [PubMed] [Google Scholar]
- Hartmann R. K., Ulbrich N., Erdmann V. A. An unusual rRNA operon constellation: in Thermus thermophilus HB8 the 23S/5S rRNA operon is a separate entity from the 16S rRNA operon. Biochimie. 1987 Oct;69(10):1097–1104. doi: 10.1016/0300-9084(87)90009-5. [DOI] [PubMed] [Google Scholar]
- Harvey S., Hill C. W. Exchange of spacer regions between rRNA operons in Escherichia coli. Genetics. 1990 Aug;125(4):683–690. doi: 10.1093/genetics/125.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Hill C. W., Squires C., Squires C. L. Loss of the spacer loop sequence from the rrnB operon in the Escherichia coli K-12 subline that bears the relA1 mutation. J Bacteriol. 1988 Mar;170(3):1235–1238. doi: 10.1128/jb.170.3.1235-1238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Turk E., Wright E. M. Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann J. A., Sprague G. F., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989 Jul 20;340(6230):205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Kinney T., Adams J. The maintenance of Plasmid-containing organisms in populations of Escherichia coli. J Gen Microbiol. 1981 Mar;123(1):129–141. doi: 10.1099/00221287-123-1-129. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Gray J. A. Effects of chromosomal inversion on cell fitness in Escherichia coli K-12. Genetics. 1988 Aug;119(4):771–778. doi: 10.1093/genetics/119.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Morgan A. F. Genome organization in Pseudomonas. Annu Rev Microbiol. 1986;40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Hintermann G., Kieser T., Wright H. M. Integrated DNA sequences in three streptomycetes form related autonomous plasmids after transfer to Streptomyces lividans. Plasmid. 1984 Jan;11(1):1–16. doi: 10.1016/0147-619x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Hornemann U., Otto C. J., Hoffman G. G., Bertinuson A. C. Spectinomycin resistance and associated DNA amplification in Streptomyces achromogenes subsp. rubradiris. J Bacteriol. 1987 Jun;169(6):2360–2366. doi: 10.1128/jb.169.6.2360-2366.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornemann U., Otto C. J., Zhang X. Y. DNA amplification in Streptomyces achromogenes subsp. rubradiris is accompanied by a deletion, and the amplified sequences are conditionally stable and can be eliminated by two pathways. J Bacteriol. 1989 Nov;171(11):5817–5822. doi: 10.1128/jb.171.11.5817-5822.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D., Gabor M. H. Biparental products of bacterial protoplast fusion showing unequal parental chromosome expression. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3553–3557. doi: 10.1073/pnas.77.6.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Deonier R. C. Mapping of IS1 elements flanking the argF gene region on the Escherichia coli K-12 chromosome. Mol Gen Genet. 1981;181(2):222–229. doi: 10.1007/BF00268430. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Reverchon S., Robert-Baudouy J. Expanded linkage map of Erwinia chrysanthemi strain 3937. Mol Microbiol. 1989 May;3(5):573–581. doi: 10.1111/j.1365-2958.1989.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Huisman O., Faelen M., Girard D., Jaffé A., Toussaint A., Rouvière-Yaniv J. Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol. 1989 Jul;171(7):3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler A., Birch A., Krek W., Piret J., Hütter R. Heterogeneous genomic amplification in Streptomyces glaucescens: structure, location and junction sequence analysis. Mol Gen Genet. 1989 Jun;217(2-3):437–446. doi: 10.1007/BF02464915. [DOI] [PubMed] [Google Scholar]
- Ichige A., Matsutani S., Oishi K., Mizushima S. Establishment of gene transfer systems for and construction of the genetic map of a marine Vibrio strain. J Bacteriol. 1989 Apr;171(4):1825–1834. doi: 10.1128/jb.171.4.1825-1834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Innes R. W., Hirose M. A., Kuempel P. L. Induction of nitrogen-fixing nodules on clover requires only 32 kilobase pairs of DNA from the Rhizobium trifolii symbiosis plasmid. J Bacteriol. 1988 Sep;170(9):3793–3802. doi: 10.1128/jb.170.9.3793-3802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N., Hirose K., Asagi M., Sato G. Incompatibility of citrate utilization plasmids isolated from Escherichia coli. J Gen Microbiol. 1981 Mar;123(1):193–196. doi: 10.1099/00221287-123-1-193. [DOI] [PubMed] [Google Scholar]
- Ishiguro N., Sato G. Nucleotide sequence of insertion sequence IS3411, which flanks the citrate utilization determinant of transposon Tn3411. J Bacteriol. 1988 Apr;170(4):1902–1906. doi: 10.1128/jb.170.4.1902-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives C. L., Bott K. F. Characterization of chromosomal DNA amplifications with associated tetracycline resistance in Bacillus subtilis. J Bacteriol. 1990 Sep;172(9):4936–4944. doi: 10.1128/jb.172.9.4936-4944.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaoua S., Guespin-Michel J. F., Breton A. M. Mode of insertion of the broad-host-range plasmid RP4 and its derivatives into the chromosome of Myxococcus xanthus. Plasmid. 1987 Sep;18(2):111–119. doi: 10.1016/0147-619x(87)90038-2. [DOI] [PubMed] [Google Scholar]
- Jarvis E. D., Widom R. L., LaFauci G., Setoguchi Y., Richter I. R., Rudner R. Chromosomal organization of rRNA operons in Bacillus subtilis. Genetics. 1988 Nov;120(3):625–635. doi: 10.1093/genetics/120.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Kaluza K., Hahn M., Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985 May;162(2):535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Imamoto F. Requirement of integration host factor (IHF) for growth of Escherichia coli deficient in HU protein. Gene. 1990 Apr 30;89(1):133–137. doi: 10.1016/0378-1119(90)90216-e. [DOI] [PubMed] [Google Scholar]
- Kano Y., Osato K., Wada M., Imamoto F. Cloning and sequencing of the HU-2 gene of Escherichia coli. Mol Gen Genet. 1987 Sep;209(2):408–410. doi: 10.1007/BF00329674. [DOI] [PubMed] [Google Scholar]
- Kano Y., Wada M., Nagase T., Imamoto F. Genetic characterization of the gene hupB encoding the HU-1 protein of Escherichia coli. Gene. 1986;45(1):37–44. doi: 10.1016/0378-1119(86)90129-0. [DOI] [PubMed] [Google Scholar]
- Kauc L., Goodgal S. H. The size and a physical map of the chromosome of Haemophilus parainfluenzae. Gene. 1989 Nov 30;83(2):377–380. doi: 10.1016/0378-1119(89)90125-x. [DOI] [PubMed] [Google Scholar]
- Kauc L., Mitchell M., Goodgal S. H. Size and physical map of the chromosome of Haemophilus influenzae. J Bacteriol. 1989 May;171(5):2474–2479. doi: 10.1128/jb.171.5.2474-2479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavenoff R., Bowen B. C. Electron microscopy of membrane-free folded chromosomes from Escherichia coli. Chromosoma. 1976 Dec 16;59(2):89–101. doi: 10.1007/BF00328479. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M., Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. 1987 Jul 30-Aug 5Nature. 328(6129):454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Kitten T., Barbour A. G. Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6077–6081. doi: 10.1073/pnas.87.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V., Blake D. J., Brownlee G. G. Completion of the detailed restriction map of the E. coli genome by the isolation of overlapping cosmid clones. Nucleic Acids Res. 1989 Aug 11;17(15):5901–5912. doi: 10.1093/nar/17.15.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kohno K., Wada M., Kano Y., Imamoto F. Promoters and autogenous control of the Escherichia coli hupA and hupB genes. J Mol Biol. 1990 May 5;213(1):27–36. doi: 10.1016/S0022-2836(05)80119-6. [DOI] [PubMed] [Google Scholar]
- Kolstø A. B., Grønstad A., Oppegaard H. Physical map of the Bacillus cereus chromosome. J Bacteriol. 1990 Jul;172(7):3821–3825. doi: 10.1128/jb.172.7.3821-3825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Kubo A., Nisioka T. Shufflon: multi-inversion of four contiguous DNA segments of plasmid R64 creates seven different open reading frames. Nucleic Acids Res. 1987 Feb 11;15(3):1165–1172. doi: 10.1093/nar/15.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Mawn C. B. Physical analysis and mapping of the Mycoplasma pneumoniae chromosome. J Bacteriol. 1990 Sep;172(9):4790–4797. doi: 10.1128/jb.172.9.4790-4797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H., Amouyal M., Nordheim A., Müller-Hill B. DNA supercoiling changes the spacing requirement of two lac operators for DNA loop formation with lac repressor. EMBO J. 1988 Feb;7(2):547–556. doi: 10.1002/j.1460-2075.1988.tb02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger M., Wahl R., Rice P. Compilation of DNA sequences of Escherichia coli (update 1990). Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2549–2587. doi: 10.1093/nar/18.suppl.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L., Pelletier A. J., Hill T. M. Tus and the terminators: the arrest of replication in prokaryotes. Cell. 1989 Nov 17;59(4):581–583. doi: 10.1016/0092-8674(89)90001-9. [DOI] [PubMed] [Google Scholar]
- Kunkel B., Losick R., Stragier P. The Bacillus subtilis gene for the development transcription factor sigma K is generated by excision of a dispensable DNA element containing a sporulation recombinase gene. Genes Dev. 1990 Apr;4(4):525–535. doi: 10.1101/gad.4.4.525. [DOI] [PubMed] [Google Scholar]
- Lamfrom H., Sarabhai A., Abelson J. Cloning of Beneckea genes in Escherichia coli. J Bacteriol. 1978 Jan;133(1):354–363. doi: 10.1128/jb.133.1.354-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latreille J., Barlogie B., Dosik G., Johnston D. A., Drewinko B., Alexanian R. Cellular DNA content as a marker of human multiple myeloma. Blood. 1980 Mar;55(3):403–408. [PubMed] [Google Scholar]
- Laundon C. H., Griffith J. D. Curved helix segments can uniquely orient the topology of supertwisted DNA. Cell. 1988 Feb 26;52(4):545–549. doi: 10.1016/0092-8674(88)90467-9. [DOI] [PubMed] [Google Scholar]
- Le Bourgeois P., Mata M., Ritzenthaler P. Genome comparison of Lactococcus strains by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1989 May;50(1-2):65–69. doi: 10.1016/0378-1097(89)90460-6. [DOI] [PubMed] [Google Scholar]
- Leblond P., Demuyter P., Moutier L., Laakel M., Decaris B., Simonet J. M. Hypervariability, a new phenomenon of genetic instability, related to DNA amplification in Streptomyces ambofaciens. J Bacteriol. 1989 Jan;171(1):419–423. doi: 10.1128/jb.171.1.419-423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Omer C. A., Brasch M. A., Cohen S. N. Analysis of recombination occurring at SLP1 att sites. J Bacteriol. 1988 Dec;170(12):5806–5813. doi: 10.1128/jb.170.12.5806-5813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A. F., Harvey S., Hill C. W. Mapping and spacer identification of rRNA operons of Salmonella typhimurium. J Bacteriol. 1984 Nov;160(2):682–686. doi: 10.1128/jb.160.2.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P., Danchin A. Mutations in the bglY gene increase the frequency of spontaneous deletions in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1990 Jan;87(1):360–363. doi: 10.1073/pnas.87.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. R. Periodic selection, infectious gene exchange and the genetic structure of E. coli populations. Genetics. 1981 Sep;99(1):1–23. doi: 10.1093/genetics/99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M. The population biology of bacterial plasmids: a priori conditions for the existence of mobilizable nonconjugative factors. Genetics. 1980 Feb;94(2):425–443. doi: 10.1093/genetics/94.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Liebart J. C., Paolozzi L., Camera M. G., Pedrini A. M., Ghelardini P. The expression of the DNA ligase gene of Escherichia coli is stimulated by relaxation of chromosomal supercoiling. Mol Microbiol. 1989 Mar;3(3):269–273. doi: 10.1111/j.1365-2958.1989.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Evidence for unlinked rrn operons in the Planctomycete Pirellula marina. J Bacteriol. 1989 Sep;171(9):5025–5030. doi: 10.1128/jb.171.9.5025-5030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. DNA opens up--supercoiling and heavy breathing. Trends Genet. 1988 Apr;4(4):111–114. doi: 10.1016/0168-9525(88)90099-6. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Kemper B. Cruciform-resolvase interactions in supercoiled DNA. Cell. 1984 Feb;36(2):413–422. doi: 10.1016/0092-8674(84)90234-4. [DOI] [PubMed] [Google Scholar]
- Lin R. J., Capage M., Hill C. W. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 25;177(1):1–18. doi: 10.1016/0022-2836(84)90054-8. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Link C. D., Reiner A. M. Genotypic exclusion: a novel relationship between the ribitol-arabitol and galactitol genes of E. coli. Mol Gen Genet. 1983;189(2):337–339. doi: 10.1007/BF00337827. [DOI] [PubMed] [Google Scholar]
- Long C. M., Virolle M. J., Chang S. Y., Chang S., Bibb M. J. alpha-Amylase gene of Streptomyces limosus: nucleotide sequence, expression motifs, and amino acid sequence homology to mammalian and invertebrate alpha-amylases. J Bacteriol. 1987 Dec;169(12):5745–5754. doi: 10.1128/jb.169.12.5745-5754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J. M., Bouché J. P., Legendre F., Louarn J., Patte J. Characterization and properties of very large inversions of the E. coli chromosome along the origin-to-terminus axis. Mol Gen Genet. 1985;201(3):467–476. doi: 10.1007/BF00331341. [DOI] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydersen B. K., Pettijohn D. E. Interactions stabilizing DNA tertiary structure in the Escherichia coli chromosome investigated with ionizing radiation. Chromosoma. 1977 Jul 8;62(3):199–215. doi: 10.1007/BF00286044. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. The William Allan memorial award address: X-chromosome inactivation and the location and expression of X-linked genes. Am J Hum Genet. 1988 Jan;42(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- Madon J., Moretti P., Hütter R. Site-specific integration and excision of pMEA100 in Nocardia mediterranei. Mol Gen Genet. 1987 Sep;209(2):257–264. doi: 10.1007/BF00329651. [DOI] [PubMed] [Google Scholar]
- Mahan M. J., Roth J. R. Reciprocality of recombination events that rearrange the chromosome. Genetics. 1988 Sep;120(1):23–35. doi: 10.1093/genetics/120.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden M. C., Davis E. O., Baldwin S. A., Moore D. C., Henderson P. J. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987 Feb 12;325(6105):641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Chattoraj D. K., Faulds D. H., Stahl M. M., Stahl F. W. Hotspots for generalized recombination in the Escherichia coli chromosome. J Mol Biol. 1978 Jun 5;121(4):473–491. doi: 10.1016/0022-2836(78)90395-9. [DOI] [PubMed] [Google Scholar]
- Marrs C. F., Ruehl W. W., Schoolnik G. K., Falkow S. Pilin-gene phase variation of Moraxella bovis is caused by an inversion of the pilin genes. J Bacteriol. 1988 Jul;170(7):3032–3039. doi: 10.1128/jb.170.7.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Houmard J., Castets A. M., Tandeau de Marsac N. Highly repetitive DNA sequences in cyanobacterial genomes. J Bacteriol. 1990 May;172(5):2755–2761. doi: 10.1128/jb.172.5.2755-2761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Petter R., Thompson C. Intergeneric conjugation between Escherichia coli and Streptomyces species. J Bacteriol. 1989 Jun;171(6):3583–3585. doi: 10.1128/jb.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino E., Bolivar F. The ribonucleoside diphosphate reductase gene (nrdA) of Escherichia coli carries a repetitive extragenic palindromic (REP) sequence in its 3' structural terminus. Mol Microbiol. 1989 Jun;3(6):839–841. doi: 10.1111/j.1365-2958.1989.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Hirsch-Twizer S., Goldman S., Yakobson E., Eisenberg H., Dennis P. P. Isolation and characterization of the rRNA gene clusters of Halobacterium marismortui. J Bacteriol. 1989 Jun;171(6):3479–3485. doi: 10.1128/jb.171.6.3479-3485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
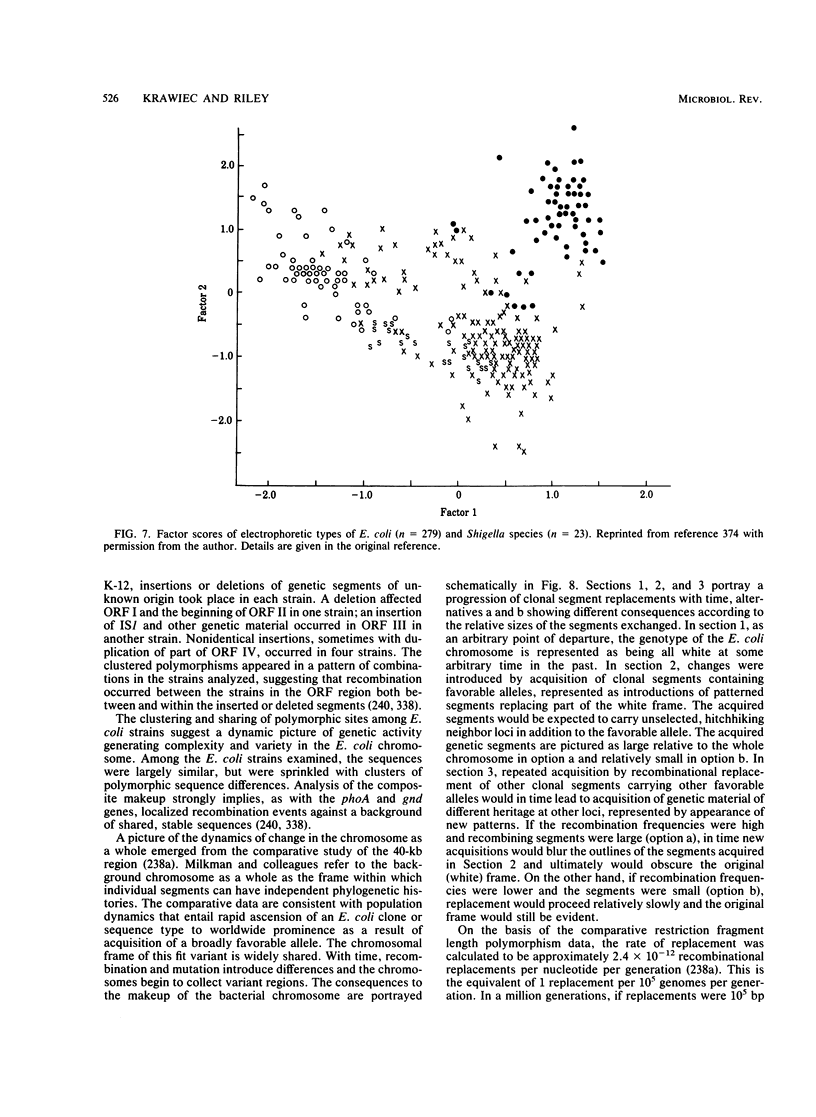
- Milkman R., Bridges M. M. Molecular evolution of the Escherichia coli chromosome. III. Clonal frames. Genetics. 1990 Nov;126(3):505–517. doi: 10.1093/genetics/126.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman R., Crawford I. P. Clustered third-base substitutions among wild strains of Escherichia coli. Science. 1983 Jul 22;221(4608):378–380. doi: 10.1126/science.6346486. [DOI] [PubMed] [Google Scholar]
- Milkman R. Electrophoretic variation in Escherichia coli from natural sources. Science. 1973 Dec 7;182(4116):1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- Milkman R., Stoltzfus A. Molecular evolution of the Escherichia coli chromosome. II. Clonal segments. Genetics. 1988 Oct;120(2):359–366. doi: 10.1093/genetics/120.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. Methyl-directed DNA mismatch correction. J Biol Chem. 1989 Apr 25;264(12):6597–6600. [PubMed] [Google Scholar]
- Muramatsu S., Kato M., Kohara Y., Mizuno T. Insertion sequence IS5 contains a sharply curved DNA structure at its terminus. Mol Gen Genet. 1988 Nov;214(3):433–438. doi: 10.1007/BF00330477. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Clonal population structure of encapsulated Haemophilus influenzae. Infect Immun. 1988 Aug;56(8):1837–1845. doi: 10.1128/iai.56.8.1837-1845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Rapp V. J., Selander R. K. Clonal diversity in Haemophilus pleuropneumoniae. Infect Immun. 1987 May;55(5):1207–1215. doi: 10.1128/iai.55.5.1207-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Osawa S. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc Natl Acad Sci U S A. 1987 Jan;84(1):166–169. doi: 10.1073/pnas.84.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Médigue C., Bouché J. P., Hénaut A., Danchin A. Mapping of sequenced genes (700 kbp) in the restriction map of the Escherichia coli chromosome. Mol Microbiol. 1990 Feb;4(2):169–187. doi: 10.1111/j.1365-2958.1990.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Nagpal P., Jafri S., Reddy M. A., Das H. K. Multiple chromosomes of Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3133–3138. doi: 10.1128/jb.171.6.3133-3138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A., Amemura M., Makino K. Unusual nucleotide arrangement with repeated sequences in the Escherichia coli K-12 chromosome. J Bacteriol. 1989 Jun;171(6):3553–3556. doi: 10.1128/jb.171.6.3553-3556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem Y., Cabrera M., Cupples C. G., Miller J. H. The mutY gene: a mutator locus in Escherichia coli that generates G.C----T.A transversions. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack D., Roth M., Geuther R., Müller G., Undisz K., Hoffmeier C., Gáspár S. Maintenance and genetic stability of vector plasmids pBR322 and pBR325 in Escherichia coli K12 strains grown in a chemostat. Mol Gen Genet. 1981;184(1):121–124. doi: 10.1007/BF00271207. [DOI] [PubMed] [Google Scholar]
- Noll K. M. Chromosome map of the thermophilic archaebacterium Thermococcus celer. J Bacteriol. 1989 Dec;171(12):6720–6725. doi: 10.1128/jb.171.12.6720-6725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman K., Nakamura K., Ohtsubo H., Ohtsubo E. Distribution of the insertion sequence IS1 in gram-negative bacteria. Nature. 1981 Feb 12;289(5798):609–612. doi: 10.1038/289609a0. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Nyman K., Doroszkiewicz W., Ohtsubo E. Multiple copies of iso-insertion sequences of IS1 in Shigella dysenteriae chromosome. Nature. 1981 Aug 13;292(5824):640–643. doi: 10.1038/292640a0. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Cohen S. N. Plasmid formation in Streptomyces: excision and integration of the SLP1 replicon at a specific chromosomal site. Mol Gen Genet. 1984;196(3):429–438. doi: 10.1007/BF00436190. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Cohen S. N. Structural analysis of plasmid and chromosomal loci involved in site-specific excision and integration of the SLP1 element of Streptomyces coelicolor. J Bacteriol. 1986 Jun;166(3):999–1006. doi: 10.1128/jb.166.3.999-1006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Stein D., Cohen S. N. Site-specific insertion of biologically functional adventitious genes into the Streptomyces lividans chromosome. J Bacteriol. 1988 May;170(5):2174–2184. doi: 10.1128/jb.170.5.2174-2184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Fontaine A. A native cruciform DNA structure probed in bacteria by recombinant T7 endonuclease. J Biol Chem. 1987 Aug 15;262(23):11364–11368. [PubMed] [Google Scholar]
- Parker L. L., Hall B. G. Characterization and nucleotide sequence of the cryptic cel operon of Escherichia coli K12. Genetics. 1990 Mar;124(3):455–471. doi: 10.1093/genetics/124.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. H., Foster T. J., Pattee P. A. Physical and genetic mapping of the protein A gene in the chromosome of Staphylococcus aureus 8325-4. J Gen Microbiol. 1989 Jul;135(7):1799–1807. doi: 10.1099/00221287-135-7-1799. [DOI] [PubMed] [Google Scholar]
- Pelletier A. J., Hill T. M., Kuempel P. L. Location of sites that inhibit progression of replication forks in the terminus region of Escherichia coli. J Bacteriol. 1988 Sep;170(9):4293–4298. doi: 10.1128/jb.170.9.4293-4298.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K., de Cespédès G., Horaud T. Heterogeneity of chromosomal genes encoding chloramphenicol resistance in streptococci. Plasmid. 1988 Jan;19(1):71–74. doi: 10.1016/0147-619x(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Pernodet J. L., Boccard F., Alegre M. T., Gagnat J., Guérineau M. Organization and nucleotide sequence analysis of a ribosomal RNA gene cluster from Streptomyces ambofaciens. Gene. 1989 Jun 30;79(1):33–46. doi: 10.1016/0378-1119(89)90090-5. [DOI] [PubMed] [Google Scholar]
- Pernodet J. L., Simonet J. M., Guérineau M. Plasmids in different strains of Streptomyces ambofaciens: free and integrated form of plasmid pSAM2. Mol Gen Genet. 1984;198(2):35–41. doi: 10.1007/BF00328697. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Van de Putte P. Genetic switches by DNA inversions in prokaryotes. Biochim Biophys Acta. 1984 Jun 16;782(2):111–119. doi: 10.1016/0167-4781(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Maniloff J. Determination of microbial genome sizes by two-dimensional denaturing gradient gel electrophoresis. Nucleic Acids Res. 1989 Apr 25;17(8):2889–2895. doi: 10.1093/nar/17.8.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Black S., Pannuri S., Carlson A. Use of the Escherichia coli SSB gene to prevent bioreactor takeover by plasmidless cells. Biotechnology (N Y) 1990 Jan;8(1):47–51. doi: 10.1038/nbt0190-47. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Kent H. M., Robson R. L., Chesshyre J. A. The genomes of Desulfovibrio gigas and D. vulgaris. J Gen Microbiol. 1984 Jul;130(7):1597–1601. doi: 10.1099/00221287-130-7-1597. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Vasil M. L. Possible insertion sequences in a mosaic genome organization upstream of the exotoxin A gene in Pseudomonas aeruginosa. J Bacteriol. 1990 Apr;172(4):2020–2028. doi: 10.1128/jb.172.4.2020-2028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J. DNA topoisomerase I mutants. Increased heterogeneity in linking number and other replicon-dependent changes in DNA supercoiling. J Mol Biol. 1985 Sep 5;185(1):51–63. doi: 10.1016/0022-2836(85)90182-2. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. Topoisomerase I mutants: the gene on pBR322 that encodes resistance to tetracycline affects plasmid DNA supercoiling. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8952–8956. doi: 10.1073/pnas.83.23.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M., Flory J., Wu A., Kahn R., DasGupta C., Gonda D., Bianchi M., Tsang S. S. Three phases in homologous pairing: polymerization of recA protein on single-stranded DNA, synapsis, and polar strand exchange. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):821–828. doi: 10.1101/sqb.1983.047.01.094. [DOI] [PubMed] [Google Scholar]
- Radman M., Wagner R. Mismatch repair in Escherichia coli. Annu Rev Genet. 1986;20:523–538. doi: 10.1146/annurev.ge.20.120186.002515. [DOI] [PubMed] [Google Scholar]
- Rahmouni A. R., Wells R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989 Oct 20;246(4928):358–363. doi: 10.1126/science.2678475. [DOI] [PubMed] [Google Scholar]
- Rayssiguier C., Thaler D. S., Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989 Nov 23;342(6248):396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- Reanney D. Extrachromosomal elements as possible agents of adaptation and development. Bacteriol Rev. 1976 Sep;40(3):552–590. doi: 10.1128/br.40.3.552-590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
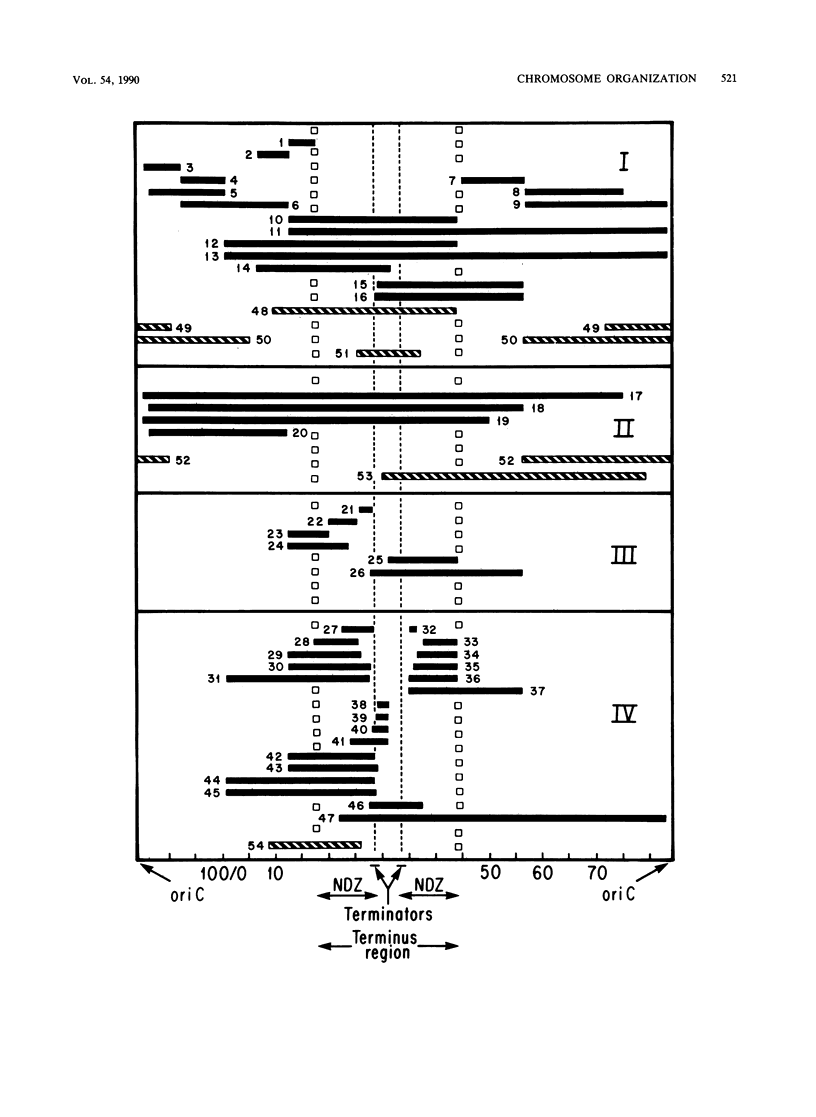
- Rebollo J. E., François V., Louarn J. M. Detection and possible role of two large nondivisible zones on the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9391–9395. doi: 10.1073/pnas.85.24.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. The lactose system in Klebsiella aerogenes V9A. 4. A comparison of the lac operons of Klebsiella and Escherichia coli. Genet Res. 1974 Dec;24(3):323–331. doi: 10.1017/s0016672300015329. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Riley M., Solomon L., Zipkas D. Relationship between gene function and gene location in Escherichia coli. J Mol Evol. 1978 May 12;11(1):47–56. doi: 10.1007/BF01768024. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Rudd K. E., Miller W., Ostell J., Benson D. A. Alignment of Escherichia coli K12 DNA sequences to a genomic restriction map. Nucleic Acids Res. 1990 Jan 25;18(2):313–321. doi: 10.1093/nar/18.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U., Grothues D., Bautsch W., Tümmler B. A physical genome map of Pseudomonas aeruginosa PAO. EMBO J. 1989 Dec 20;8(13):4081–4089. doi: 10.1002/j.1460-2075.1989.tb08592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Shimel B., Ellis S. Characterization of Azotobacter vinelandii deoxyribonucleic acid and folded chromosomes. J Bacteriol. 1979 Jun;138(3):871–877. doi: 10.1128/jb.138.3.871-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadosky A. B., Davidson A., Lin R. J., Hill C. W. rhs gene family of Escherichia coli K-12. J Bacteriol. 1989 Feb;171(2):636–642. doi: 10.1128/jb.171.2.636-642.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. A., Dykhuizen D. E., Hartl D. L. Confidence interval for the number of selectively neutral amino acid polymorphisms. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6225–6228. doi: 10.1073/pnas.84.17.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. Statistical tests for detecting gene conversion. Mol Biol Evol. 1989 Sep;6(5):526–538. doi: 10.1093/oxfordjournals.molbev.a040567. [DOI] [PubMed] [Google Scholar]
- Scaife J. F-prime factor formation in E. coli K12. Genet Res. 1966 Oct;8(2):189–196. doi: 10.1017/s0016672300010041. [DOI] [PubMed] [Google Scholar]
- Schleif R. DNA looping. Science. 1988 Apr 8;240(4849):127–128. doi: 10.1126/science.3353710. [DOI] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Gene location affects expression level in Salmonella typhimurium. J Bacteriol. 1987 Jun;169(6):2872–2875. doi: 10.1128/jb.169.6.2872-2875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Selection and endpoint distribution of bacterial inversion mutations. Genetics. 1983 Nov;105(3):539–557. doi: 10.1093/genetics/105.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. P., Nichols B. P., Yanofsky C. Procedure for production of hybrid genes and proteins and its use in assessing significance of amino acid differences in homologous tryptophan synthetase alpha polypeptides. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2169–2173. doi: 10.1073/pnas.78.4.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. W., Smith G. R. Conservation of Chi cutting activity in terrestrial and marine enteric bacteria. J Mol Biol. 1986 Jun 20;189(4):585–595. doi: 10.1016/0022-2836(86)90489-4. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Kalinowski J., Simon R., Seep-Feldhaus A. H., Pühler A. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J Bacteriol. 1990 Mar;172(3):1663–1666. doi: 10.1128/jb.172.3.1663-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Hagblom P., Seifert H. S., So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall A. M., Roth J. R. Recombination between homologies in direct and inverse orientation in the chromosome of Salmonella: intervals which are nonpermissive for inversion formation. Genetics. 1989 Aug;122(4):737–747. doi: 10.1093/genetics/122.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall A., Mahan M. J., Roth J. R. Rearrangement of the bacterial chromosome: forbidden inversions. Science. 1988 Sep 9;241(4871):1314–1318. doi: 10.1126/science.3045970. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Marchal C., Sparling P. F., So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988 Nov 24;336(6197):392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- Sela S., Clark-Curtiss J. E., Bercovier H. Characterization and taxonomic implications of the rRNA genes of Mycobacterium leprae. J Bacteriol. 1989 Jan;171(1):70–73. doi: 10.1128/jb.171.1.70-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Musser J. M., Caugant D. A., Gilmour M. N., Whittam T. S. Population genetics of pathogenic bacteria. Microb Pathog. 1987 Jul;3(1):1–7. doi: 10.1016/0882-4010(87)90032-5. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol Gen Genet. 1984;194(1-2):79–90. doi: 10.1007/BF00383501. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24(1-2):28–38. doi: 10.1007/BF02099948. [DOI] [PubMed] [Google Scholar]
- Sharp P. M. Processes of genome evolution reflected by base frequency differences among Serratia marcescens genes. Mol Microbiol. 1990 Jan;4(1):119–122. doi: 10.1111/j.1365-2958.1990.tb02020.x. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Shields D. C., Wolfe K. H., Li W. H. Chromosomal location and evolutionary rate variation in enterobacterial genes. Science. 1989 Nov 10;246(4931):808–810. doi: 10.1126/science.2683084. [DOI] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986 Mar;112(3):441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair M. I., Maxwell P. C., Lyon B. R., Holloway B. W. Chromosomal location of TOL plasmid DNA in Pseudomonas putida. J Bacteriol. 1986 Dec;168(3):1302–1308. doi: 10.1128/jb.168.3.1302-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Condemine G. New approaches for physical mapping of small genomes. J Bacteriol. 1990 Mar;172(3):1167–1172. doi: 10.1128/jb.172.3.1167-1172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Smith D., Yarus M. tRNA-tRNA interactions within cellular ribosomes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4397–4401. doi: 10.1073/pnas.86.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Amundsen S. K., Chaudhury A. M., Cheng K. C., Ponticelli A. S., Roberts C. M., Schultz D. W., Taylor A. F. Roles of RecBC enzyme and chi sites in homologous recombination. Cold Spring Harb Symp Quant Biol. 1984;49:485–495. doi: 10.1101/sqb.1984.049.01.055. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in E. coli: multiple pathways for multiple reasons. Cell. 1989 Sep 8;58(5):807–809. doi: 10.1016/0092-8674(89)90929-x. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Snyder U. K., Thompson J. F., Landy A. Phasing of protein-induced DNA bends in a recombination complex. Nature. 1989 Sep 21;341(6239):255–257. doi: 10.1038/341255a0. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Cannon J. G., So M. Phase and antigenic variation of pili and outer membrane protein II of Neisseria gonorrhoeae. J Infect Dis. 1986 Feb;153(2):196–201. doi: 10.1093/infdis/153.2.196. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Bacteria-yeast conjugation. Generic trans-kingdom sex? Nature. 1989 Jul 20;340(6230):190–191. doi: 10.1038/340190a0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Bacterial genetics. A unicorn in the garden. Nature. 1988 Sep 8;335(6186):112–113. doi: 10.1038/335112a0. [DOI] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Evidence that adenine methylation influences DNA-protein interactions in Escherichia coli. J Bacteriol. 1985 Oct;164(1):490–493. doi: 10.1128/jb.164.1.490-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart F. M., Gordon D. M., Levin B. R. Fluctuation analysis: the probability distribution of the number of mutants under different conditions. Genetics. 1990 Jan;124(1):175–185. doi: 10.1093/genetics/124.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus A., Leslie J. F., Milkman R. Molecular evolution of the Escherichia coli chromosome. I. Analysis of structure and natural variation in a previously uncharacterized region between trp and tonB. Genetics. 1988 Oct;120(2):345–358. doi: 10.1093/genetics/120.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storts D. R., Markovitz A. Construction and characterization of mutations in hupB, the gene encoding HU-beta (HU-1) in Escherichia coli K-12. J Bacteriol. 1988 Apr;170(4):1541–1547. doi: 10.1128/jb.170.4.1541-1547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Baseman J. B. Genome size of Mycoplasma genitalium. J Bacteriol. 1990 Aug;172(8):4705–4707. doi: 10.1128/jb.172.8.4705-4707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. Directional mutation pressure and neutral molecular evolution. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2653–2657. doi: 10.1073/pnas.85.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. A dominant influence of flanking sequences on a local structural transition in DNA. Cell. 1986 Dec 5;47(5):817–827. doi: 10.1016/0092-8674(86)90524-6. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Ono Y., Nagata A., Yamada T. Molecular cloning and characterization of an rRNA operon in Streptomyces lividans TK21. J Bacteriol. 1988 Apr;170(4):1631–1636. doi: 10.1128/jb.170.4.1631-1636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yoshinaga K., Ono Y., Nagata A., Yamada T. Organization of rRNA genes in Mycobacterium bovis BCG. J Bacteriol. 1987 Feb;169(2):839–843. doi: 10.1128/jb.169.2.839-843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschke C., Klinkert M. Q., Wolters J., Herrmann R. Organization of the ribosomal RNA genes in Mycoplasma hyopneumoniae: the 5S rRNA gene is separated from the 16S and 23S rRNA genes. Mol Gen Genet. 1986 Dec;205(3):428–433. doi: 10.1007/BF00338078. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Courvalin P. Conjugative plasmid transfer from Enterococcus faecalis to Escherichia coli. J Bacteriol. 1988 Sep;170(9):4388–4391. doi: 10.1128/jb.170.9.4388-4391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse-Dinh Y. C. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res. 1985 Jul 11;13(13):4751–4763. doi: 10.1093/nar/13.13.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Genetic analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWW0. Mol Gen Genet. 1987 Dec;210(2):270–276. doi: 10.1007/BF00325693. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Identification and characterization of Tn4653, a transposon covering the toluene transposon Tn4651 on TOL plasmid pWW0. Mol Gen Genet. 1988 Jul;213(1):72–77. doi: 10.1007/BF00333400. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion element IS5 in the Escherichia coli K-12 chromosome. Chromosomal rearrangements mediated by IS5. J Mol Biol. 1990 May 20;213(2):229–237. doi: 10.1016/S0022-2836(05)80186-X. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion elements IS1, IS2 and IS3 on the Escherichia coli K-12 chromosome. Role of the insertion elements in formation of Hfrs and F' factors and in rearrangement of bacterial chromosomes. J Mol Biol. 1989 Aug 20;208(4):601–614. doi: 10.1016/0022-2836(89)90151-4. [DOI] [PubMed] [Google Scholar]
- Van Vliet F., Boyen A., Glansdorff N. On interspecies gene transfer: the case of the argF gene of Escherichia coli. Ann Inst Pasteur Microbiol. 1988 Jul-Aug;139(4):493–496. doi: 10.1016/0769-2609(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Ventra L., Weiss A. S. Transposon-mediated restriction mapping of the Bacillus subtilis chromosome. Gene. 1989 May 15;78(1):29–36. doi: 10.1016/0378-1119(89)90311-9. [DOI] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Kano Y., Ogawa T., Okazaki T., Imamoto F. Construction and characterization of the deletion mutant of hupA and hupB genes in Escherichia coli. J Mol Biol. 1988 Dec 5;204(3):581–591. doi: 10.1016/0022-2836(88)90357-9. [DOI] [PubMed] [Google Scholar]
- Wada M., Kutsukake K., Komano T., Imamoto F., Kano Y. Participation of the hup gene product in site-specific DNA inversion in Escherichia coli. Gene. 1989;76(2):345–352. doi: 10.1016/0378-1119(89)90174-1. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Giaever G. N. Action at a distance along a DNA. Science. 1988 Apr 15;240(4850):300–304. doi: 10.1126/science.3281259. [DOI] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J. N., Maskell D. J., Butler P. D., Lindberg A. A., Moxon E. R. Characterization of repetitive sequences controlling phase variation of Haemophilus influenzae lipopolysaccharide. J Bacteriol. 1990 Jun;172(6):3304–3309. doi: 10.1128/jb.172.6.3304-3309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Collier D. A., Hanvey J. C., Shimizu M., Wohlrab F. The chemistry and biology of unusual DNA structures adopted by oligopurine.oligopyrimidine sequences. FASEB J. 1988 Nov;2(14):2939–2949. [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Cloning of the complete Mycoplasma pneumoniae genome. Nucleic Acids Res. 1989 Sep 12;17(17):7029–7043. doi: 10.1093/nar/17.17.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Iglewski B. H. Codon usage in Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Oct 11;16(19):9323–9335. doi: 10.1093/nar/16.19.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead T. R., Rabinowitz J. C. Nucleotide sequence of the Clostridium acidiurici ("Clostridium acidi-urici") gene for 10-formyltetrahydrofolate synthetase shows extensive amino acid homology with the trifunctional enzyme C1-tetrahydrofolate synthase from Saccharomyces cerevisiae. J Bacteriol. 1988 Jul;170(7):3255–3261. doi: 10.1128/jb.170.7.3255-3261.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam T. S., Ochman H., Selander R. K. Multilocus genetic structure in natural populations of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1751–1755. doi: 10.1073/pnas.80.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom R. L., Jarvis E. D., LaFauci G., Rudner R. Instability of rRNA operons in Bacillus subtilis. J Bacteriol. 1988 Feb;170(2):605–610. doi: 10.1128/jb.170.2.605-610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. J., Charles H. P. Polymorphism in Escherichia coli: rtl atl and gat regions behave as chromosomal alternatives. J Gen Microbiol. 1983 Jan;129(1):75–84. doi: 10.1099/00221287-129-1-75. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Xia X. M., Enomoto M. A naturally occurring large chromosomal inversion in Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):376–379. doi: 10.1007/BF00430454. [DOI] [PubMed] [Google Scholar]
- Yamagishi A., Oshima T. Circular chromosomal DNA in the sulfur-dependent archaebacterium Sulfolobus acidocaldarius. Nucleic Acids Res. 1990 Mar 11;18(5):1133–1136. doi: 10.1093/nar/18.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ames G. F. DNA gyrase binds to the family of prokaryotic repetitive extragenic palindromic sequences. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8850–8854. doi: 10.1073/pnas.85.23.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Folley L. S. Sense codons are found in specific contexts. J Mol Biol. 1985 Apr 20;182(4):529–540. doi: 10.1016/0022-2836(85)90239-6. [DOI] [PubMed] [Google Scholar]
- Yee T., Inouye M. Two-dimensional DNA electrophoresis applied to the study of DNA methylation and the analysis of genome size in Myxococcus xanthus. J Mol Biol. 1982 Jan 15;154(2):181–196. doi: 10.1016/0022-2836(82)90059-6. [DOI] [PubMed] [Google Scholar]
- York M. K., Stodolsky M. Characterization of P1argF derivatives from Escherichia coli K12 transduction. I. IS1 elements flank the argF gene segment. Mol Gen Genet. 1981;181(2):230–240. doi: 10.1007/BF00268431. [DOI] [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]
- Zeigler D. R., Dean D. H. Orientation of genes in the Bacillus subtilis chromosome. Genetics. 1990 Aug;125(4):703–708. doi: 10.1093/genetics/125.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Béjar S., Louarn J., Louarn J. M., Bouché J. P. Inhibition of replication forks exiting the terminus region of the Escherichia coli chromosome occurs at two loci separated by 5 min. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1759–1763. doi: 10.1073/pnas.84.7.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]


