Highlights
-
•
Emmprin regulates pathogenic elements relevant to virus-associated cancer, including drug resistance and cell migration.
-
•
Kaposi's sarcoma-associated herpesvirus (KSHV) regulates emmprin expression and downstream function.
-
•
Targeting emmprin or its interacting proteins at the cell surface suppresses KSHV-induced pathogenesis in vitro.
Keywords: KSHV, Kaposi sarcoma, Primary effusion lymphoma, Emmprin, CD147
Abstract
Emmprin (CD147; basigin) is a multifunctional glycoprotein expressed at higher levels by cancer cells and stromal cells in the tumor microenvironment. Through direct effects within tumor cells and promotion of tumor–stroma interactions, emmprin participates in induction of tumor cell invasiveness, angiogenesis, metastasis and chemoresistance. Although its contribution to cancer progression has been widely studied, the role of emmprin in viral oncogenesis still remains largely unclear, and only a small body of available literature implicates emmprin-associated mechanisms in viral pathogenesis and tumorigenesis. We summarize these data in this review, focusing on the role of emmprin in pathogenesis associated with the Kaposi sarcoma-associated herpesvirus (KSHV), a common etiology for cancers arising in the setting of immune suppression. We also discuss future directions for mechanistic studies exploring roles for emmprin in viral cancer pathogenesis.
1. Introduction
Emmprin (CD147; basigin), a heavily glycosylated transmembrane protein, is a member of the immunoglobulin superfamily [1] and was initially identified on the surface of human cancer cells where it stimulates production of matrix metalloproteinases (MMPs) by adjacent stromal cells in the tumor microenvironment [2]. Emmprin is expressed within a variety of normal tissues, and existing data indicate its putative roles in a number of physiologic processes, including blood–brain barrier function, T cell activation, menstruation, and tissue repair [3], [4]. Emmprin interacts with a number of binding partners on the cell surface, including the hyaluronan receptor CD44, cyclophilin A, monocarboxylate transporters (MCTs), and ATP-binding cassette (ABC) transporters [4], [5], [6]. Through these interactions, emmprin regulates a variety of pathogenic events associated with cancer, including signal transduction, efflux of chemotherapeutic agents, tumor cell invasiveness and metastasis. Underscoring its association with cancer, emmprin expression is increased within many tumors relative to normal tissue, and its overexpression correlates with malignant progression [3], [4]. A number of studies have indicated that targeting emmprin or its interactions with other cellular proteins reduces tumor progression in vivo [7], [8], [9]. One emerging strategy involves the use of small hyaluronan oligosaccharides (oHA) which interfere with interactions between CD44 and the pericellular polysaccharide hyaluronan (HA), thereby inhibiting emmprin/CD44-associated tumor progression and metastases [10]. Roles for emmprin and its binding partners have not been clearly defined for cancers of viral etiology, and understanding whether oncogenic viruses themselves regulate emmprin expression and function may yield new mechanistic insights for transcriptional activation of emmprin expression and downstream pathogenesis relevant to all cancers expressing this protein. In this review, we will discuss the small body of published literature indicating a role for emmprin in viral pathogenesis, focusing on published data implicating emmprin as a key regulator of cell migration and chemoresistance for cells infected by a human oncogenic virus, the Kaposi sarcoma-associated herpesvirus (KSHV). We will also discuss putative mechanisms for KSHV regulation of emmprin expression, and in the context of other published data, offer logical future directions for exploring mechanisms associated with emmprin and viral cancer pathogenesis.
2. Emmprin and viral pathogenesis
A limited number of published studies indicate that emmprin may serve as a cell surface receptor or as a co-factor facilitating virus entry. Pushkarsky et al. found that emmprin increases human immunodeficiency virus-1 (HIV-1) infection by interacting with virus-associated cyclophilin A [11]. Emmprin may also play a role in cell entry for measles virus and severe acute respiratory syndrome coronavirus (SARS-CoV) [12], [13].
Emmprin has been implicated in pathogenesis for two viruses associated with hepatocellular carcinoma (HCC): hepatitis B virus (HBV) and hepatitis C virus (HCV). Emmprin and cyclophilin A interact with the HBV small surface protein, and in a murine model for HBV infection, either cyclosporine (which inhibits the chemotactic effect of cyclophilin A) or a monoclonal antibody targeting emmprin reduce inflammation in the liver and correlative increases in serum alanine aminotransferase and aspartate aminotransferase caused by the virus [14]. Translational studies further reveal higher serum cyclophilin A levels within patients chronically infected with HBV relative to healthy individuals [14]. The HCV core protein promotes migration and invasion for hepatocytes via induction of emmprin expression [15], and emmprin may mediate HCV-associated cirrhosis [16]. A summary of emmprin interactions with human viruses is provided in Table 1 .
Table 1.
Overview of emmprin interactions with human viruses.
| Virus | Emmprin function | References |
|---|---|---|
| Human immunodeficiency virus-1 (HIV-1) | Receptor for virus entry (interaction with cyclophilin A) | [11] |
| Measles virus | Receptor for virus entry | [12] |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Co-factor for virus entry | [13] |
| Hepatitis B virus (HBV) | Promotes hepatic inflammation (interaction with cyclophilin A) | [14] |
| Hepatitis C virus (HCV) | Promotes migration and invasion induced by HCV core protein | [15], [16] |
| Kaposi sarcoma-associated herpesvirus (KSHV) | Promotes migration and invasiveness for KSHV-infected cells; promotes chemoresistance for KSHV-infected tumor cells | [31], [32], [33], [65] |
KSHV is the etiologic agent for Kaposi’s sarcoma (KS) and primary effusion lymphoma (PEL), malignancies arising primarily in patients infected with the human immunodeficiency virus (HIV) or in those receiving organ transplants [17], [18]. The KSHV genome contains ∼87 open reading frames (ORFs), and KSHV-encoded proteins regulate a variety of pathogenic events associated with cancer progression, including cell migration and angiogenesis [19]. Thus far, this knowledge has not led to the development of targeted therapies for KSHV-associated malignancies, and the standard of care remains use of non-targeted cytotoxic agents that incur significant morbidity and only limited clinical benefit [20], [21], [22]. Data now reveal that emmprin mediates pathogenesis associated with KSHV infection, including increased invasiveness and chemotherapeutic resistance for infected cells. A schematic summary of known and putative functions for emmprin within KSHV-infected cells is shown in Fig. 1 , and these concepts are outlined in more detail below.
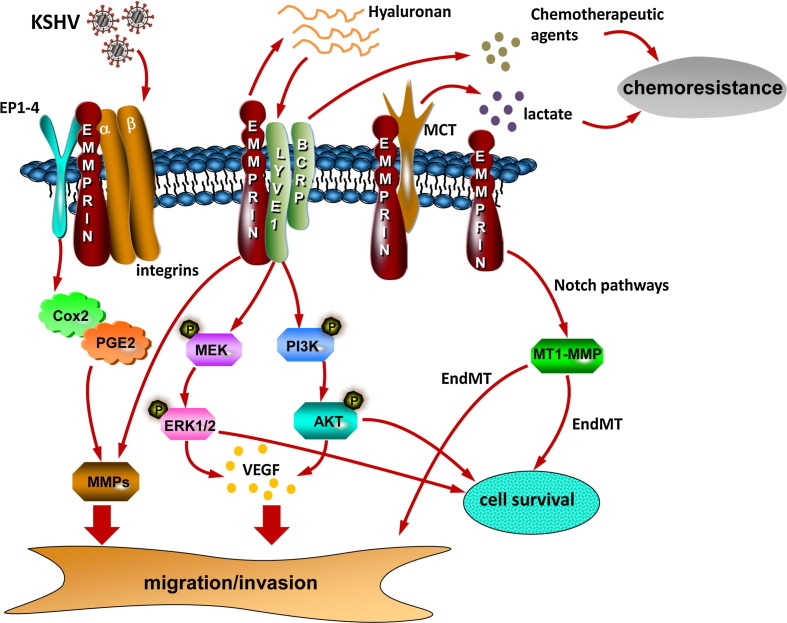
Fig. 1.
Schematic of known and putative contributions of emmprin to KSHV-associated cellular pathogenesis. Published data indicate that emmprin mediates critical pathogenic determinants for KSHV-infected cells, including cell migration/invasion, survival, and chemoresistance. Cell invasiveness is mediated through emmprin induction of MMPs and VEGF secretion which is itself dependent upon emmprin stimulation of ERK and Akt, possibly through emmprin-mediated secretion of hyaluronan and hyaluronan interactions with LYVE-1. Emmprin contributes to survival and chemoresistance for KSHV-infected tumor cells through its interactions with LYVE-1 and BCRP and efflux of chemotherapeutic agents. Emmprin stimulation of ERK and Akt may also maintain anti-apoptotic signaling. Whether interactions between emmprin and other known binding partners on the cell surface, including integrins, EP1-4, and MCTs mediate KSHV pathogenesis remains to be determined. Also, it remains unclear whether emmprin is involved in KSHV-induced EndMT through Notch pathways and MT1-MMP. KSHV = Kaposi sarcoma-associated herpesvirus; PGE2 = prostaglandin E2; EP1-4 = prostaglandin E2 receptors; LYVE-1 = lymphatic vessel endothelial hyaluronan receptor-1; BCRP = breast cancer resistance protein; MCT = monocarboxylate transporter; MMP = matrix metalloproteinase; VEGF = vascular endothelial growth factor; MEK = mitogen-activated protein kinase; ERK = extracellular signal-regulated kinase; Endothelial-to-mesenchymal transformation (EndMT); membrane-type-1 matrix metalloproteinase (MT1-MMP).
3. Emmprin and KSHV-associated cell migration/invasion
Acquisition of a migratory or invasive phenotype represents one hallmark of KSHV-infected endothelial cells, with implications for both viral dissemination and angiogenesis within KS lesions. KSHV-infected, skin-derived fibroblasts also promote endothelial cell migration through paracrine mechanisms [23]. Published data implicate involvement of a number of factors in KSHV-induced migration/invasion, including MMPs, interleukin-8 (IL-8), IL-6, vascular endothelial growth factor (VEGF), cyclooxygenase-2 (Cox-2), integrin β3, Angiopoietin-like 4, and Angiogenin [24], [25], [26], [27], [28], [29], [30]. We initially reported that KSHV-induced migration/invasion for human umbilical vein endothelial cells (HUVEC) and KSHV-infected PEL cells are dependent upon KSHV induction of emmprin expression [31]. We have also observed a similar pattern for KSHV-infected fibroblasts, including human foreskin-derived fibroblasts (HFF), human gingival fibroblasts (HGF), and periodontal ligament fibroblasts (PDLF) [31], [32], [33]. Emmprin-focused studies utilizing fibroblasts are relevant given that tumor-associated fibroblasts enhance tumor progression through their secretion of pro-migratory or pro-angiogenic factors [34].
To characterize specific migration-associated factors secreted into the extracellular space following KSHV induction of emmprin expression, we have used RNA interference and adenoviral transduction to manipulate emmprin expression [31], [32], [33]. We found that KSHV-induced expression or secretion of MMP-1, MMP-2, MMP-7, MMP-9, VEGF-A, and IL-6 by HUVEC and HFF is emmprin-dependent [31], [32]. Moreover, exogenous VEGF-A, but not IL-6, restores invasiveness for KSHV-infected HUVEC in which emmprin expression has been suppressed. In agreement with other published data [25], [26], we also found that KSHV induces IL-8 secretion by HUVEC, HGF and PDLF [32], [33]. However, neither suppression of emmprin expression during KSHV infection, nor ectopic emmprin expression, affects IL-8 secretion by these cells [32], [33]. These data for IL-8 reflect the complexity and redundancy of KSHV pathogenesis, as the virus initiates multiple mechanisms (emmprin-dependent and -independent) to accomplish the same pathogenic endpoint.
Although highly active antiretroviral therapy (HAART) for the treatment of HIV infection results in reduction of KSHV viremia for a subset of patients [35] (presumably through improved immune recognition of KSHV-infected cells), HAART does not appear to significantly reduce KSHV replication within the oropharynx [36], [37]. Moreover, oral KS lesions contain higher KSHV viral loads relative to skin KS lesions [38] and may portend higher mortality for HIV-infected patients [39], [40]. Therefore, comparison of primary cells from the oral cavity with those from other sources may prove useful for unique pathways related to oral KSHV persistence. In contrast to our findings for endothelial cells, we found that infection of HGF and PDLF induces emmprin-dependent expression of MMP-1 and MMP-9, but not MMP-2 and MMP-7 [33]. These results suggest that KSHV-induced, emmprin-dependent secretion of pro-migratory factors may be cell-type specific, and perhaps unique for cells from the oral cavity.
Other published data implicate emmprin binding partners in KSHV-induced migration/invasion, including Prostaglandin E2 (PGE2) receptors [43] and integrins [49], [50], [51], [52]. KSHV infection induces Cox-2 expression and PGE2 production within human endothelial cells and fibroblasts, and these factors contribute to angiogenesis and invasion by KSHV-infected cells [28], [41]. Cox-2, PGE2, and the PGE2 receptors (EP1-4) are all expressed in KS tumors [28], [42]. Inhibition of Cox-2, PGE2, or PGE2 receptors suppresses expression and/or activity of MMP1, MMP2, MMP3, MMP7 and MMP9 [43]. Integrins serve as cell surface receptors for KSHV entry, and integrin-mediated signal transduction is activated during de novo KSHV infection [29], [44], [45], [46], [47], [48]. Furthermore, emmprin interacts with a variety of integrins to facilitate signal transduction and promotion of cell mobility and invasion for a variety of cancer cells [49], [50], [51], [52]. These data indicate that a more comprehensive understanding of emmprin interactions with putative binding partners on the surface of virus-infected cells may uncover mechanisms for emmprin-associated migration of tumor cells and virus-infected stromal cells in the tumor microenvironment.
Endothelial-to-mesenchymal transition (EndMT) of cells in the tumor microenvironment is an important pathophysiologic correlate for cancer progression [53], [54]. Two recent studies demonstrate that EndMT is induced by KSHV infection and contributes to viral tumorigenesis [54], [55]. Cheng et al. report that KSHV-induced EndMT can be initiated by the viral FLICE inhibitory protein (vFLIP) or viral G-protein coupled receptor (vGPCR) through Notch pathway activation, leading to invasiveness for cells which is dependent upon membrane-type-1 matrix metalloproteinase (MT1-MMP) [55]. These authors also report that mesenchymal markers and MT1-MMP are co-distributed with LANA in the same cells within primary KS biopsies. Gasperini et al. reported that canonical Notch signaling, including Slug and ZEB1, is required for KSHV-induced EndMT but not through the TGF-β signaling pathway. Their results further indicate that KSHV-induced EndMT increases invasiveness and survival for infected endothelial cells, an effect blocked using Notch-specific inhibitors [54]. Several published studies have reported that emmprin is involved in EndMT [56], [57]. Additionally, the expression of functional emmprin and MMP-2 is significantly decreased in Notch1-deficient breast cancer cells which display impaired migration and invasion [58]. Another study has also demonstrated that upregulation of emmprin is sufficient to induce MT1-MMP expression, invasiveness and formation of invadopodia-like structures in breast epithelial cells [59]. Whether emmprin plays a key role in KSHV-induced EndMT, is a subject of ongoing investigation in our laboratory.
4. Emmprin and chemoresistance for KSHV-infected tumors
PEL is a rapidly progressive form of lymphoma arising primarily in patients infected with HIV, although cases have also been documented in other immune compromised hosts [60]. Standard therapeutic approaches for PEL are ineffective, and the prognosis for this disease remains poor with a median survival of approximately 6 months [60], [61], [62]. Many PEL tumors demonstrate resistance to chemotherapy, and putative mechanisms involve p53 mutagenesis and the KSHV-encoded latency-associated nuclear antigen-2 (LANA2) [63], [64]. We have found that PEL resistance to paclitaxel and doxorubicin is emmprin-dependent, and this effect involves emmprin interactions with at least two cell surface proteins: a receptor for hyaluronan known as the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and the drug transporter ABCG2/BCRP [65]. We have demonstrated expression of LYVE-1 and BCRP on the PEL cell surface using complementary approaches, and their expression is directly proportional to intrinsic PEL chemoresistance characterized previously [63], [65]. Inhibiting expression of either LYVE-1 or BCRP restores PEL chemosensitivity, while overexpression of emmprin renders chemosensitive cells chemoresistant. Emmprin co-localizes with another hyaluronan receptor, CD44, on the surface of a variety of cancer cells [6], and CD44 is closely associated with metastasis, chemoresistance, and cancer progression [66], [67]. In contrast to our findings for LYVE-1, we found that only a small subpopulation of PEL cells in culture express CD44 (unpublished data), in general agreement with other work indicating a lack of significant CD44 expression on the PEL cell surface [68]. It is of interest to determine whether CD44-expressing cells within these PEL cultures represent cancer stem-like cells which exhibit increased expression of emmprin and chemoresistance relative to CD44-negative cells [66], [69], [70]. Emmprin also interacts with several monocarboxylate transporters (MCTs) to facilitate lactate transport across the plasma membrane [71], [72], [73], [74], and MCT activity facilitates both the “Warburg effect” (the use of glycolysis as an energy source despite availability of oxygen) and chemoresistance [75], [76], [77], [78]. More recent data have revealed preferential utilization of glycolysis within KSHV-infected PEL tumors, as well as induction of the Warburg Effect following de novo KSHV infection of endothelial cells [79], [80]. Therefore, it will be of interest to determine whether emmprin regulation of MCT function and lactate efflux impacts PEL chemosensitivity. In addition, LYVE-1 is expressed within KS lesions [81], [82], [83], and a substantial proportion of KS tumors exhibit minimal or no response to chemotherapy [20], [21], [22]. Consequently, LYVE-1 may represent an attractive therapeutic target for either KS or PEL. Characterization of expression of emmprin, hyaluronan receptors, MCTs, and drug efflux pumps within KS tumors, and in the context of de novo KSHV infection of human primary cells relevant to KS, should provide additional clues to the role of these pathways in KS resistance to chemotherapy.
5. Induction of emmprin expression by KSHV
We have found that the KSHV-encoded latency-associated nuclear antigen (LANA) induces emmprin expression in primary cells [31]. In its capacity as a transcription factor, LANA regulates expression of several cellular and viral genes through direct interaction with gene promoters [84], [85], or through interactions with other transcription factors, including the zinc finger transcription factor Sp1 [86], [87]. The emmprin promoter region contains several binding sites for Sp1 and the transcription factor known as the early growth response gene 2 (Egr-2) [88], and we have found that direct targeting of either Sp1 or Egr-2 reduces KSHV- or LANA-mediated activation of emmprin expression in human primary cells from the oral cavity [33]. Therefore, it is possible that LANA either binds directly to the emmprin promoter, or transactivates emmprin indirectly through interactions with Sp1 or Egr-2. LANA or other KSHV genes may also induce emmprin expression through activation of intracellular signal transduction. NF-κB and JNK activation increases emmprin expression and function in tumor-associated macrophages [89], and NF-κB and JNK signaling are induced by vFLIP and vGPCR [90], [91], [92], [93]. Positive regulators of emmprin expression include amphiregulin and EGF-EGFR interactions [94], and negative regulators include Pinin, a nuclear and cell adhesion-related protein [95], and Caveolin-1 which reduces emmprin glycosylation and associated MMP activity [96]. Whether these factors play a role in KSHV induction of emmprin expression remains to be determined. KSHV (intact virus) or KSHV gene transfer may prove useful for identifying mechanisms for emmprin overexpression by cancer cells and intracellular pathways responsible for specific functional outcomes of emmprin overexpression.
6. Emmprin and KSHV-induced signal transduction
Upregulation of emmprin induces signal transduction associated with EGFR, ErbB2, FAK, Akt, and MAPK (ERK and p38) [94], [97], [98], [99], [100]. We have found that ectopic overexpression of emmprin in endothelial cells results in activation of PI3K/Akt and ERK phosphorylation [33], and that pharmacologic inhibitors of these pathways block MMP production and invasiveness for both emmprin-overexpressing and KSHV-infected cells [32]. The mechanisms by which emmprin activates these signaling pathways in KSHV-infected cells is unknown. One possibility involves the stimulation of secretion of hyaluronan which triggers downstream signal transduction through binding to its cognate receptors (LYVE-1 or CD44) [98], [99], [100]. Consistent with this, chemoresistant PEL cells exhibit greater hyaluronan secretion and emmprin expression relative to chemosensitive PEL cells [65]. Moreover, targeting emmprin reduces hyaluronan secretion by PEL cells [65]. Another possibility is that emmprin interacts with and stabilizes binding partners on the cell surface which themselves induce signal transduction. Additional studies are clearly needed to define specific roles for signaling intermediates, hyaluronan, and other mechanisms for emmprin-induced signaling by KSHV-infected cells.
7. Concluding remarks
Emmprin drives distinct but interrelated pathologic processes associated with tumor formation. Although its contribution to cancer progression has been widely studied, the role of emmprin in viral oncogenesis requires further characterization. Nevertheless, published data now indicate a comprehensive role for emmprin in KSHV-associated cellular pathogenesis, as well as pathogenesis for hepatitis viruses. Future work highlighting the impact of targeting emmprin and related molecules may uncover innovative therapeutic and preventive strategies for KSHV- and other virus-associated malignancies.
Conflict of Interest
All the authors declare no conflicts of interest.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01-CA142362 to CP and CA082867 to BPT), a Center for Biomedical Research Excellence Award (P20-RR021970 to CP and ZQ), and the National Natural Science Foundation (81272191 to ZQ) and NNSF for Young Scientists of China (81101791 to ZQ).
Contributor Information
Chris Parsons, Email: cpars1@lsuhsc.edu.
Zhiqiang Qin, Email: zqin@lsuhsc.edu.
References
- 1.Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 2.Ellis S.M., Nabeshima K., Biswas C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989;49:3385–3391. [PubMed] [Google Scholar]
- 3.Yan L., Zucker S., Toole B.P. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb. Haemost. 2005;93:199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 4.Nabeshima K., Iwasaki H., Koga K., Hojo H., Suzumiya J., Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 5.Iacono K.T., Brown A.L., Greene M.I., Saouaf S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toole B.P., Slomiany M.G. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist. Updat. 2008;11:110–121. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weidle U.H., Scheuer W., Eggle D., Klostermann S., Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–169. [PubMed] [Google Scholar]
- 8.Hao J.L., Cozzi P.J., Khatri A., Power C.A., Li Y. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer. Curr. Cancer Drug Targets. 2010;10:287–306. doi: 10.2174/156800910791190193. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy K.M., Dewhirst M.W. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–148. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toole B.P., Slomiany M.G. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushkarsky T., Zybarth G., Dubrovsky L., Yurchenko V., Tang H., Guo H., Toole B., Sherry B., Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. USA. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe A., Yoneda M., Ikeda F., Terao-Muto Y., Sato H., Kai C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 2010;84:4183–4193. doi: 10.1128/JVI.02168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y., Shaw P.X., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian X., Zhao C., Zhu H., She W., Zhang J., Liu J., Li L., Zheng S., Wen Y.M., Xie Y. Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection. J. Virol. 2010;84:3373–3381. doi: 10.1128/JVI.02555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X., Xiu B., Xu L., Yang X., He J., Leong D., He F., Zhang H. Hepatitis C virus core protein promotes the migration and invasion of hepatocyte via activating transcription of extracellular matrix metalloproteinase inducer. Virus Res. 2011;158:146–153. doi: 10.1016/j.virusres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Shackel N.A., McGuinness P.H., Abbott C.A., Gorrell M.D., McCaughan G.W. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am. J. Pathol. 2002;160:641–654. doi: 10.1016/S0002-9440(10)64884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y., Cesarman E., Pessin M.S., Lee F., Culpepper J., Knowles D.M., Moore P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 18.Cesarman E., Chang Y., Moore P.S., Said J.W., Knowles D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 19.Mesri E.A., Cesarman E., Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat. Rev. Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanni T., Sprinz E., Machado M.W., Santana Rde C., Fonseca B.A., Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat. Rev. 2006;32:445–455. doi: 10.1016/j.ctrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Von Roenn J.H. Clinical presentations and standard therapy of AIDS-associated Kaposi’s sarcoma. Hematol. Oncol. Clin. North Am. 2003;17:747–762. doi: 10.1016/s0889-8588(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen H.Q., Magaret A.S., Kitahata M.M., Van Rompaey S.E., Wald A., Casper C. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. AIDS. 2008;22:937–945. doi: 10.1097/QAD.0b013e3282ff6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Z., Dai L., Toole B., Robertson E., Parsons C. Regulation of Nm23-H1 and cell invasiveness by Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2011;85:3596–3606. doi: 10.1128/JVI.01596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian L.W., Xie J., Ye F., Gao S.J. Kaposi’s sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 2007;81:7001–7010. doi: 10.1128/JVI.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q., Matta H., Lu G., Chaudhary P.M. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-kappaB activation. Oncogene. 2006;25:2717–2726. doi: 10.1038/sj.onc.1209298. [DOI] [PubMed] [Google Scholar]
- 26.Montaner S., Sodhi A., Servitja J.M., Ramsdell A.K., Barac A., Sawai E.T., Gutkind J.S. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–2911. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- 27.Pati S., Cavrois M., Guo H.G., Foulke J.S., Jr., Kim J., Feldman R.A., Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J. Virol. 2001;75:8660–8673. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma-Walia N., Paul A.G., Bottero V., Sadagopan S., Veettil M.V., Kerur N., Chandran B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiMaio T.A., Gutierrez K.D., Lagunoff M. Latent KSHV infection of endothelial cells induces integrin beta3 to activate angiogenic phenotypes. PLoS Pathog. 2011;7:e1002424. doi: 10.1371/journal.ppat.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadagopan S., Sharma-Walia N., Veettil M.V., Bottero V., Levine R., Vart R.J., Chandran B. Kaposi’s sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis. J. Virol. 2009;83:3342–3364. doi: 10.1128/JVI.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Z., Dai L., Slomiany M.G., Toole B.P., Parsons C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010;70:3884–3889. doi: 10.1158/0008-5472.CAN-09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai L., Bratoeva M., Toole B.P., Qin Z., Parsons C. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int. J. Cancer. 2012;131:834–843. doi: 10.1002/ijc.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai L., Qin Z., Defee M., Toole B.P., Kirkwood K.L., Parsons C. Kaposi sarcoma-associated herpesvirus (KSHV) induces a functional tumor-associated phenotype for oral fibroblasts. Cancer Lett. 2012;318:214–220. doi: 10.1016/j.canlet.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford Y., Kasman I., Yu L., Zhong C., Wu X., Modrusan Z., Kaminker J., Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Gill J., Bourboulia D., Wilkinson J., Hayes P., Cope A., Marcelin A.G., Calvez V., Gotch F., Boshoff C., Gazzard B. Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma–associated herpesvirus infection in patients with and without Kaposi sarcoma. J. Acquir. Immune Defic. Syndr. 2002;31:384–390. doi: 10.1097/00126334-200212010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Miller C.S., Berger J.R., Mootoor Y., Avdiushko S.A., Zhu H., Kryscio R.J. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J. Clin. Microbiol. 2006;44:2409–2415. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casper C., Redman M., Huang M.L., Pauk J., Lampinen T.M., Hawes S.E., Critchlow C.W., Morrow R.A., Corey L., Kiviat N., Wald A. HIV infection and human herpesvirus-8 oral shedding among men who have sex with men. J. Acquir. Immune Defic. Syndr. 2004;35:233–238. doi: 10.1097/00126334-200403010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Campbell T.B., Borok M., Gwanzura L., MaWhinney S., White I.E., Ndemera B., Gudza I., Fitzpatrick L., Schooley R.T. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi’s sarcoma clinical stage. AIDS. 2000;14:2109–2116. doi: 10.1097/00002030-200009290-00006. [DOI] [PubMed] [Google Scholar]
- 39.Rohrmus B., Thoma-Greber E.M., Bogner J.R., Rocken M. Outlook in oral and cutaneous Kaposi’s sarcoma. Lancet. 2000;356:2160. doi: 10.1016/S0140-6736(00)03503-0. [DOI] [PubMed] [Google Scholar]
- 40.Gorsky M., Epstein J.B. A case series of acquired immunodeficiency syndrome patients with initial neoplastic diagnoses of intraoral Kaposi’s sarcoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000;90:612–617. doi: 10.1067/moe.2000.109518. [DOI] [PubMed] [Google Scholar]
- 41.Sharma-Walia N., Raghu H., Sadagopan S., Sivakumar R., Veettil M.V., Naranatt P.P., Smith M.M., Chandran B. Cyclooxygenase 2 induced by Kaposi’s sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J. Virol. 2006;80:6534–6552. doi: 10.1128/JVI.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George Paul A., Sharma-Walia N., Kerur N., White C., Chandran B. Piracy of prostaglandin E2/EP receptor-mediated signaling by Kaposi’s sarcoma-associated herpes virus (HHV-8) for latency gene expression: strategy of a successful pathogen. Cancer Res. 2010;70:3697–3708. doi: 10.1158/0008-5472.CAN-09-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J., Banu S.K., Subbarao T., Starzinski-Powitz A., Arosh J.A. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol. Cell. Endocrinol. 2011;332:306–313. doi: 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Kerur N., Veettil M.V., Sharma-Walia N., Sadagopan S., Bottero V., Paul A.G., Chandran B. Characterization of entry and infection of monocytic THP-1 cells by Kaposi’s sarcoma associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology. 2010;406:103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veettil M.V., Sadagopan S., Sharma-Walia N., Wang F.Z., Raghu H., Varga L., Chandran B. Kaposi’s sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 2008;82:12126–12144. doi: 10.1128/JVI.01146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garrigues H.J., Rubinchikova Y.E., Dipersio C.M., Rose T.M. Integrin alphaVbeta3 Binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 2008;82:1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma-Walia N., Naranatt P.P., Krishnan H.H., Zeng L., Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akula S.M., Pramod N.P., Wang F.Z., Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 49.Zhao P., Zhang W., Wang S.J., Yu X.L., Tang J., Huang W., Li Y., Cui H.Y., Guo Y.S., Tavernier J., Zhang S.H., Jiang J.L., Chen Z.N. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54:2012–2024. doi: 10.1002/hep.24592. [DOI] [PubMed] [Google Scholar]
- 50.Dai J.Y., Dou K.F., Wang C.H., Zhao P., Lau W.B., Tao L., Wu Y.M., Tang J., Jiang J.L., Chen Z.N. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer. 2009;9:337. doi: 10.1186/1471-2407-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang J., Wu Y.M., Zhao P., Yang X.M., Jiang J.L., Chen Z.N. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell. Mol. Life Sci. 2008;65:2933–2942. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berditchevski F., Chang S., Bodorova J., Hemler M.E. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 1997;272:29174–29180. doi: 10.1074/jbc.272.46.29174. [DOI] [PubMed] [Google Scholar]
- 53.Zeisberg E.M., Tarnavski O., Zeisberg M., Dorfman A.L., McMullen J.R., Gustafsson E., Chandraker A., Yuan X., Pu W.T., Roberts A.B., Neilson E.G., Sayegh M.H., Izumo S., Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 54.Gasperini P., Espigol-Frigole G., McCormick P.J., Salvucci O., Maric D., Uldrick T.S., Polizzotto M.N., Yarchoan R., Tosato G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 2012;72:1157–1169. doi: 10.1158/0008-5472.CAN-11-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng F., Pekkonen P., Laurinavicius S., Sugiyama N., Henderson S., Gunther T., Rantanen V., Kaivanto E., Aavikko M., Sarek G., Hautaniemi S., Biberfeld P., Aaltonen L., Grundhoff A., Boshoff C., Alitalo K., Lehti K., Ojala P.M. KSHV-initiated notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe. 2011;10:577–590. doi: 10.1016/j.chom.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Ullmann U., Gilles C., De Rycke M., Van de Velde H., Sermon K., Liebaers I. GSK-3-specific inhibitor-supplemented hESC medium prevents the epithelial-mesenchymal transition process and the up-regulation of matrix metalloproteinases in hESCs cultured in feeder-free conditions. Mol. Hum. Reprod. 2008;14:169–179. doi: 10.1093/molehr/gan001. [DOI] [PubMed] [Google Scholar]
- 57.Wu J., Ru N.Y., Zhang Y., Li Y., Wei D., Ren Z., Huang X.F., Chen Z.N., Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–4427. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Fu L., Gu F., Ma Y. Notch1 is involved in migration and invasion of human breast cancer cells. Oncol. Rep. 2011;26:1295–1303. doi: 10.3892/or.2011.1399. [DOI] [PubMed] [Google Scholar]
- 59.Grass G.D., Bratoeva M., Toole B.P. Regulation of invadopodia formation and activity by CD147. J. Cell Sci. 2012;125:777–788. doi: 10.1242/jcs.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.B., Rahemtullah A., Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 61.Simonelli C., Spina M., Cinelli R., Talamini R., Tedeschi R., Gloghini A., Vaccher E., Carbone A., Tirelli U. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J. Clin. Oncol. 2003;21:3948–3954. doi: 10.1200/JCO.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Boulanger E., Gerard L., Gabarre J., Molina J.M., Rapp C., Abino J.F., Cadranel J., Chevret S., Oksenhendler E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J. Clin. Oncol. 2005;23:4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 63.Petre C.E., Sin S.H., Dittmer D.P. Functional p53 signaling in Kaposi’s sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 2007;81:1912–1922. doi: 10.1128/JVI.01757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munoz-Fontela C., Marcos-Villar L., Hernandez F., Gallego P., Rodriguez E., Arroyo J., Gao S.J., Avila J., Rivas C. Induction of paclitaxel resistance by the Kaposi’s sarcoma-associated herpesvirus latent protein LANA2. J. Virol. 2008;82:1518–1525. doi: 10.1128/JVI.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin Z., Dai L., Bratoeva M., Slomiany M.G., Toole B.P., Parsons C. Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia. 2011;25:1598–1609. doi: 10.1038/leu.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toole B.P. Hyaluronan-CD44 Interactions in cancer: paradoxes and possibilities. Clin. Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 68.Boshoff C., Gao S.J., Healy L.E., Matthews S., Thomas A.J., Coignet L., Warnke R.A., Strauchen J.A., Matutes E., Kamel O.W., Moore P.S., Weiss R.A., Chang Y. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671–1679. [PubMed] [Google Scholar]
- 69.Slomiany M.G., Dai L., Tolliver L.B., Grass G.D., Zeng Y., Toole B.P. Inhibition of functional hyaluronan-CD44 interactions in CD133-positive primary human ovarian carcinoma cells by small hyaluronan oligosaccharides. Clin. Cancer Res. 2009;15:7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai L., Guinea M.C., Slomiany M.G., Bratoeva M., Grass G.D., Tolliver L.B., Maria B.L., Toole B.P. CD147-dependent heterogeneity in malignant and chemoresistant properties of cancer cells. Am. J. Pathol. 2013;182:577–585. doi: 10.1016/j.ajpath.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirk P., Wilson M.C., Heddle C., Brown M.H., Barclay A.N., Halestrap A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Philp N.J., Ochrietor J.D., Rudoy C., Muramatsu T., Linser P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest. Ophthalmol. Vis. Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 73.Wilson M.C., Meredith D., Fox J.E., Manoharan C., Davies A.J., Halestrap A.P. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) J. Biol. Chem. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 74.Slomiany M.G., Grass G.D., Robertson A.D., Yang X.Y., Maria B.L., Beeson C., Toole B.P. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69:1293–1301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 76.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 77.Koukourakis M.I., Pitiakoudis M., Giatromanolaki A., Tsarouha A., Polychronidis A., Sivridis E., Simopoulos C. Oxygen and glucose consumption in gastrointestinal adenocarcinomas: correlation with markers of hypoxia, acidity and anaerobic glycolysis. Cancer Sci. 2006;97:1056–1060. doi: 10.1111/j.1349-7006.2006.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelicano H., Martin D.S., Xu R.H., Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 79.Bhatt A.P., Jacobs S.R., Freemerman A.J., Makowski L., Rathmell J.C., Dittmer D.P., Damania B. Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA. 2012;109:11818–11823. doi: 10.1073/pnas.1205995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado T., Carroll P.A., Punjabi A.S., Margineantu D., Hockenbery D.M., Lagunoff M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. USA. 2010;107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carroll P.A., Brazeau E., Lagunoff M. Kaposi’s sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology. 2004;328:7–18. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.An F.Q., Folarin H.M., Compitello N., Roth J., Gerson S.L., McCrae K.R., Fakhari F.D., Dittmer D.P., Renne R. Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 2006;80:4833–4846. doi: 10.1128/JVI.80.10.4833-4846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pyakurel P., Pak F., Mwakigonja A.R., Kaaya E., Heiden T., Biberfeld P. Lymphatic and vascular origin of Kaposi’s sarcoma spindle cells during tumor development. Int. J. Cancer. 2006;119:1262–1267. doi: 10.1002/ijc.21969. [DOI] [PubMed] [Google Scholar]
- 84.Renne R., Barry C., Dittmer D., Compitello N., Brown P.O., Ganem D. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001;75:458–468. doi: 10.1128/JVI.75.1.458-468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lan K., Kuppers D.A., Verma S.C., Robertson E.S. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 2004;78:6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma S.C., Borah S., Robertson E.S. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 2004;78:10348–10359. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen W., Hilton I.B., Staudt M.R., Burd C.E., Dittmer D.P. Distinct p53, p53:LANA, and LANA complexes in Kaposi’s Sarcoma–associated Herpesvirus Lymphomas. J. Virol. 2010;84:3898–3908. doi: 10.1128/JVI.01321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang L., Major T., Bocan T. Characterization of the promoter of human extracellular matrix metalloproteinase inducer (EMMPRIN) Gene. 2002;282:75–86. doi: 10.1016/s0378-1119(01)00847-2. [DOI] [PubMed] [Google Scholar]
- 89.Hagemann T., Wilson J., Kulbe H., Li N.F., Leinster D.A., Charles K., Klemm F., Pukrop T., Binder C., Balkwill F.R. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 90.Matta H., Chaudhary P.M. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc. Natl. Acad. Sci. USA. 2004;101:9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chugh P., Matta H., Schamus S., Zachariah S., Kumar A., Richardson J.A., Smith A.L., Chaudhary P.M. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc. Natl. Acad. Sci. USA. 2005;102:12885–12890. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannon M., Philpott N.J., Cesarman E. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 2003;77:57–67. doi: 10.1128/JVI.77.1.57-67.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cannon M. The KSHV and other human herpesviral G protein-coupled receptors. Curr. Top. Microbiol. Immunol. 2007;312:137–156. doi: 10.1007/978-3-540-34344-8_5. [DOI] [PubMed] [Google Scholar]
- 94.Menashi S., Serova M., Ma L., Vignot S., Mourah S., Calvo F. Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res. 2003;63:7575–7580. [PubMed] [Google Scholar]
- 95.Shi Y., Simmons M.N., Seki T., Oh S.P., Sugrue S.P. Change in gene expression subsequent to induction of Pnn/DRS/memA: increase in p21(cip1/waf1) Oncogene. 2001;20:4007–4018. doi: 10.1038/sj.onc.1204507. [DOI] [PubMed] [Google Scholar]
- 96.Tang W., Chang S.B., Hemler M.E. Links between CD147 function, glycosylation, and caveolin-1. Mol. Biol. Cell. 2004;15:4043–4050. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim M., Martinez T., Jablons D., Cameron R., Guo H., Toole B., Li J.D., Basbaum C. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998;441:88–92. doi: 10.1016/s0014-5793(98)01474-4. [DOI] [PubMed] [Google Scholar]
- 98.Marieb E.A., Zoltan-Jones A., Li R., Misra S., Ghatak S., Cao J., Zucker S., Toole B.P. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64:1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 99.Misra S., Ghatak S., Zoltan-Jones A., Toole B.P. Regulation of multidrug resistance in cancer cells by hyaluronan. J. Biol. Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 100.Ghatak S., Misra S., Toole B.P. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J. Biol. Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]