Abstract
In Arabidopsis thaliana, like in other dicots, the shoot epidermis originates from protodermis, the outermost cell layer of shoot apical meristem. We examined leaf epidermis in transgenic A. thaliana plants in which CDKA;1.N146, a negative dominant allele of A-type cyclin-dependent kinase, was expressed from the SHOOTMERISTEMLESS promoter, i.e., in the shoot apical meristem. Using cleared whole mount preparations of expanding leaves and sequential in vivo replicas of expanding leaf surface, we show that dominant-negative CDKA;1 expression results in defects in epidermis continuity: loss of individual cells and occurrence of gaps between anticlinal walls of neighboring pavement cells. Another striking feature is ingrowth-like invaginations of anticlinal cell walls of pavement cells. Their formation is related to various processes: expansion of cells surrounding the sites of cell loss, defected cytokinesis, and presumably also, the actual ingrowth of an anticlinal cell wall. The mutant exhibits also increased variation in cell size and locally reduced waviness of anticlinal walls of pavement cells. These unusual features of leaf epidermis phenotype may shed a new light on our knowledge on morphogenesis of jigsaw puzzle-shaped pavement cells and on the CDKA;1 role in regulation of plant development via influence on cytoskeleton and plant cell wall.
Keywords: Arabidopsis thaliana, CDKA;1, Cell loss, Cell wall invaginations, Gaps in epidermis, Leaf epidermis
Introduction
All the shoot organs are generated at the shoot apical meristem (SAM). In Arabidopsis thaliana (L.) Heynh., like in other dicots, the shoot epidermis originates from the outermost SAM cell layer, i.e., protodermis. Defects in the protodermal cells are therefore expected to influence the shoot epidermis phenotype. We examined the leaf epidermis in transgenic A. thaliana plants in which CDKA;1.N146, a negative dominant allele of A-type cyclin-dependent kinase (CDKA;1), was expressed from the SHOOTMERISTEMLESS (STM) promoter, resulting in transgene expression in the SAM (Joubès et al. 2004; Gaamouche et al. 2010). CDKA;1 is the major cell division controlling CDK in A. thaliana. Next to driving different cell cycle transitions, its activity is postulated to control also cytokinesis through affecting microtubule-associated proteins (Vanstraelen et al. 2006; Dudits et al. 2007; Sasabe et al. 2011). Gaamouche et al. (2010) showed that in the examined transgenic lines, some of the SAM cells differentiate prematurely, and mean epidermal cell size is increased, while their number is reduced in comparison to wild type. Mature leaves of transgenic plants are smaller, and their shapes are less regular than in the wild type. Here, we report novel features of leaf epidermis phenotype of these lines, some of which seem difficult to explain by transgene action. These features, upon further investigation, may shed a new light on our knowledge on morphogenesis of leaf epidermal cells and on the CDKA;1 role in regulation of plant development via influence on cytoskeleton and plant cell wall.
Material and methods
Plant material and growth conditions
We examined A. thaliana transgenic lines on the cv. Columbia-0 (Col-0) background that expressed the dominant negative CDKA;1.N146 allele under the control of STM promoter (P STM). Two independent homozygous lines were used: P STM :CDKA;1.N146 B3–4 and P STM :CDKA;1.N146 B3–5, further referred to as B3–4 and B3–5, respectively. The same lines were examined by Gaamouche et al. (2010).
Potted plants were grown for 4–6 weeks (from sowing) in a growth room under short-day conditions (9 h day; 15 h night), temperature 19–21 °C, and illumination 60 μmol m−2 s−1.
Histological procedures
Entire leaves were fixed in formalin–aceto–alcohol (with 70 % ethanol; Johansen 1940), rinsed in 70 % ethanol, and cleared in 5–10 % sodium hydroxide solution for 24–36 h at 37 °C. The whole mount-cleared material was observed in 50 % glycerol under Nomarsky light microscopy (Nikon Eclipse 80i). Some specimens were additionally stained in ruthenium red (Johansen 1940) to better visualize anticlinal cell walls. Abaxial and adaxial epidermis was examined in 18 young leaves from the mutant (eight from B-3 and ten from B-5 line) and ten from Col-0 plants, always the seventh, eighth, ninth, or tenth rosette leaf, with laminas 1–5 mm long. All the leaves were in the expansion phase of development distinguished by Granier and Tardieu (2009).
In order to visualize nuclei and walls of epidermal cells, entire leaves (two from Col-0 and four from B3–5) were fixed in aceto–alcohol (Johansen 1940) and stained with Schiff reagent (S 5133, Sigma-Aldrich) and Vectashield with DAPI (H-1200, Vector Laboratories, Inc.) as described earlier (Elsner et al. 2012). Epidermis of these leaves was examined in the epi-fluorescence microscopy (Olympus BX41).
Sequential replicas and scanning electron microscopy
To analyze epidermal cell fates, the sequential replica method was used (Williams and Green 1988; Elsner et al. 2012). Briefly, silicon polymer molds were taken from the surface of individual leaves and filled with epoxy resin. Such obtained casts were sputter coated and observed under scanning electron microscopy (SEM; Philips XL 30 TMP ESEN). Sequences of three replicas were taken at 48-h intervals from abaxial epidermis of seven B-5 and seven Col-0 leaves. All these were the seventh rosette leaves, with laminas 2.5–6 mm long. They were in the expansion phase of development (Granier and Tardieu 2009).
Results and discussion
Loss of individual cells and occurrence of gaps in leaf epidermis of plants with dominant-negative CDKA;1 expression
The most spectacular and common feature of leaf epidermis observed in both independent P STM :CDKA;1.N146 lines are defects in epidermis continuity. In cleared material, there are sites where some pavement cells are missing (e.g., marked by x in Fig. 1a). Outlines of these sites resemble the jigsaw puzzle shape of pavement cells. They are partly closed due to expansion of surrounding epidermal cells (Fig. 1a, b) and/or by underlying mesophyll cells growing toward the leaf surface (M1 and M2 in Fig. 1b). Most likely, in the mutant epidermis, there are also gaps (G in Fig. 1c, d, f), i.e., places where anticlinal walls of adjacent cells are not in contact. They resemble epidermal gaps reported for the distorted group of mutants (e.g., Basu et al. 2005, 2008; reviewed in Szymanski 2005; Qian et al. 2009). However, since, generally, a cell pattern in the mutant is not so regular as in the wild type (compare Fig. 1a, c, d with h, i), it is often not easy to distinguish between the sites of cell loss, partly closed by neighboring cell expansion, and gaps.
Fig. 1.
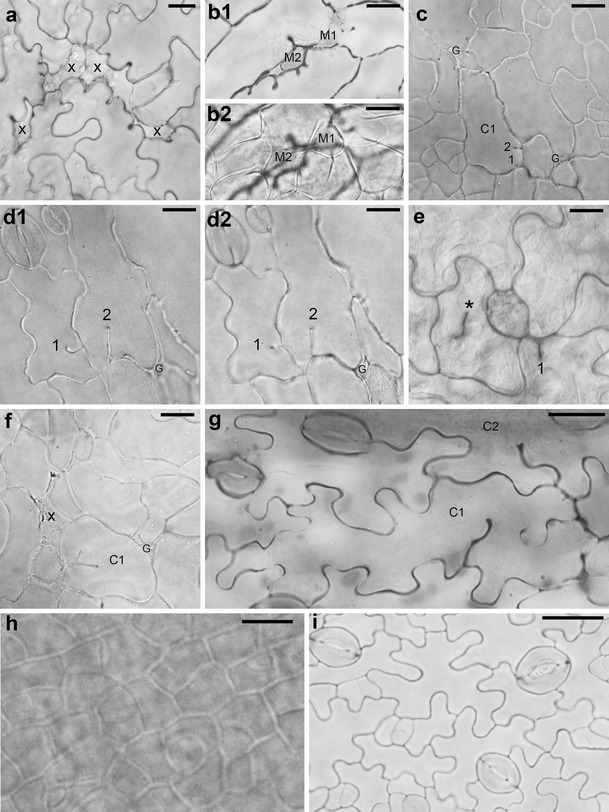
a–i Micrographs showing optical sections of abaxial (a, c–i) or adaxial (b) epidermis of cleared whole mount preparations of P STM :CDKA;1.N146 (a–g) and Col-0 (h–i) leaves. a Sites of cell loss (x) surrounded by cells with anticlinal wall invaginations (line B3–4; leaf with lamina c. 1 mm long). b 1, 2 Two optical sections of the leaf fragment. b 1 Through epidermis, cell loss sites that are partly filled by rounded cells (M1 and M2) are shown; b 2 through underlying palisade mesophyll, the M1 and M2 can be recognized as mesophyll cells (B3–5 leaf; c. 4 mm). c Epidermis fragment with not yet well-developed wall waviness showing gaps (G) that are most likely not related to cell loss and a cell (C1) with two short ingrowth-like invaginations (1 and 2) not adjacent to a gap (B3–5, c. 2 mm). d 1, 2 Two optical sections of the epidermis region with two invaginations (1 and 2) not adjacent to a gap (G). The comparison of the sections shows that in d 1, closer to the leaf surface, the invagination 1 is longer than that in d 2 (deeper section) unlike the invagination 2 (B3–5, c. 3.5 mm). e An epidermis fragment showing a defected cytokinesis (asterisk labels the anticlinal wall fragment not attached to anticlinal wall of the mother cell) and an invagination 1 in the vicinity of a putative gap (B3–4, c. 2 mm). f Young epidermis fragment showing a cell (C1) with a branched invagination, a putative cell loss site (x), and a gap (G) (B3–5, c. 2 mm). g Epidermis fragment with well-developed wall waviness showing a cell (C1) with two wavy ingrowth-like invaginations that are not in the vicinity of a gap or cell loss site, and a fragment of a neighboring cell (C2) with a single wavy invagination (B3–5, c. 2.5 mm). h Fragment of wild-type epidermis with dividing cells and wall waviness not yet developed (c. 2 mm). i Wild-type epidermis with well-developed wall waviness and active meristemoids (c. 3.5 mm). Scale bars 20 μm (a, h, i) and 10 μm (b–g)
In sequential replicas, the cell loss sites appear as cavities (asterisks in Fig. 2a; a cavity between cells 1, 2, and 14 in Fig. 2b, or 3–5 and 13 in Fig. 2d, all at t = 0 h). Moreover, during the replica taking, additional cells are lost (marked by x in Fig. 2b–e). Such cell loss very rarely accompanies replica taking from Col-0 leaves (replicas in Fig. 2h, i are second in the sequence). Replicas also show closure of cell loss sites due to expansion of surrounding cells (expanding cell 1 in Fig. 2b; 2 in Fig. 2c; or 2, 3, and 6 in Fig. 2e) or underlying mesophyll (M1 and M2 in Fig. 2d). The gaps, however, were not observed in replicas of the mutant epidermis. It may be because gaps become apparent when there is no turgor in cells as in the cleared material, while replicas represent turgid cell shapes.
Fig. 2.
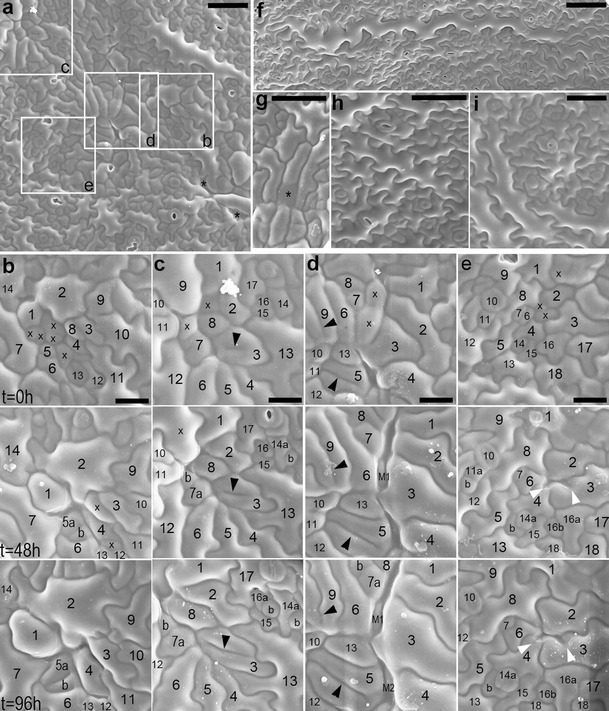
a–i SEM micrographs showing abaxial epidermis replicas of proximal portions of B3–5 (a–g) and Col-0 (h, i) leaves. a Epidermis region (the first replica of the sequence) from which fragments outlined by rectangles are shown in b–e (leaf lamina c. 3 mm long). Asterisks mark sites of cell loss. b–e Fragments of epidermis region shown in a. Each column is a sequence of consecutive replicas; time at which they were taken is given on left of each row. In each column, the cells have the same numbers; a cell progeny is labeled with letters; cells are marked with x if they are lost in the next replica. b Five cells are lost after the first replica taking; cell 1 grows so that this space becomes closed. c Cell 3 has an interior fragment of anticlinal wall (arrowhead) not attached to its outer anticlinal walls. Some cells are lost, and their sites are filled by surrounding cells. d Cells 5 and 9 have long ingrowth-like invaginations (arrowheads). In the elongated gaps formed due to cell loss, underlying mesophyll cells (M1 and M2) are appearing. e Anticlinal walls of cells 3 and 4 form invagination (arrowheads) after a neighboring cell loss. A space between cells 2–4 and 6 is closing. f A giant cell surrounded by over 30 much smaller neighbors (leaf c. 6 mm). This cell is more or less parallel to leaf margin, located c. 400 μm from the margin. g Fragment of replica, the first in the sequence, showing a cell (asterisk) which outer periclinal wall has probably collapsed (leaf c. 2.5 mm long). h The second replica taken from wild-type epidermis (leaf c. 3 mm). i The second replica taken from wild-type epidermis of the leaf older than that in h (leaf c. 5 mm). Scale bars 50 μm (a, g–i), 20 μm (b–e), and 100 μm (f)
What happens with epidermal cells that are “lost” from the leaf surface is intriguing. Since epoxy resin casts obtained with the replica method represent detailed geometry of a tissue in its fresh and turgid state (Williams and Green 1988), the fact that the site of cell loss is a cavity visible in a replica implies that the outer periclinal wall of such a cell is either collapsed (bends into the cell lumen) or missing. Indeed, in some replicas, such sites look as if the walls were collapsed (labeled by asterisk in Fig. 2g), probably because protoplasts of the cells die. However, collapse of the outer periclinal wall is unexpected since this wall is thicker and stiffer than the inner periclinal wall (see Fig. 26 in Zhao and Sack 1999; Fig. 6 in Donnelly et al. 1999; reviewed in Savaldi-Goldstein and Chory 2008), and thus after the cell death, the inner rather than the outer wall would be expected to collapse. Other sites of cell loss look as if the cell, with its periclinal walls, disappeared, like a cavity between cells 3–5 and 13 in Fig. 2d (at t = 0 h). Such scenario is further supported by the appearance of a mesophyll cell in this cell loss site in the third replica of the sequence. One would expect that both the outer periclinal wall collapse and its loss should be disabled by a cuticle layer, which in wild type forms a continuous film covering the epidermis surface that is not discretized to individual cells. Although in A. thaliana leaves, it is very thin (Franke et al. 2005); the cuticle has special mechanical properties, and in other species, it has been shown to stiffen epidermal cell outer walls (reviewed in Domínguez et al. 2011). Therefore, we postulate that there are cuticle defects in the mutant. Such defects could possibly also explain death of some protoplasts.
Both the formation of gaps and cell loss may be a manifestation of defects in adhesion of anticlinal walls of adjacent cells, as it is postulated for the distorted group of mutants (Szymanski 2005). In plants, unlike in animals, walls of adjacent cells adhere from the moment of their formation during cytokinesis (Knox 1992; Jarvis et al. 2003). If indeed there is a defect in mutant cell adhesion, the observed cell loss during replica taking could in fact be, at least in some cases, a removal of entire cells (protoplast and surrounding walls) with the replica. Noteworthy, also such scenario would mean that the cuticle could not be continuous between cells.
Ingrowth-like invaginations of anticlinal cell walls related or unrelated to the cell loss
Another striking and common feature of epidermal pavement cells in the mutant is the occurrence of ingrowth-like invaginations of anticlinal cell walls. They are present already in young epidermis in which pavement cells are not yet puzzle shaped (Fig. 1c, f) and in the more differentiated cells (Fig. 1a, d, g). The invaginations vary in length (compare invaginations in Fig. 1c, d, g), number per cell (Fig. 1a, d), and shape (simple in Fig. 1d and branched in Fig. 1f; with uniform or not uniform length, invaginations 2 and 1 in Fig. 1d, respectively; straight or wavy, in Fig. 1d, g, respectively). More invaginations, especially the short ones, are apparent in cleared material than in replicas. However, in replicas, anticlinal walls can be recognized only as groves on the examined surface, which may be not deep enough in the case of short invaginations.
There are at least two modes of the invagination formation. Some invaginations are evidently related to the loss of a neighboring cell (around x in Fig. 1a; arrowheads in Fig. 2e). They are formed when a cell expands, filling a space appearing after a neighboring cell loss; if the anticlinal wall at the contact between the remaining and lost cells is wavy, it folds during the space closure. Invaginations related to cell loss often have a drop-like outline of inner margin (Fig. 1a, b). If in the course of the cell loss its outer periclinal wall collapses, as suggested above, its anticlinal walls remain, and invaginations are folds of double anticlinal walls.
Invaginations can be formed also due to defects in cytokinesis that lead to formation of cell wall stubs (Cutler and Ehrhardt 2002; Söllner et al. 2002). It is likely the case in the mutant, e.g., invaginations in C1 and C2 in Fig. 1g or in cells 5 and 9 in Fig. 2d (arrowheads). In order to investigate whether defected cytokinesis takes place in the mutant, we checked the number of nuclei in cells with invaginations. This examination did not reveal any cells with more than one nucleus. There are reports, however, showing that after defected cytokinesis the two sister nuclei may fuse (Weingartner et al. 2004). Therefore, not encountering double-nuclei cells is not an argument against defected cytokinesis. The occurrence of cytokinesis defects in the mutant is nevertheless confirmed by rarely occurring anticlinal wall fragments not contacting anticlinal walls of the mother cell (asterisk in Fig. 1e, arrowhead in Fig. 2c). It seems that invaginations may also be formed as ingrowths of cell walls, similar to that of mesophyll cells of Pinus sylvestris L. needle (Hoss and Wernicke 1995; Hejnowicz 2011). It may be true for cells of young leaf epidermis, with not yet wavy anticlinal walls, which have several short invaginations that do not contact any gap or cell loss site (C1 in Fig. 1c) or that have branched invaginations (C1 in Fig. 1f). Noteworthy, both invaginations formed during defected cytokinesis, and wall ingrowths are folds of anticlinal wall of a single cell (Hoss and Wernicke 1995; Yang et al. 1999; Söllner et al. 2002).
Since some invaginations presumably unrelated to cell loss are wavy (Fig. 1g), the mechanism leading to formation of waviness of double anticlinal walls that are walls at contact of two cells (Panteris and Galatis 2005; Kotzer and Wasteneys 2006; Hejnowicz 2011) operates also in case of such ingrowth-like invaginations formed by an individual cell wall.
Occurrence of giant pavement cells and reduced wall waviness
The leaf epidermis of P STM :CDKA;1.N146 lines exhibits higher local variation in size of pavement cells than Col-0 epidermis (compare Fig. 2a with h, i). Often, especially in older leaves, there are very large pavement cells surrounded by numerous much smaller ones. In extreme cases, which are, however, infrequent, giant cells (up to 1 mm long) occur (Fig. 2f), one to four per adaxial or abaxial leaf side. These cells have single nuclei. Judging from their frequency, size, and nucleus number, we regard the giant cells as those that had started differentiation and ceased mitotic divisions when still within the SAM, i.e., in the transgene expression domain. Their presence would thus be a direct effect of the transgene action.
Local variation of pavement cell shape is also increased in the mutant. Waviness of anticlinal walls of pavement cells is reduced, but only in some places (compare frames d, e in Fig. 2a with h, i). The sites where the waviness reduction occurs are not related to leaf venation but appear as patches surrounded by anticlinal cell walls with typically developed waviness. Like in the case of gaps, such patchy phenotype was described also for distorted group of mutants, and similar to gaps was explained by presumable defects in vesicle trafficking involved in cell wall formation (Szymanski 2005).
Conclusions
Most of the epidermal traits of the P STM :CDKA;1.N146 leaves are related to cytoskeleton function and vesicular trafficking. Postulated cuticle defects and defects in anticlinal wall adhesion may result from problems with delivery of cell wall components during or after cytokinesis. Also, formation of wavy anticlinal walls in pavement cells and ingrowth-like cell wall invaginations are processes in which cytoskeleton plays an important role. The described phenotype may thus result from some effects that the modified CDKA;1 activity has on its interactions with cytoskeleton via cytoskeleton-associated proteins. It has been postulated that during cytokinesis, CDKAs interfere with microtubule-associated proteins. The present results suggest that there may also be an interaction between CDKAs and cytoskeleton during interphase.
It remains unclear in which cases the observed traits are direct effects of transgene function in the SAM, and which result from a cascade of events initiated in the SAM and continued after cells departure from the meristem. Examples of the former case are giant cells and evident cytokinesis defects, which occur in few cells per leaf only. More puzzling are much more common cell loss, gaps between cells, and invaginations in anticlinal walls of pavement cells that most likely appear in a generation of cells which is separated in time from SAM cells by several mitotic cell cycles.
Acknowledgments
We thank Prof. Zygmunt Hejnowicz and Dr. Agata Burian for their discussions during preparation of this manuscript, and Dr. Ewa Teper (Laboratory of Scanning Electron Microscopy, Faculty of Earth Sciences, University of Silesia) for the help in preparation of SEM micrographs. Part of this work was financially supported by the MAESTRO research grant no. 2011/02/A/NZ3/00079 from the National Science Centre, Poland.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Basu D, Le J, El-Essai SE-D, et al. DISTORTED3/SCAR2 is a putative Arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. Plant Cell. 2005;17:502–524. doi: 10.1105/tpc.104.027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB. A SPIKE1 signaling controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. PNAS. 2008;10:4044–4049. doi: 10.1073/pnas.0710294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW. Polarized cytokinesis in vacuolate cells of Arabidopsis. PNAS. 2002;99:2812–2817. doi: 10.1073/pnas.052712299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez E, Cuartero J, Heredia A. An overview on plant cuticle biomechanics. Plant Sci. 2011;181:77–84. doi: 10.1016/j.plantsci.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- Dudits D, Cserháti M, Miskolczi P, Horváth GV. The growing family of plant cyclin-dependent kinases with multiple functions in cellular and developmental regulation. In: Inzé D, editor. Cell cycle control and plant development. Oxford: Blackwell; 2007. pp. 1–30. [Google Scholar]
- Elsner J, Michalski M, Kwiatkowska D. Spatiotemporal variation of leaf epidermal cell growth: a quantitative analysis of Arabidopsis thaliana wild-type and triple cyclinD3 mutant plants. Ann Bot. 2012;109:897–910. doi: 10.1093/aob/mcs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, et al. Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochem. 2005;66:2643–2658. doi: 10.1016/j.phytochem.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Gaamouche T, Manes C-LO, Kwiatkowska D, et al. Cyclin-dependent kinase activity maintains the shoot apical meristem cells in an undifferentiated state. Plant J. 2010;64:26–37. doi: 10.1111/j.1365-313X.2010.04317.x. [DOI] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Multi-scale phenotyping of leaf expansion in response to environmental changes: the whole is more than the sum of parts. Plant Cell Environ. 2009;32:1175–1184. doi: 10.1111/j.1365-3040.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z. Plants as mechano-osmotic transducers. In: Wojtaszek P, editor. Mechanical integration of plant cells and plants. Berlin Heidelberg: Springer; 2011. pp. 241–267. [Google Scholar]
- Hoss S, Wernicke W. Microtubules and the establishment of apparent cell wall invaginations in mesophyll cells of Pinus silvestris L. J Plant Physiol. 1995;147:474–476. doi: 10.1016/S0176-1617(11)82186-3. [DOI] [Google Scholar]
- Jarvis MC, Briggs SPH, Knox JP. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003;26:977–989. doi: 10.1046/j.1365-3040.2003.01034.x. [DOI] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. [Google Scholar]
- Joubès J, De Schutter K, Verkest A, Inzé D, De Veylder L. Conditional, recombinase-mediated expression of genes in plant cell cultures. Plant J. 2004;37:889–896. doi: 10.1111/j.1365-313X.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- Knox JP. Cell adhesion, cell separation and plant morphogenesis. Plant J. 1992;2:137–141. doi: 10.1111/j.1365-313X.1992.00137.x. [DOI] [Google Scholar]
- Kotzer AM, Wasteneys GO. Mechanisms behind the puzzle: microtubule-microfilament cross-talk in pavement cell formation. Can J Bot. 2006;84:594–603. doi: 10.1139/b06-023. [DOI] [Google Scholar]
- Panteris E, Galatis B. The morphogenesis of lobed plant cells in the mesophyll and epidermis: organization and distinct roles of cortical microtubules and actin filaments. New Phytol. 2005;167:721–732. doi: 10.1111/j.1469-8137.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- Qian P, Hou S, Guo G. Molecular mechanisms controlling pavement cell shape in Arabidopsis leaves. Plant Cell Rep. 2009;28:1147–1157. doi: 10.1007/s00299-009-0729-8. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Boudolf V, De Veylder L, Inzé D, Genschil P, Machida Y. Phosphorylation of a mitotic kinesin-like protein and a MAPKKK by cyclin-dependent kinases (CDKs) is involved in the transition to cytokinesis in plants. PNAS. 2011;108:17844–17849. doi: 10.1073/pnas.1110174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Chory J. Growth coordination and the shoot epidermis. Curr Opin Plant Biol. 2008;11:42–48. doi: 10.1016/j.pbi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner R, Glässer, Wanner G, Somerville CR, Jürgens G, Assaad FF. Cytokinesis-defective mutants of Arabidopsis. Plant Physiol. 2002;129:678–690. doi: 10.1104/pp.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB. Breaking the WAVE complex: the point of Arabidopsis trichomes. Curr Opin Plant Biol. 2005;8:103–112. doi: 10.1016/j.pbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Inzé D, Geelen D. Mitosis-specific kinesins in Arabidopsis. Trends Plant Sci. 2006;11:167–175. doi: 10.1016/j.tplants.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Weingartner M, Criqui M-C, Mészáros T, et al. Expression of a nondegradable cyclin B1 affects plant development and leads to endomitosis by inhibiting the formation of a phragmoplast. Plant Cell. 2004;16:643–657. doi: 10.1105/tpc.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MH, Green PB. Sequential scanning electron microscopy of a growing plant meristem. Protoplasma. 1988;147:77–79. doi: 10.1007/BF01403879. [DOI] [Google Scholar]
- Yang M, Nadeau JA, Zhao L, Sack FD. Characterization of a cytokinesis defective (cyd1) mutant of Arabidopsis. J Exp Bot. 1999;50:1437–1446. doi: 10.1093/jxb/50.338.1437. [DOI] [PubMed] [Google Scholar]
- Zhao L, Sack FD. Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Amer J Bot. 1999;86:929–939. doi: 10.2307/2656609. [DOI] [PubMed] [Google Scholar]


