Abstract
When the growth of the gram-negative bacterial cell wall is considered in relation to the synthesis of the other components of the cell, a new understanding of the pattern of wall synthesis emerges. Rather than a switch in synthesis between the side wall and pole, there is a partitioning of synthesis such that the volume of the cell increases exponentially and thus perfectly encloses the exponentially increasing cytoplasm. This allows the density of the cell to remain constant during the division cycle. This model is explored at both the cellular and molecular levels to give a unified description of wall synthesis which has the following components: (i) there is no demonstrable turnover of peptidoglycan during cell growth, (ii) the side wall grows by diffuse intercalation, (iii) pole synthesis starts by some mechanism and is preferentially synthesized compared with side wall, and (iv) the combined side wall and pole syntheses enclose the newly synthesized cytoplasm at a constant cell density. The central role of the surface stress model in wall growth is distinguished from, and preferred to, models that propose cell-cycle-specific signals as triggers of changes in the rate of wall synthesis. The actual rate of wall synthesis during the division cycle is neither exponential nor linear, but is close to exponential when compared with protein synthesis during the division cycle.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Garrido T., Pla J., Vicente M. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990 Nov;9(11):3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M., Herrero E., Esteve M. I., Guerrero R. Surface density of major outer membrane proteins in Salmonella typhimurium in different growth conditions. J Gen Microbiol. 1980 Oct;120(2):355–367. doi: 10.1099/00221287-120-2-355. [DOI] [PubMed] [Google Scholar]
- Bauza M. T., De Loach J. R., Aguanno J. J., Larrabee A. R. Acyl carrier protein prosthetic group exchange and phospholipid synthesis in synchronized cultures of a pantothenate auxotroph Escherichia coli. Arch Biochem Biophys. 1976 May;174(1):344–349. doi: 10.1016/0003-9861(76)90354-4. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Cole R. M. Cell wall replication in Escherichia coli, studies by immunofluorescence and immunoelectron microscopy. J Bacteriol. 1966 Oct;92(4):1245–1251. doi: 10.1128/jb.92.4.1245-1251.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J. Cell surface growth in Escherichia coli: distribution of matrix protein. J Bacteriol. 1978 Aug;135(2):307–310. doi: 10.1128/jb.135.2.307-310.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Doanachie W. D. Growth of the Escherichia coli cell surface. J Bacteriol. 1977 Mar;129(3):1524–1536. doi: 10.1128/jb.129.3.1524-1536.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Topography of outer membrane growth in E. coli. Nat New Biol. 1973 Sep 12;245(141):38–39. doi: 10.1038/newbio245038a0. [DOI] [PubMed] [Google Scholar]
- Begg K. J., Takasuga A., Edwards D. H., Dewar S. J., Spratt B. G., Adachi H., Ohta T., Matsuzawa H., Donachie W. D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990 Dec;172(12):6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Protein d, an iron-transport protein induced by filtration of cultures of Escherichia coli. FEBS Lett. 1977 Apr 1;76(1):20–24. doi: 10.1016/0014-5793(77)80112-9. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Changes in the composition of Escherichia coli murein as it ages during exponential growth. J Bacteriol. 1983 Aug;155(2):447–453. doi: 10.1128/jb.155.2.447-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Raichler J., Park J. T. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J Bacteriol. 1983 Sep;155(3):983–988. doi: 10.1128/jb.155.3.983-988.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Reichler J., Park J. T. Evidence for multisite growth of Escherichia coli murein involving concomitant endopeptidase and transpeptidase activities. J Bacteriol. 1983 Oct;156(1):386–392. doi: 10.1128/jb.156.1.386-392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. II. CELL WALL GROWTH AND CROSS WALL FORMATION OF ESCHERICHIA COLI AND STREPTOCOCCUS FAECALIS. Can J Microbiol. 1964 Jun;10:473–482. doi: 10.1139/m64-057. [DOI] [PubMed] [Google Scholar]
- COLE R. M. CELL WALL REPLICATION IN SALMONELLA TYPHOSA. Science. 1964 Feb 21;143(3608):820–822. doi: 10.1126/science.143.3608.820. [DOI] [PubMed] [Google Scholar]
- COLLINS J. F., RICHMOND M. H. Rate of growth of Bacillus cereus between divisions. J Gen Microbiol. 1962 Apr;28:15–33. doi: 10.1099/00221287-28-1-15. [DOI] [PubMed] [Google Scholar]
- Carty C. E., Ingram L. O. Lipid synthesis during the Escherichia coli cell cycle. J Bacteriol. 1981 Jan;145(1):472–478. doi: 10.1128/jb.145.1.472-478.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka J., Strnadová M. Turnover of murein in a diaminopimelic acid dependent mutant of Escherichia coli. Folia Microbiol (Praha) 1972;17(6):446–455. doi: 10.1007/BF02872729. [DOI] [PubMed] [Google Scholar]
- Chang C. F., Shuman H., Somlyo A. P. Electron probe analysis, X-ray mapping, and electron energy-loss spectroscopy of calcium, magnesium, and monovalent ions in log-phase and in dividing Escherichia coli B cells. J Bacteriol. 1986 Sep;167(3):935–939. doi: 10.1128/jb.167.3.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G. G., Holland I. B. Envelope synthesis during the cell cycle in Escherichia coli B/r. J Mol Biol. 1976 Aug 5;105(2):245–261. doi: 10.1016/0022-2836(76)90110-8. [DOI] [PubMed] [Google Scholar]
- Cook W. R., Kalb V. F., Jr, Peace A. A., Bernlohr R. W. Is cyclic guanosine 3',5'-monophosphate a cell cycle regulator? J Bacteriol. 1980 Mar;141(3):1450–1453. doi: 10.1128/jb.141.3.1450-1453.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. R., Kepes F., Joseleau-Petit D., MacAlister T. J., Rothfield L. I. Proposed mechanism for generation and localization of new cell division sites during the division cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7144–7148. doi: 10.1073/pnas.84.20.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Cell division and DNA replication following a shift to a richer medium. J Mol Biol. 1969 Jul 14;43(1):1–11. doi: 10.1016/0022-2836(69)90074-6. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cooper S., Hsieh M. L., Guenther B. Mode of peptidoglycan synthesis in Salmonella typhimurium: single-strand insertion. J Bacteriol. 1988 Aug;170(8):3509–3512. doi: 10.1128/jb.170.8.3509-3512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
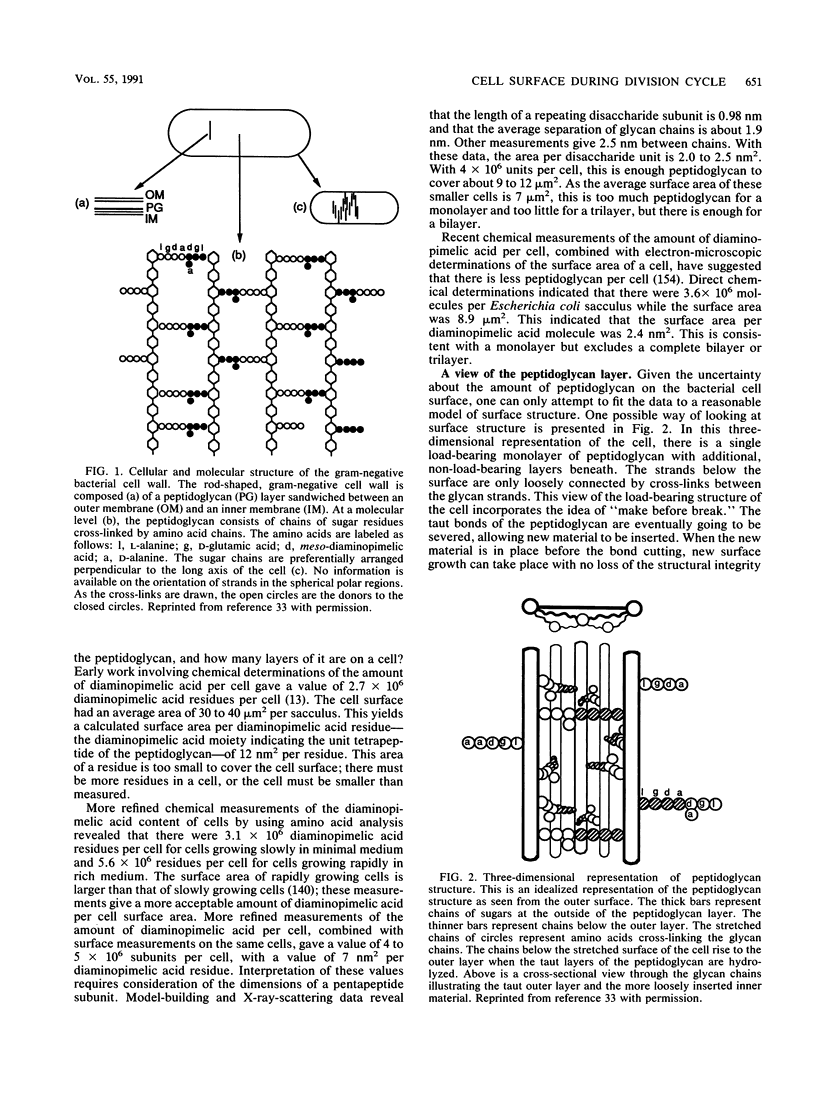
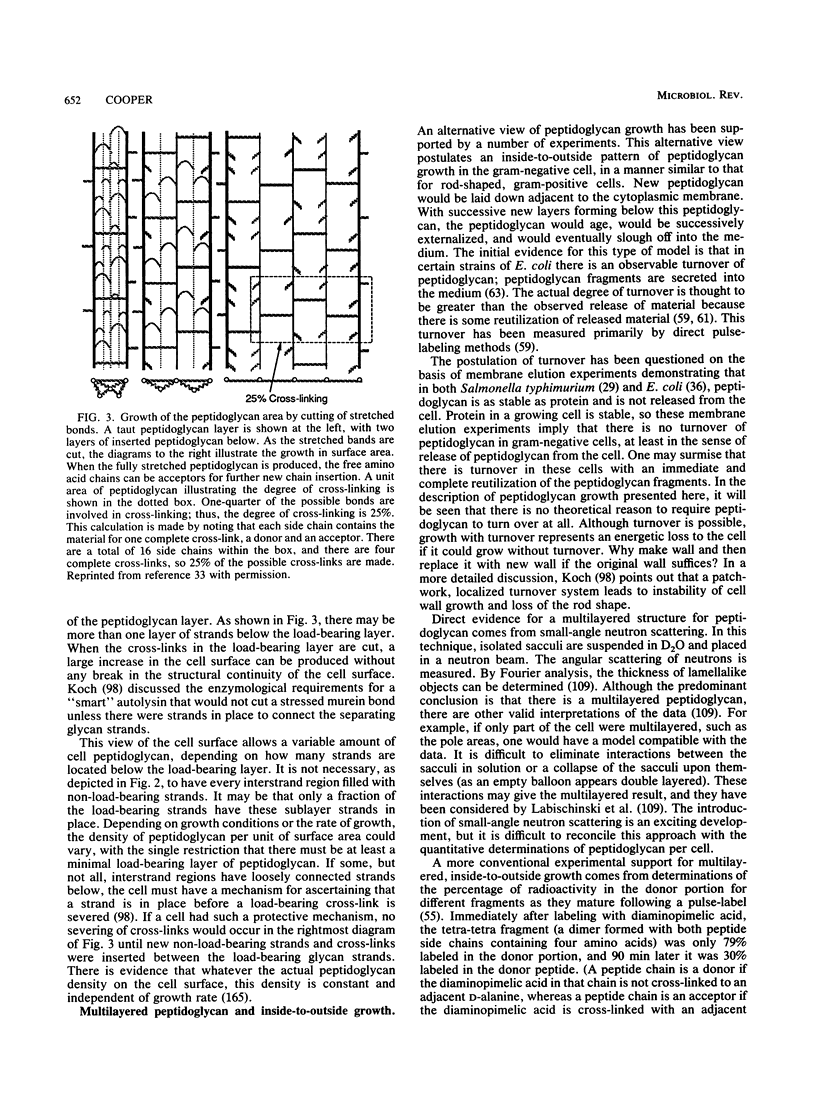
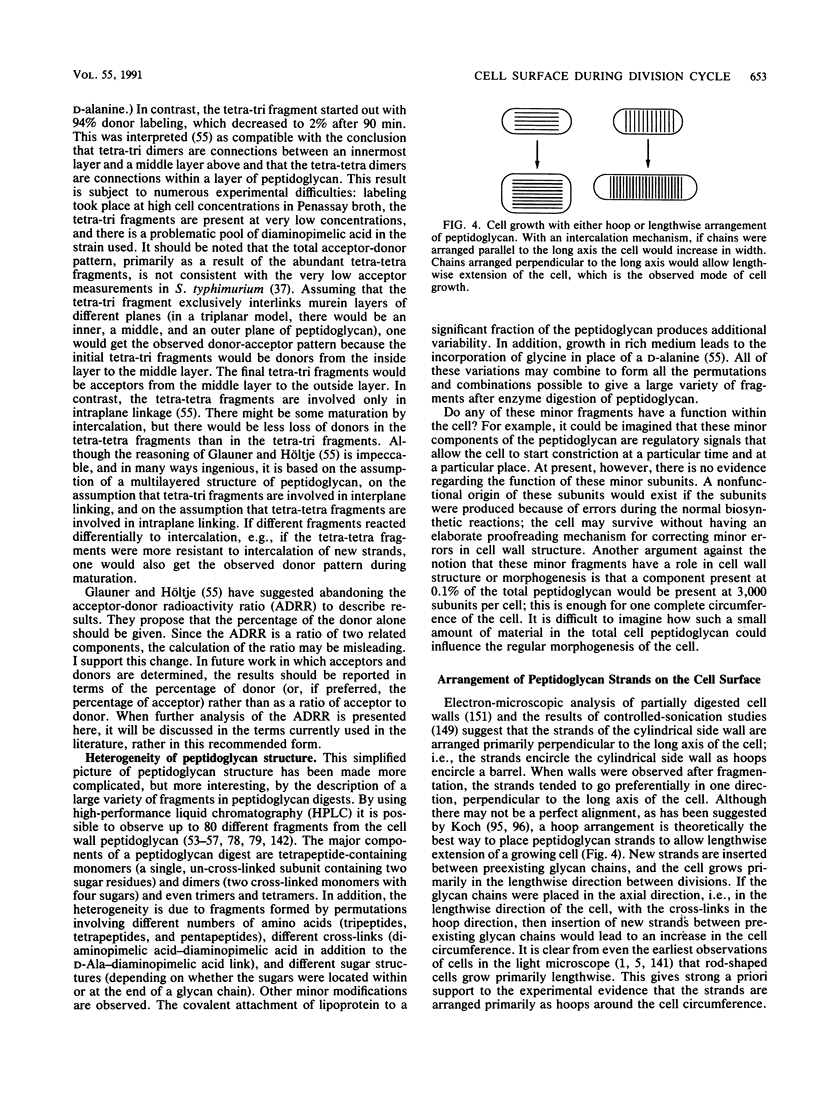
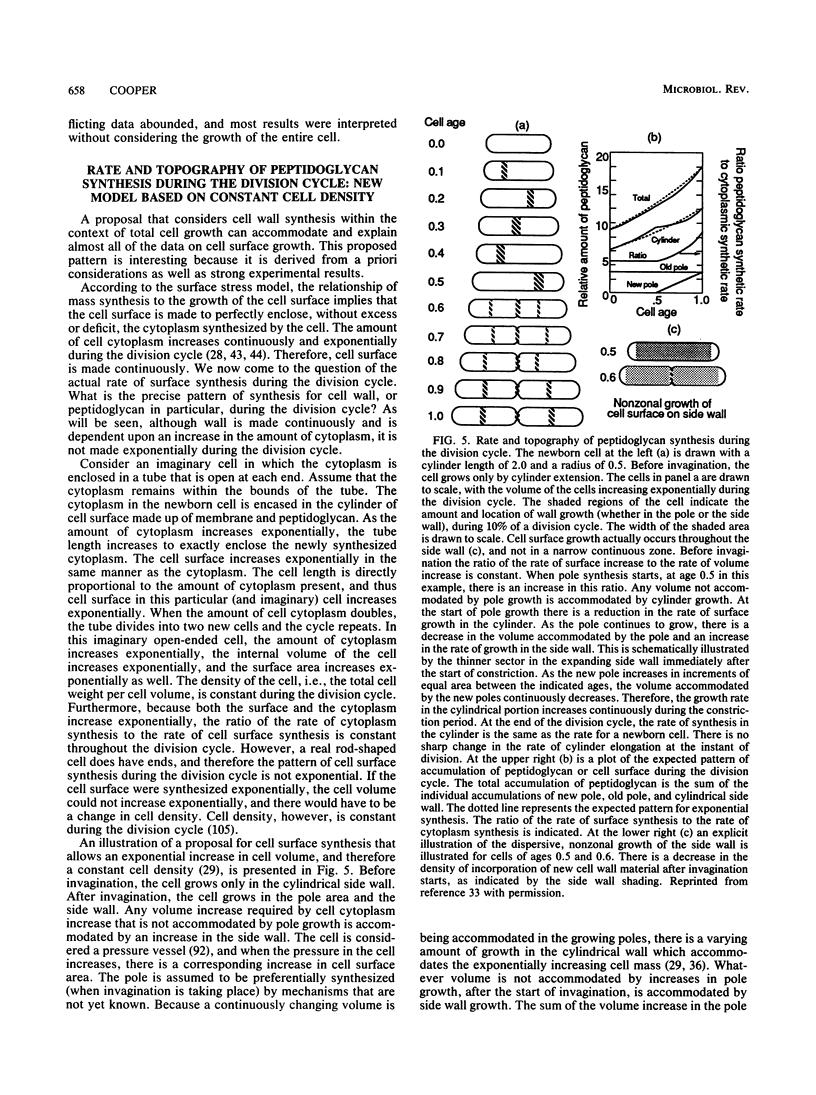
- Cooper S., Hsieh M. L. The rate and topography of cell wall synthesis during the division cycle of Escherichia coli using N-acetylglucosamine as a peptidoglycan label. J Gen Microbiol. 1988 Jun;134(6):1717–1721. doi: 10.1099/00221287-134-6-1717. [DOI] [PubMed] [Google Scholar]
- Cooper S. Leucine uptake and protein synthesis are exponential during the division cycle of Escherichia coli B/r. J Bacteriol. 1988 Jan;170(1):436–438. doi: 10.1128/jb.170.1.436-438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Rate and topography of cell wall synthesis during the division cycle of Salmonella typhimurium. J Bacteriol. 1988 Jan;170(1):422–430. doi: 10.1128/jb.170.1.422-430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Relationship between the acceptor/donor radioactivity ratio and cross-linking in bacterial peptidoglycan: application to surface synthesis during the division cycle. J Bacteriol. 1990 Sep;172(9):5506–5510. doi: 10.1128/jb.172.9.5506-5510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. Temperature dependent alteration in bacterial protein composition. Biochem Biophys Res Commun. 1975 Feb 3;62(3):584–586. doi: 10.1016/0006-291x(75)90438-6. [DOI] [PubMed] [Google Scholar]
- Cooper S. The Escherichia coli cell cycle. Res Microbiol. 1990 Jan;141(1):17–29. doi: 10.1016/0923-2508(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Cooper S. What is the bacterial growth law during the division cycle? J Bacteriol. 1988 Nov;170(11):5001–5005. doi: 10.1128/jb.170.11.5001-5005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Stable ribonucleic acid synthesis during the cell division cycle in slowly growing Escherichia coli B-r. J Biol Chem. 1972 Jan 10;247(1):204–208. [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driehuis F., Wouters J. T. Effect of growth rate and cell shape on the peptidoglycan composition in Escherichia coli. J Bacteriol. 1987 Jan;169(1):97–101. doi: 10.1128/jb.169.1.97-101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988 Aug 1;172(2):451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Higgins C. F. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1987 Aug;169(8):3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W. Recycling of murein by Escherichia coli. J Bacteriol. 1985 Jul;163(1):305–310. doi: 10.1128/jb.163.1.305-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Cleavage and resynthesis of peptide cross bridges in Escherichia coli murein. J Bacteriol. 1983 Oct;156(1):136–140. doi: 10.1128/jb.156.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985 Apr;162(1):391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U., Teather R. M. Cell envelope composition of Escherichia coli K12: a comparison of the cell poles and the lateral wall. Eur J Biochem. 1974 Sep 16;47(3):567–572. doi: 10.1111/j.1432-1033.1974.tb03727.x. [DOI] [PubMed] [Google Scholar]
- Grover N. B., Woldringh C. L., Koppes L. J. Elongation and surface extension of individual cells of Escherichia coli B/r: comparison of theoretical and experimental size distributions. J Theor Biol. 1987 Dec 7;129(3):337–348. doi: 10.1016/s0022-5193(87)80006-1. [DOI] [PubMed] [Google Scholar]
- Grover N. B., Woldringh C. L., Zaritsky A., Rosenberger R. F. Elongation of rod-shaped bacteria. J Theor Biol. 1977 Jul 21;67(2):181–193. doi: 10.1016/0022-5193(77)90192-8. [DOI] [PubMed] [Google Scholar]
- Grover N. B., Zaritsky A., Woldringh C. L., Rosenberger R. F. Dimensional rearrangement of rod-shaped bacteria following nutritional shift-up. I. Theory. J Theor Biol. 1980 Oct 7;86(3):421–439. doi: 10.1016/0022-5193(80)90343-4. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1239–1244. doi: 10.1128/jb.129.3.1239-1244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Oscillations in the synthesis of cell wall components in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1234–1238. doi: 10.1128/jb.129.3.1234-1238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harz H., Burgdorf K., Höltje J. V. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal Biochem. 1990 Oct;190(1):120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Casaregola S., Norris V. Cytoskeletal elements and calcium: do they play a role in the Escherichia coli cell cycle? Res Microbiol. 1990 Jan;141(1):131–136. doi: 10.1016/0923-2508(90)90104-x. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. H., Young K. D. Cell cycle-independent lysis of Escherichia coli by cefsulodin, an inhibitor of penicillin-binding proteins 1a and 1b. J Bacteriol. 1991 Jan;173(1):1–5. doi: 10.1128/jb.173.1.1-5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Gudas L. J. Cell cycle-specific incorporation of lipoprotein into the outer membrane of Escherichia coli. J Bacteriol. 1976 Jan;125(1):374–375. doi: 10.1128/jb.125.1.374-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau-Petit D., Kepes F., Kepes A. Cyclic changes of the rate of phospholipid synthesis during synchronous growth of Escherichia coli. Eur J Biochem. 1984 Mar 15;139(3):605–611. doi: 10.1111/j.1432-1033.1984.tb08047.x. [DOI] [PubMed] [Google Scholar]
- Joseleau-Petit D., Kepes F., Peutat L., D'Ari R., Rothfield L. I. Biosynthesis of a membrane adhesion zone fraction throughout the cell cycle of Escherichia coli. J Bacteriol. 1990 Nov;172(11):6573–6575. doi: 10.1128/jb.172.11.6573-6575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau-Petit D., Képès F., Peutat L., D'Ari R., Képès A. DNA replication initiation, doubling of rate of phospholipid synthesis, and cell division in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3701–3706. doi: 10.1128/jb.169.8.3701-3706.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes F., Kepes A. Long - lasting synchrony of the division of enteric bacteria. Biochem Biophys Res Commun. 1981 Apr 15;99(3):761–767. doi: 10.1016/0006-291x(81)91230-4. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Additional arguments for the key role of "smart" autolysins in the enlargement of the wall of gram-negative bacteria. Res Microbiol. 1990 Jun;141(5):529–541. doi: 10.1016/0923-2508(90)90017-k. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol Rev. 1988 Sep;52(3):337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Blumberg G. Distribution of bacteria in the velocity gradient centrifuge. Biophys J. 1976 May;16(5):389–405. doi: 10.1016/S0006-3495(76)85696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. The variable T model for gram-negative morphology. J Gen Microbiol. 1984 Sep;130(9):2325–2338. doi: 10.1099/00221287-130-9-2325. [DOI] [PubMed] [Google Scholar]
- Koch A. L. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985 Dec;31(12):1071–1084. doi: 10.1139/m85-204. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Meyer M., Oonk H. B., de Jong M. A., Nanninga N. Correlation between size and age at different events in the cell division cycle of Escherichia coli. J Bacteriol. 1980 Sep;143(3):1241–1252. doi: 10.1128/jb.143.3.1241-1252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Overbeeke N., Nanninga N. DNA replication pattern and cell wall growth in Escherichia coli PAT 84. J Bacteriol. 1978 Mar;133(3):1053–1061. doi: 10.1128/jb.133.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Woldringh C. L., Grover N. B. Predicted steady-state cell size distributions for various growth models. J Theor Biol. 1987 Dec 7;129(3):325–335. doi: 10.1016/s0022-5193(87)80005-x. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Baldwin W. W., Graetzer R. Buoyant density constancy during the cell cycle of Escherichia coli. J Bacteriol. 1983 Sep;155(3):1027–1032. doi: 10.1128/jb.155.3.1027-1032.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Baldwin W. W., Schroeter S. J., Graetzer R. Independence of buoyant cell density and growth rate in Escherichia coli. J Bacteriol. 1984 Apr;158(1):296–299. doi: 10.1128/jb.158.1.296-299.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Buoyant density variation during the cell cycle in microorganisms. Crit Rev Microbiol. 1987;14(1):73–97. doi: 10.3109/10408418709104436. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Woldringh C. L. Cell elongation and division probability during the Escherichia coli growth cycle. J Bacteriol. 1983 Mar;153(3):1379–1387. doi: 10.1128/jb.153.3.1379-1387.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labischinski H., Goodell E. W., Goodell A., Hochberg M. L. Direct proof of a "more-than-single-layered" peptidoglycan architecture of Escherichia coli W7: a neutron small-angle scattering study. J Bacteriol. 1991 Jan;173(2):751–756. doi: 10.1128/jb.173.2.751-756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo M. M., Canepari P., Satta G. Bacterial cell shape regulation: testing of additional predictions unique to the two-competing-sites model for peptidoglycan assembly and isolation of conditional rod-shaped mutants from some wild-type cocci. J Bacteriol. 1990 Jul;172(7):3758–3771. doi: 10.1128/jb.172.7.3758-3771.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister T. J., Cook W. R., Weigand R., Rothfield L. I. Membrane-murein attachment at the leading edge of the division septum: a second membrane-murein structure associated with morphogenesis of the gram-negative bacterial division septum. J Bacteriol. 1987 Sep;169(9):3945–3951. doi: 10.1128/jb.169.9.3945-3951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Salas E., Martín J. A., Vicente M. Relationship of Escherichia coli density to growth rate and cell age. J Bacteriol. 1981 Jul;147(1):97–100. doi: 10.1128/jb.147.1.97-100.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Wachi M., Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990 Jan;141(1):89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Meyer M., De Jong M. A., Demets R., Nanninga N. Length growth of two Escherichia coli B/r substrains. J Bacteriol. 1979 Apr;138(1):17–23. doi: 10.1128/jb.138.1.17-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozharov A. D., Shchipakin V. N., Fishov I. L., Evtodienko YuV Changes in the composition of membrane phospholipids during the cell cycle of Escherichia coli. FEBS Lett. 1985 Jul 1;186(1):103–106. doi: 10.1016/0014-5793(85)81348-x. [DOI] [PubMed] [Google Scholar]
- Mulder E., Woldringh C. L. Actively replicating nucleoids influence positioning of division sites in Escherichia coli filaments forming cells lacking DNA. J Bacteriol. 1989 Aug;171(8):4303–4314. doi: 10.1128/jb.171.8.4303-4314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Cell division and peptidoglycan assembly in Escherichia coli. Mol Microbiol. 1991 Apr;5(4):791–795. doi: 10.1111/j.1365-2958.1991.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Norris V. Phospholipid flip-out controls the cell cycle of Escherichia coli. J Theor Biol. 1989 Jul 10;139(1):117–128. doi: 10.1016/s0022-5193(89)80061-x. [DOI] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Olijhoek A. J., Klencke S., Pas E., Nanninga N., Schwarz U. Volume growth, murein synthesis, and murein cross-linkage during the division cycle of Escherichia coli PA3092. J Bacteriol. 1982 Dec;152(3):1248–1254. doi: 10.1128/jb.152.3.1248-1254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O. Dimensions of Escherichia coli at various growth rates: model for envelope growth. J Bacteriol. 1978 Aug;135(2):559–574. doi: 10.1128/jb.135.2.559-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Melzer M., Querini C., Rickert M., Krajewski C. Comparison among patterns of macromolecular synthesis in Escherichia coli B/r at growth rates of less and more than one doubling per hour at 37 degrees C. J Bacteriol. 1981 Nov;148(2):684–696. doi: 10.1128/jb.148.2.684-696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O. Phospholipid synthesis during the cell division cycle of Escherichia coli. J Bacteriol. 1979 May;138(2):453–460. doi: 10.1128/jb.138.2.453-460.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard R. H. Review lecture on the growth and form of a bacterial cell. Philos Trans R Soc Lond B Biol Sci. 1974 Feb 21;267(886):303–336. doi: 10.1098/rstb.1974.0003. [DOI] [PubMed] [Google Scholar]
- Ramey W. D., Ishiguro E. E. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli. J Bacteriol. 1978 Jul;135(1):71–77. doi: 10.1128/jb.135.1.71-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A., Joseleau-Petit D., D'Ari R. Transcription of the ftsZ gene and cell division in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1392–1399. doi: 10.1128/jb.172.3.1392-1399.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger R. F., Grover N. B., Zaritsky A., Woldringh C. L. Control of microbial surface-growth by density. Nature. 1978 Jan 19;271(5642):244–245. doi: 10.1038/271244a0. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Grover N. B., Zaritsky A., Woldringh C. L. Surface growth in rod-shaped bacteria. J Theor Biol. 1978 Aug 21;73(4):711–721. doi: 10.1016/0022-5193(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Rothfield L. I., Cook W. R. Periseptal annuli: organelles involved in the bacterial cell division process. Microbiol Sci. 1988 Jun;5(6):182–185. [PubMed] [Google Scholar]
- Rothfield L. I., DeBoer P., Cook W. R. Localization of septation sites. Res Microbiol. 1990 Jan;141(1):57–63. doi: 10.1016/0923-2508(90)90098-b. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Surface extension and the cell cycle in prokaryotes. Adv Microb Physiol. 1978;18:105–176. doi: 10.1016/s0065-2911(08)60416-6. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., VanBogelen R. A., Neidhardt F. C. Heat shock response in Escherichia coli influences cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6959–6963. doi: 10.1073/pnas.83.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R. Changes in peptidoglycan composition and penicillin-binding proteins in slowly growing Escherichia coli. J Bacteriol. 1987 Nov;169(11):5308–5310. doi: 10.1128/jb.169.11.5308-5310.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TUBERGEN R. P., SETLOW R. B. Quantitative radioautographic studies on exponentially growing cultures of Escherichia coli. The distribution of parental DNA, RNA, protein, and cell wall among progeny cells. Biophys J. 1961 Sep;1:589–625. doi: 10.1016/s0006-3495(61)86911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Beachey E. H., Keck W., Stoub A. M., Poldermans J. E. Oriented fragmentation of Escherichia coli sacculi by sonication. J Bacteriol. 1980 Jan;141(1):327–332. doi: 10.1128/jb.141.1.327-332.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N., Keck W., Schwarz U. Arrangement of glycan chains in the sacculus of Escherichia coli. J Bacteriol. 1978 Nov;136(2):723–729. doi: 10.1128/jb.136.2.723-729.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N. Pattern of meso-dl-2,6-diaminopimelic acid incorporation during the division cycle of Escherichia coli. J Bacteriol. 1980 Oct;144(1):327–336. doi: 10.1128/jb.144.1.327-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Nanninga N. Rate and topography of peptidoglycan synthesis during cell division in Escherichia coli: concept of a leading edge. J Bacteriol. 1989 Jun;171(6):3412–3419. doi: 10.1128/jb.171.6.3412-3419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976 Jan;125(1):248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., Mulder E., Huls P. G., Vischer N. Toporegulation of bacterial division according to the nucleoid occlusion model. Res Microbiol. 1991 Feb-Apr;142(2-3):309–320. doi: 10.1016/0923-2508(91)90046-d. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., Valkendurg J. A., Pas E., Taschner P. E., Huls P., Wientjes F. B. Physiological and geometrical conditions for cell division in Escherichia coli. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):131–138. doi: 10.1016/s0769-2609(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A. On dimensional determination of rod-shaped bacteria. J Theor Biol. 1975 Oct;54(2):243–248. doi: 10.1016/s0022-5193(75)80129-9. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa E. J., de Pedro M. A., Vázquez D. Penicillin binding proteins: role in initiation of murein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5632–5635. doi: 10.1073/pnas.82.17.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]


