Abstract
Regulation of chromosomally determined nutrient cation and anion uptake systems shows important similarities to regulation of plasmid-determined toxic ion resistance systems that mediate the outward transport of deleterious ions. Chromosomally determined transport systems result in accumulation of K+, Mg2+, Fe3+, Mn2+, PO4(3-), SO4(2-), and additional trace nutrients, while bacterial plasmids harbor highly specific resistance systems for AsO2-, AsO4(3-), CrO4(2-), Cd2+, Co2+, Cu2+, Hg2+, Ni2+, SbO2-, TeO3(2-), Zn2+, and other toxic ions. To study the regulation of these systems, we need to define both the trans-acting regulatory proteins and the cis-acting target operator DNA regions for the proteins. The regulation of gene expression for K+ and PO4(3-) transport systems involves two-component sensor-effector pairs of proteins. The first protein responds to an extracellular ionic (or related) signal and then transmits the signal to an intracellular DNA-binding protein. Regulation of Fe3+ transport utilizes the single iron-binding and DNA-binding protein Fur. The MerR regulatory protein for mercury resistance both represses and activates transcription. The ArsR regulatory protein functions as a repressor for the arsenic and antimony(III) efflux system. Although the predicted cadR regulatory gene has not been identified, cadmium, lead, bismuth, zinc, and cobalt induce this system in a carefully regulated manner from a single mRNA start site. The cadA Cd2+ resistance determinant encodes an E1(1)-1E2-class efflux ATPase (consisting of two polypeptides, rather than the one earlier identified). Cadmium resistance is also conferred by the czc system (which confers resistances to zinc and cobalt in Alcaligenes species) via a complex efflux pump consisting of four polypeptides. These two cadmium efflux systems are not otherwise related. For chromate resistance, reduced cellular accumulation is again the resistance mechanism, but the regulatory components are not identified. For other toxic heavy metals (with few exceptions), there exist specific plasmid resistances that remain relatively terra incognita for future exploration of bioinorganic molecular genetics and gene regulation.
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloing G., Trombe M. C., Claverys J. P. The ami locus of the gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of gram-negative bacteria. Mol Microbiol. 1990 Apr;4(4):633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
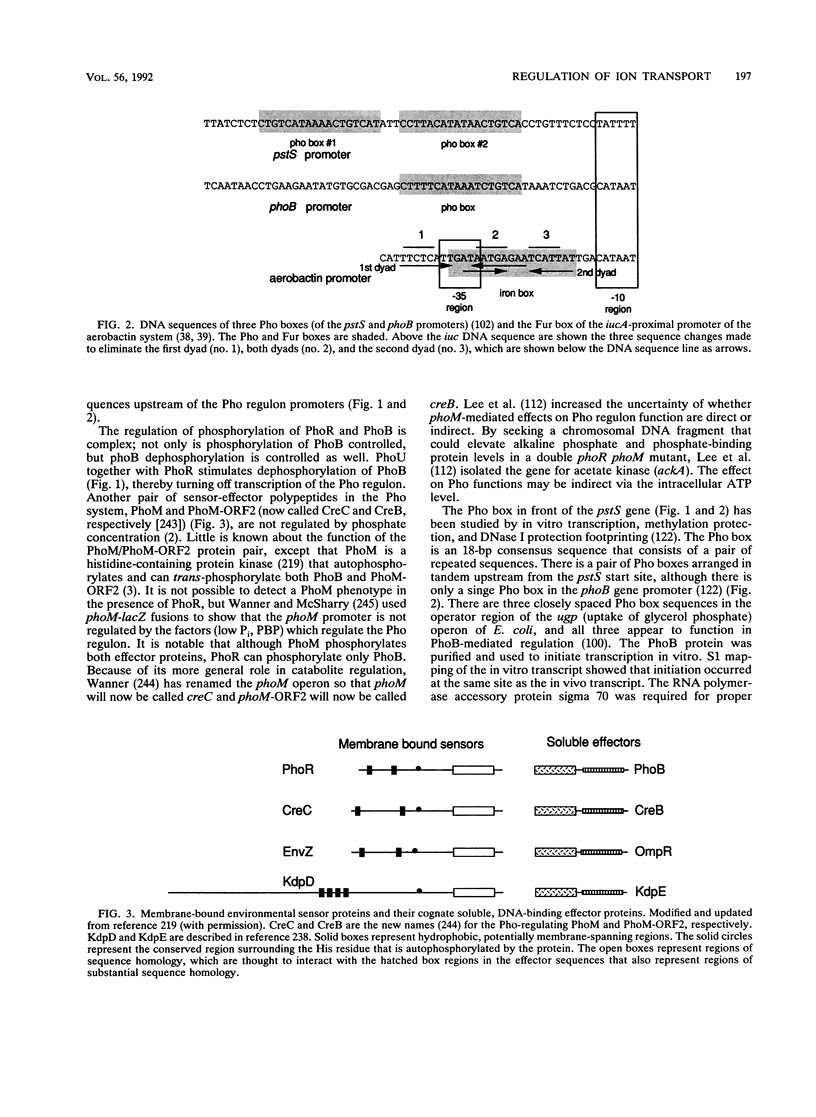
- Amemura M., Makino K., Shinagawa H., Nakata A. Cross talk to the phosphate regulon of Escherichia coli by PhoM protein: PhoM is a histidine protein kinase and catalyzes phosphorylation of PhoB and PhoM-open reading frame 2. J Bacteriol. 1990 Nov;172(11):6300–6307. doi: 10.1128/jb.172.11.6300-6307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemura M., Makino K., Shinagawa H., Nakata A. Nucleotide sequence of the phoM region of Escherichia coli: four open reading frames may constitute an operon. J Bacteriol. 1986 Oct;168(1):294–302. doi: 10.1128/jb.168.1.294-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K., Groarke J., Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J Biol Chem. 1989 Mar 5;264(7):3998–4002. [PubMed] [Google Scholar]
- Ansari A. Z., Chael M. L., O'Halloran T. V. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature. 1992 Jan 2;355(6355):87–89. doi: 10.1038/355087a0. [DOI] [PubMed] [Google Scholar]
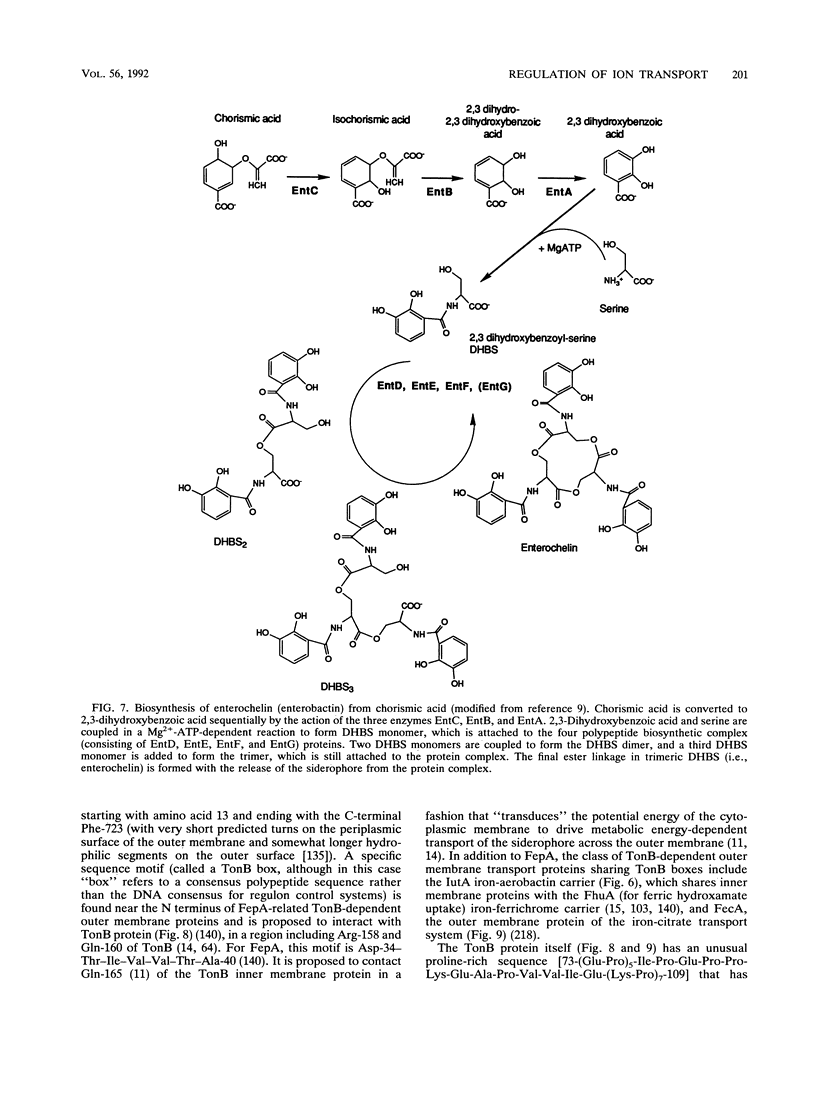
- Armstrong S. K., Pettis G. S., Forrester L. J., McIntosh M. A. The Escherichia coli enterobactin biosynthesis gene, entD: nucleotide sequence and membrane localization of its protein product. Mol Microbiol. 1989 Jun;3(6):757–766. doi: 10.1111/j.1365-2958.1989.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Babich K., Engle M., Skinner J. S., Laddaga R. A. Deletion mutant analysis of the Staphylococcus aureus plasmid pI258 mercury-resistance determinant. Can J Microbiol. 1991 Aug;37(8):624–631. doi: 10.1139/m91-106. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. E., Nau C. D., Brown J. T., Konisky J., Kadner R. J. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J Bacteriol. 1990 Jul;172(7):3826–3829. doi: 10.1128/jb.172.7.3826-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Conway K. E., George S., Pratt P. Characterization of pXV10A, a Copper Resistance Plasmid in Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1990 Jan;56(1):170–175. doi: 10.1128/aem.56.1.170-175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Misra T. K., Winnie J. N., Schmidt A., Seiff M., Silver S. The nucleotide sequence of the mercuric resistance operons of plasmid R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Mol Gen Genet. 1986 Jan;202(1):143–151. doi: 10.1007/BF00330531. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Rouch D. A., Lee B. T. Copper resistance determinants in bacteria. Plasmid. 1992 Jan;27(1):41–51. doi: 10.1016/0147-619x(92)90005-u. [DOI] [PubMed] [Google Scholar]
- Cervantes C., Ohtake H., Chu L., Misra T. K., Silver S. Cloning, nucleotide sequence, and expression of the chromate resistance determinant of Pseudomonas aeruginosa plasmid pUM505. J Bacteriol. 1990 Jan;172(1):287–291. doi: 10.1128/jb.172.1.287-291.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes C., Silver S. Plasmid chromate resistance and chromate reduction. Plasmid. 1992 Jan;27(1):65–71. doi: 10.1016/0147-619x(92)90007-w. [DOI] [PubMed] [Google Scholar]
- Cha J. S., Cooksey D. A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Chen C. M., Ye Q. Z., Zhu Z. M., Wanner B. L., Walsh C. T. Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and C-P lyase activity in Escherichia coli B. J Biol Chem. 1990 Mar 15;265(8):4461–4471. [PubMed] [Google Scholar]
- Chenault S. S., Earhart C. F. Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol Microbiol. 1991 Jun;5(6):1405–1413. doi: 10.1111/j.1365-2958.1991.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Ching M. H., Kaur P., Karkaria C. E., Steiner R. F., Rosen B. P. Substrate-induced dimerization of the ArsA protein, the catalytic component of an anion-translocating ATPase. J Biol Chem. 1991 Feb 5;266(4):2327–2332. [PubMed] [Google Scholar]
- Cooksey D. A., Azad H. R., Cha J. S., Lim C. K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl Environ Microbiol. 1990 Feb;56(2):431–435. doi: 10.1128/aem.56.2.431-435.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey D. A. Characterization of a Copper Resistance Plasmid Conserved in Copper-Resistant Strains of Pseudomonas syringae pv. tomato. Appl Environ Microbiol. 1987 Feb;53(2):454–456. doi: 10.1128/aem.53.2.454-456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Webb D., Godovac-Zimmermann J., Rosenberg H. Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol. 1988 May;170(5):2283–2286. doi: 10.1128/jb.170.5.2283-2286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Webb D., Rosenberg H. Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J Bacteriol. 1989 Mar;171(3):1531–1534. doi: 10.1128/jb.171.3.1531-1534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy M., Neilands J. B. Structural dynamics and functional domains of the fur protein. Biochemistry. 1991 Aug 20;30(33):8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Nikaido H. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J Biol Chem. 1990 Mar 15;265(8):4254–4260. [PubMed] [Google Scholar]
- Dean D. A., Davidson A. L., Nikaido H. Maltose transport in membrane vesicles of Escherichia coli is linked to ATP hydrolysis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9134–9138. doi: 10.1073/pnas.86.23.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diels L., Mergeay M. DNA probe-mediated detection of resistant bacteria from soils highly polluted by heavy metals. Appl Environ Microbiol. 1990 May;56(5):1485–1491. doi: 10.1128/aem.56.5.1485-1491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberz G., Eitinger T., Friedrich B. Genetic determinants of a nickel-specific transport system are part of the plasmid-encoded hydrogenase gene cluster in Alcaligenes eutrophus. J Bacteriol. 1989 Mar;171(3):1340–1345. doi: 10.1128/jb.171.3.1340-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitinger T., Friedrich B. Cloning, nucleotide sequence, and heterologous expression of a high-affinity nickel transport gene from Alcaligenes eutrophus. J Biol Chem. 1991 Feb 15;266(5):3222–3227. [PubMed] [Google Scholar]
- Elkins M. F., Earhart C. F. Nucleotide sequence and regulation of the Escherichia coli gene for ferrienterobactin transport protein FepB. J Bacteriol. 1989 Oct;171(10):5443–5451. doi: 10.1128/jb.171.10.5443-5451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore M. J., Lamb A. J., Ritchie G. Y., Douglas R. M., Munro A., Gajewska A., Booth I. R. Activation of potassium efflux from Escherichia coli by glutathione metabolites. Mol Microbiol. 1990 Mar;4(3):405–412. doi: 10.1111/j.1365-2958.1990.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Epstein W., Walderhaug M. O., Polarek J. W., Hesse J. E., Dorus E., Daniel J. M. The bacterial Kdp K(+)-ATPase and its relation to other transport ATPases, such as the Na+/K(+)- and Ca2(+)-ATPases in higher organisms. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):479–487. doi: 10.1098/rstb.1990.0026. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Fisher S., Buxbaum L., Toth K., Eisenstadt E., Silver S. Regulation of manganese accumulation and exchange in Bacillus subtilis W23. J Bacteriol. 1973 Mar;113(3):1373–1380. doi: 10.1128/jb.113.3.1373-1380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Brown N. L. Identification of the merR gene of R100 by using mer-lac gene and operon fusions. J Bacteriol. 1985 Sep;163(3):1153–1157. doi: 10.1128/jb.163.3.1153-1157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Nakahara H., Weiss A. A., Silver S. Transposon A-generated mutations in the mercuric resistance genes of plasmid R100-1. J Bacteriol. 1979 Oct;140(1):167–181. doi: 10.1128/jb.140.1.167-181.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. The genetics and biochemistry of mercury resistance. Crit Rev Microbiol. 1987;15(2):117–140. doi: 10.3109/10408418709104455. [DOI] [PubMed] [Google Scholar]
- Frantz B., O'Halloran T. V. DNA distortion accompanies transcriptional activation by the metal-responsive gene-regulatory protein MerR. Biochemistry. 1990 May 22;29(20):4747–4751. doi: 10.1021/bi00472a001. [DOI] [PubMed] [Google Scholar]
- Gardel C., Johnson K., Jacq A., Beckwith J. The secD locus of E.coli codes for two membrane proteins required for protein export. EMBO J. 1990 Oct;9(10):3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Bagga D. A., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol. 1991 Nov;5(11):2753–2762. doi: 10.1111/j.1365-2958.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Green L. S., Laudenbach D. E., Grossman A. R. A region of a cyanobacterial genome required for sulfate transport. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1949–1953. doi: 10.1073/pnas.86.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin H. G., Foster T. J., Silver S., Misra T. K. Cloning and DNA sequence of the mercuric- and organomercurial-resistance determinants of plasmid pDU1358. Proc Natl Acad Sci U S A. 1987 May;84(10):3112–3116. doi: 10.1073/pnas.84.10.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günter K., Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990 Nov 12;274(1-2):85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- Hannavy K., Barr G. C., Dorman C. J., Adamson J., Mazengera L. R., Gallagher M. P., Evans J. S., Levine B. A., Trayer I. P., Higgins C. F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990 Dec 20;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. J., Quiocho F. A. A nonconservative serine to cysteine mutation in the sulfate-binding protein, a transport receptor. Science. 1991 Mar 22;251(5000):1479–1481. doi: 10.1126/science.1900953. [DOI] [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Nucleotide sequence and high-level expression of the major Escherichia coli phosphofructokinase. Eur J Biochem. 1985 Jun 3;149(2):363–373. doi: 10.1111/j.1432-1033.1985.tb08934.x. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Ballard B. T., Walsh C. T. The MerR metalloregulatory protein binds mercuric ion as a tricoordinate, metal-bridged dimer. Science. 1990 Feb 23;247(4945):946–948. doi: 10.1126/science.2305262. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Shewchuk L. M., Walsh C. T. Regulation of gene expression by mercury. Adv Inorg Biochem. 1990;8:33–61. [PubMed] [Google Scholar]
- Helmann J. D., Wang Y., Mahler I., Walsh C. T. Homologous metalloregulatory proteins from both gram-positive and gram-negative bacteria control transcription of mercury resistance operons. J Bacteriol. 1989 Jan;171(1):222–229. doi: 10.1128/jb.171.1.222-229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltzel A., Gambill D., Jackson W. J., Totis P. A., Summers A. O. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J Bacteriol. 1987 Jul;169(7):3379–3384. doi: 10.1128/jb.169.7.3379-3384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltzel A., Lee I. W., Totis P. A., Summers A. O. Activator-dependent preinduction binding of sigma-70 RNA polymerase at the metal-regulated mer promoter. Biochemistry. 1990 Oct 16;29(41):9572–9584. doi: 10.1021/bi00493a011. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H. Regulation of bacterial gene expression by metal-protein complexes. Mol Microbiol. 1990 Oct;4(10):1621–1628. doi: 10.1111/j.1365-2958.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hinton J. C., Hulton C. S., Owen-Hughes T., Pavitt G. D., Seirafi A. Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence? Mol Microbiol. 1990 Dec;4(12):2007–2012. doi: 10.1111/j.1365-2958.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Florer J. B., Maguire M. E., Miller C. G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989 Sep;171(9):4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
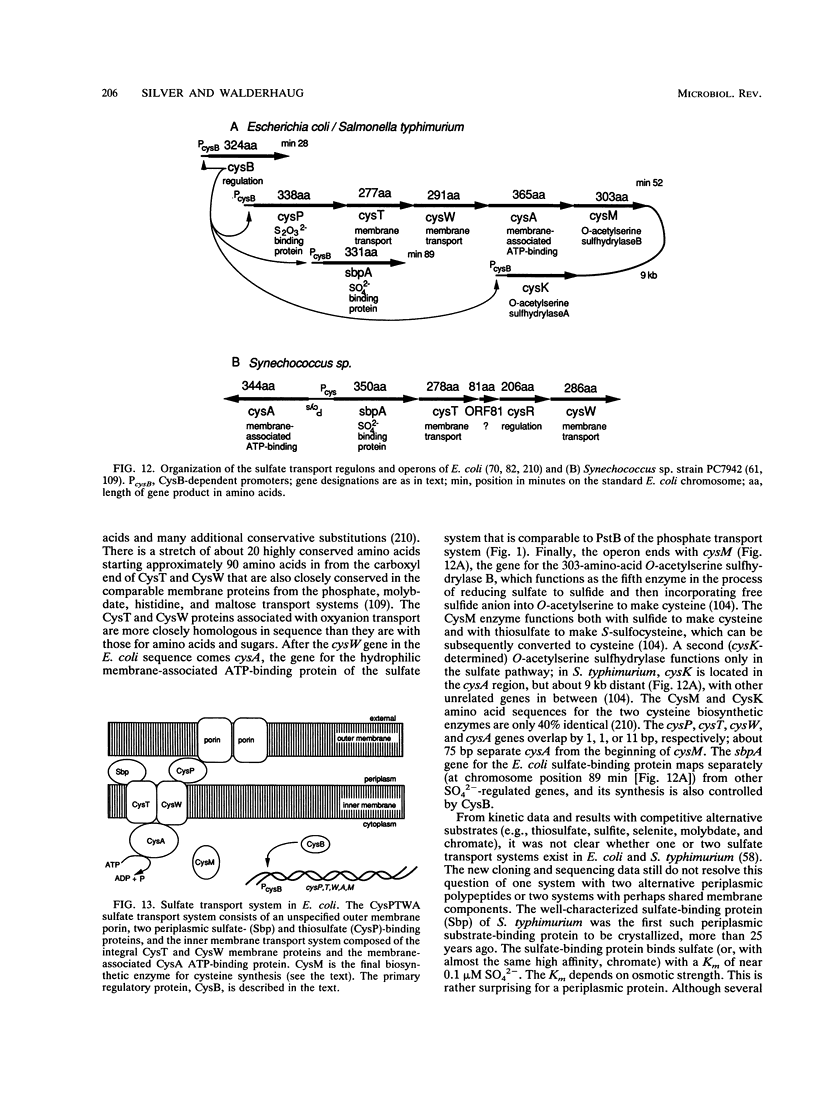
- Hryniewicz M. M., Kredich N. M. The cysP promoter of Salmonella typhimurium: characterization of two binding sites for CysB protein, studies of in vivo transcription initiation, and demonstration of the anti-inducer effects of thiosulfate. J Bacteriol. 1991 Sep;173(18):5876–5886. doi: 10.1128/jb.173.18.5876-5886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz M., Sirko A., Pałucha A., Böck A., Hulanicka D. Sulfate and thiosulfate transport in Escherichia coli K-12: identification of a gene encoding a novel protein involved in thiosulfate binding. J Bacteriol. 1990 Jun;172(6):3358–3366. doi: 10.1128/jb.172.6.3358-3366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. M., Rosen B. P. Characterization of the catalytic subunit of an anion pump. J Biol Chem. 1989 Oct 15;264(29):17349–17354. [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. The merR regulatory gene in Thiobacillus ferrooxidans is spaced apart from the mer structural genes. Mol Microbiol. 1991 Nov;5(11):2707–2718. doi: 10.1111/j.1365-2958.1991.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. Thiobacillus ferrooxidans mer operon: sequence analysis of the promoter and adjacent genes. Gene. 1990 Nov 30;96(1):115–120. doi: 10.1016/0378-1119(90)90349-v. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Shiratori T., Kusano T., Kitagawa Y. Nucleotide sequence of the Thiobacillus ferrooxidans chromosomal gene encoding mercuric reductase. Gene. 1989 Dec 7;84(1):47–54. doi: 10.1016/0378-1119(89)90138-8. [DOI] [PubMed] [Google Scholar]
- Jacobson B. L., Quiocho F. A. Sulfate-binding protein dislikes protonated oxyacids. A molecular explanation. J Mol Biol. 1988 Dec 5;204(3):783–787. doi: 10.1016/0022-2836(88)90369-5. [DOI] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Nucleotide sequence and genetic analysis of the nifB-nifQ region from Azotobacter vinelandii. J Bacteriol. 1988 Apr;170(4):1475–1487. doi: 10.1128/jb.170.4.1475-1487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkaria C. E., Chen C. M., Rosen B. P. Mutagenesis of a nucleotide-binding site of an anion-translocating ATPase. J Biol Chem. 1990 May 15;265(14):7832–7836. [PubMed] [Google Scholar]
- Karkaria C. E., Rosen B. P. Trinitrophenyl-ATP binding to the ArsA protein: the catalytic subunit of an anion pump. Arch Biochem Biophys. 1991 Jul;288(1):107–111. doi: 10.1016/0003-9861(91)90170-n. [DOI] [PubMed] [Google Scholar]
- Karkaria C. E., Steiner R. F., Rosen B. P. Ligand interactions in the ArsA protein, the catalytic component of an anion-translocating adenosinetriphosphatase. Biochemistry. 1991 Mar 12;30(10):2625–2628. doi: 10.1021/bi00224a009. [DOI] [PubMed] [Google Scholar]
- Karpel R., Alon T., Glaser G., Schuldiner S., Padan E. Expression of a sodium proton antiporter (NhaA) in Escherichia coli is induced by Na+ and Li+ ions. J Biol Chem. 1991 Nov 15;266(32):21753–21759. [PubMed] [Google Scholar]
- Karpel R., Olami Y., Taglicht D., Schuldiner S., Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10408–10414. [PubMed] [Google Scholar]
- Kasahara M., Makino K., Amemura M., Nakata A., Shinagawa H. Dual regulation of the ugp operon by phosphate and carbon starvation at two interspaced promoters. J Bacteriol. 1991 Jan;173(2):549–558. doi: 10.1128/jb.173.2.549-558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Rosen B. P. Plasmid-encoded resistance to arsenic and antimony. Plasmid. 1992 Jan;27(1):29–40. doi: 10.1016/0147-619x(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Kimura S., Makino K., Shinagawa H., Amemura M., Nakata A. Regulation of the phosphate regulon of Escherichia coli: characterization of the promoter of the pstS gene. Mol Gen Genet. 1989 Feb;215(3):374–380. doi: 10.1007/BF00427032. [DOI] [PubMed] [Google Scholar]
- Kusano T., Ji G. Y., Inoue C., Silver S. Constitutive synthesis of a transport function encoded by the Thiobacillus ferrooxidans merC gene cloned in Escherichia coli. J Bacteriol. 1990 May;172(5):2688–2692. doi: 10.1128/jb.172.5.2688-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., North A. K., Weiss D. S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991 Nov;16(11):397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- Köster W. Iron(III) hydroxamate transport across the cytoplasmic membrane of Escherichia coli. Biol Met. 1991;4(1):23–32. doi: 10.1007/BF01135553. [DOI] [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Chu L., Misra T. K., Silver S. Nucleotide sequence and expression of the mercurial-resistance operon from Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5106–5110. doi: 10.1073/pnas.84.15.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach D. E., Grossman A. R. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J Bacteriol. 1991 May;173(9):2739–2750. doi: 10.1128/jb.173.9.2739-2750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. Y., Makino K., Shinagawa H., Nakata A. Overproduction of acetate kinase activates the phosphate regulon in the absence of the phoR and phoM functions in Escherichia coli. J Bacteriol. 1990 May;172(5):2245–2249. doi: 10.1128/jb.172.5.2245-2249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones G., Ritchie D. A., Strike P. Biochemical and biophysical analysis of plasmid pMJ600-encoded tellurite [TeO2(3-)] resistance. FEMS Microbiol Lett. 1991 Jun 1;65(1):19–24. doi: 10.1016/0378-1097(91)90464-l. [DOI] [PubMed] [Google Scholar]
- Luecke H., Quiocho F. A. High specificity of a phosphate transport protein determined by hydrogen bonds. Nature. 1990 Sep 27;347(6291):402–406. doi: 10.1038/347402a0. [DOI] [PubMed] [Google Scholar]
- Lund P. A., Brown N. L. Regulation of transcription in Escherichia coli from the mer and merR promoters in the transposon Tn501. J Mol Biol. 1989 Jan 20;205(2):343–353. doi: 10.1016/0022-2836(89)90345-8. [DOI] [PubMed] [Google Scholar]
- Lund P. A., Ford S. J., Brown N. L. Transcriptional regulation of the mercury-resistance genes of transposon Tn501. J Gen Microbiol. 1986 Feb;132(2):465–480. doi: 10.1099/00221287-132-2-465. [DOI] [PubMed] [Google Scholar]
- Makino K., Kim S. K., Shinagawa H., Amemura M., Nakata A. Molecular analysis of the cryptic and functional phn operons for phosphonate use in Escherichia coli K-12. J Bacteriol. 1991 Apr;173(8):2665–2672. doi: 10.1128/jb.173.8.2665-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Kawamoto T., Yamada M., Nakata A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol. 1989 Dec 5;210(3):551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Kimura S., Nakata A., Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988 Sep 5;203(1):85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Nakata A. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J Mol Biol. 1986 Jul 5;190(1):37–44. doi: 10.1016/0022-2836(86)90073-2. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Nakata A. Nucleotide sequence of the phoR gene, a regulatory gene for the phosphate regulon of Escherichia coli. J Mol Biol. 1986 Dec 5;192(3):549–556. doi: 10.1016/0022-2836(86)90275-5. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Ambudkar S. V., Anatharam V., Sonna L. A., Varadhachary A. Anion-exchange mechanisms in bacteria. Microbiol Rev. 1990 Mar;54(1):1–17. doi: 10.1128/mr.54.1.1-17.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellano M. A., Cooksey D. A. Induction of the copper resistance operon from Pseudomonas syringae. J Bacteriol. 1988 Sep;170(9):4399–4401. doi: 10.1128/jb.170.9.4399-4401.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellano M. A., Cooksey D. A. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1988 Jun;170(6):2879–2883. doi: 10.1128/jb.170.6.2879-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Fritzinger D. C., Pridmore R. D., Barnes W. M., Haberstroh L., Silver S. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K., Brown N. L., Haberstroh L., Schmidt A., Goddette D., Silver S. Mercuric reductase structural genes from plasmid R100 and transposon Tn501: functional domains of the enzyme. Gene. 1985;34(2-3):253–262. doi: 10.1016/0378-1119(85)90134-9. [DOI] [PubMed] [Google Scholar]
- Moore M. J., Distefano M. D., Walsh C. T., Schiering N., Pai E. F. Purification, crystallization, and preliminary x-ray diffraction studies of the flavoenzyme mercuric ion reductase from Bacillus sp. strain RC607. J Biol Chem. 1989 Aug 25;264(24):14386–14388. [PubMed] [Google Scholar]
- Mukhopadhyay D., Yu H. R., Nucifora G., Misra T. K. Purification and functional characterization of MerD. A coregulator of the mercury resistance operon in gram-negative bacteria. J Biol Chem. 1991 Oct 5;266(28):18538–18542. [PubMed] [Google Scholar]
- Munro A. W., Ritchie G. Y., Lamb A. J., Douglas R. M., Booth I. R. The cloning and DNA sequence of the gene for the glutathione-regulated potassium-efflux system KefC of Escherichia coli. Mol Microbiol. 1991 Mar;5(3):607–616. doi: 10.1111/j.1365-2958.1991.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlik M. S., Brickman T. J., Ozenberger B. A., McIntosh M. A. Nucleotide sequence and transcriptional organization of the Escherichia coli enterobactin biosynthesis cistrons entB and entA. J Bacteriol. 1989 Feb;171(2):784–790. doi: 10.1128/jb.171.2.784-790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature. 1977 Mar 10;266(5598):165–167. doi: 10.1038/266165a0. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Silver S., Miki T., Rownd R. H. Hypersensitivity to Hg2+ and hyperbinding activity associated with cloned fragments of the mercurial resistance operon of plasmid NR1. J Bacteriol. 1979 Oct;140(1):161–166. doi: 10.1128/jb.140.1.161-166.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C. D., Konisky J. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J Bacteriol. 1989 Feb;171(2):1041–1047. doi: 10.1128/jb.171.2.1041-1047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Ni'Bhriain N. N., Silver S., Foster T. J. Tn5 insertion mutations in the mercuric ion resistance genes derived from plasmid R100. J Bacteriol. 1983 Aug;155(2):690–703. doi: 10.1128/jb.155.2.690-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies A., Nies D. H., Silver S. Cloning and expression of plasmid genes encoding resistances to chromate and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989 Sep;171(9):5065–5070. doi: 10.1128/jb.171.9.5065-5070.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies A., Nies D. H., Silver S. Nucleotide sequence and expression of a plasmid-encoded chromate resistance determinant from Alcaligenes eutrophus. J Biol Chem. 1990 Apr 5;265(10):5648–5653. [PubMed] [Google Scholar]
- Nies D. H., Nies A., Chu L., Silver S. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7351–7355. doi: 10.1073/pnas.86.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992 Jan;27(1):17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Metal ion uptake by a plasmid-free metal-sensitive Alcaligenes eutrophus strain. J Bacteriol. 1989 Jul;171(7):4073–4075. doi: 10.1128/jb.171.7.4073-4075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H., Silver S. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989 Feb;171(2):896–900. doi: 10.1128/jb.171.2.896-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D., Mergeay M., Friedrich B., Schlegel H. G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J Bacteriol. 1987 Oct;169(10):4865–4868. doi: 10.1128/jb.169.10.4865-4868.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Chu L., Misra T. K., Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc Natl Acad Sci U S A. 1989 May;86(10):3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Chu L., Silver S., Misra T. K. Mercury operon regulation by the merR gene of the organomercurial resistance system of plasmid pDU1358. J Bacteriol. 1989 Aug;171(8):4241–4247. doi: 10.1128/jb.171.8.4241-4247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Silver S., Misra T. K. Down regulation of the mercury resistance operon by the most promoter-distal gene merD. Mol Gen Genet. 1989 Dec;220(1):69–72. doi: 10.1007/BF00260858. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- O'Halloran T., Walsh C. Metalloregulatory DNA-binding protein encoded by the merR gene: isolation and characterization. Science. 1987 Jan 9;235(4785):211–214. doi: 10.1126/science.3798107. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Cervantes C., Silver S. Decreased chromate uptake in Pseudomonas fluorescens carrying a chromate resistance plasmid. J Bacteriol. 1987 Aug;169(8):3853–3856. doi: 10.1128/jb.169.8.3853-3856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Jagura-Burdzy G., Kredich N. M. DNA sequences of the cysB regions of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1987 May 5;262(13):5999–6005. [PubMed] [Google Scholar]
- Ostrowski J., Kredich N. M. Molecular characterization of the cysJIH promoters of Salmonella typhimurium and Escherichia coli: regulation by cysB protein and N-acetyl-L-serine. J Bacteriol. 1989 Jan;171(1):130–140. doi: 10.1128/jb.171.1.130-140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounis C., Sander C. A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins. FEBS Lett. 1991 Feb 11;279(1):73–78. doi: 10.1016/0014-5793(91)80254-z. [DOI] [PubMed] [Google Scholar]
- Owolabi J. B., Rosen B. P. Differential mRNA stability controls relative gene expression within the plasmid-encoded arsenical resistance operon. J Bacteriol. 1990 May;172(5):2367–2371. doi: 10.1128/jb.172.5.2367-2371.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Brickman T. J., McIntosh M. A. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J Bacteriol. 1989 Feb;171(2):775–783. doi: 10.1128/jb.171.2.775-783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Nahlik M. S., McIntosh M. A. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3638–3646. doi: 10.1128/jb.169.8.3638-3646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Maisler N., Taglicht D., Karpel R., Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J Biol Chem. 1989 Dec 5;264(34):20297–20302. [PubMed] [Google Scholar]
- Parkhill J., Brown N. L. Site-specific insertion and deletion mutants in the mer promoter-operator region of Tn501; the nineteen base-pair spacer is essential for normal induction of the promoter by MerR. Nucleic Acids Res. 1990 Sep 11;18(17):5157–5162. doi: 10.1093/nar/18.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugrath J. W., Quiocho F. A. Sulphate sequestered in the sulphate-binding protein of Salmonella typhimurium is bound solely by hydrogen bonds. Nature. 1985 Mar 21;314(6008):257–260. doi: 10.1038/314257a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. R., Earhart C. F. Escherichia coli K-12 envelope proteins specifically required for ferrienterobactin uptake. J Bacteriol. 1986 Jun;166(3):930–936. doi: 10.1128/jb.166.3.930-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A. Atomic structures of periplasmic binding proteins and the high-affinity active transport systems in bacteria. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):341–352. doi: 10.1098/rstb.1990.0016. [DOI] [PubMed] [Google Scholar]
- Ralston D. M., O'Halloran T. V. Ultrasensitivity and heavy-metal selectivity of the allosterically modulated MerR transcription complex. Proc Natl Acad Sci U S A. 1990 May;87(10):3846–3850. doi: 10.1073/pnas.87.10.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. N., Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990 Jul;4(7):1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Rogers S. D., Bhave M. R., Mercer J. F., Camakaris J., Lee B. T. Cloning and characterization of cutE, a gene involved in copper transport in Escherichia coli. J Bacteriol. 1991 Nov;173(21):6742–6748. doi: 10.1128/jb.173.21.6742-6748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Hsu C. M., Karkaria C. E., Owolabi J. B., Tisa L. S. Molecular analysis of an ATP-dependent anion pump. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):455–463. doi: 10.1098/rstb.1990.0024. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Monticello R. A., Edwards B. P. Molecular analysis of an anion pump: purification of the ArsC protein. Arch Biochem Biophys. 1991 Feb 1;284(2):381–385. doi: 10.1016/0003-9861(91)90312-7. [DOI] [PubMed] [Google Scholar]
- Ross W., Park S. J., Summers A. O. Genetic analysis of transcriptional activation and repression in the Tn21 mer operon. J Bacteriol. 1989 Jul;171(7):4009–4018. doi: 10.1128/jb.171.7.4009-4018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Duly D., Williams R. J. The histidines of the iron-uptake regulation protein, Fur. Eur J Biochem. 1991 Apr 10;197(1):39–42. doi: 10.1111/j.1432-1033.1991.tb15879.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Williams R. J. The binding of the ferric uptake regulation protein to a DNA fragment. Eur J Biochem. 1991 Apr 10;197(1):43–47. doi: 10.1111/j.1432-1033.1991.tb15880.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Wormald M. R., Williams R. J. Some structural features of the iron-uptake regulation protein. Eur J Biochem. 1991 Apr 10;197(1):29–38. doi: 10.1111/j.1432-1033.1991.tb15878.x. [DOI] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Tisa L. S., Rosen B. P. Identification of the membrane component of the anion pump encoded by the arsenical resistance operon of R-factor R773. Mol Microbiol. 1989 Jan;3(1):15–21. doi: 10.1111/j.1365-2958.1989.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Schiering N., Kabsch W., Moore M. J., Distefano M. D., Walsh C. T., Pai E. F. Structure of the detoxification catalyst mercuric ion reductase from Bacillus sp. strain RC607. Nature. 1991 Jul 11;352(6331):168–172. doi: 10.1038/352168a0. [DOI] [PubMed] [Google Scholar]
- Serrano R., Portillo F. Catalytic and regulatory sites of yeast plasma membrane H(+)-ATPase studied by directed mutagenesis. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):195–199. doi: 10.1016/0005-2728(90)90247-2. [DOI] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Shea C. M., McIntosh M. A. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol. 1991 Jun;5(6):1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Shewchuk L. M., Helmann J. D., Ross W., Park S. J., Summers A. O., Walsh C. T. Transcriptional switching by the MerR protein: activation and repression mutants implicate distinct DNA and mercury(II) binding domains. Biochemistry. 1989 Mar 7;28(5):2340–2344. doi: 10.1021/bi00431a053. [DOI] [PubMed] [Google Scholar]
- Siddiqui R. A., Benthin K., Schlegel H. G. Cloning of pMOL28-encoded nickel resistance genes and expression of the genes in Alcaligenes eutrophus and Pseudomonas spp. J Bacteriol. 1989 Sep;171(9):5071–5078. doi: 10.1128/jb.171.9.5071-5078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R. A., Schlegel H. G., Meyer M. Inducible and constitutive expression of pMOL28-encoded nickel resistance in Alcaligenes eutrophus N9A. J Bacteriol. 1988 Sep;170(9):4188–4193. doi: 10.1128/jb.170.9.4188-4193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Misra T. K. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- Silver S., Nucifora G., Chu L., Misra T. K. Bacterial resistance ATPases: primary pumps for exporting toxic cations and anions. Trends Biochem Sci. 1989 Feb;14(2):76–80. doi: 10.1016/0968-0004(89)90048-0. [DOI] [PubMed] [Google Scholar]
- Sirko A., Hryniewicz M., Hulanicka D., Böck A. Sulfate and thiosulfate transport in Escherichia coli K-12: nucleotide sequence and expression of the cysTWAM gene cluster. J Bacteriol. 1990 Jun;172(6):3351–3357. doi: 10.1128/jb.172.6.3351-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J. S., Ribot E., Laddaga R. A. Transcriptional analysis of the Staphylococcus aureus plasmid pI258 mercury resistance determinant. J Bacteriol. 1991 Aug;173(16):5234–5238. doi: 10.1128/jb.173.16.5234-5238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989 Sep;171(9):4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J Bacteriol. 1989 Sep;171(9):4752–4760. doi: 10.1128/jb.171.9.4752-4760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Gravina S. A., Cheung T. T., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J Biol Chem. 1991 Jan 15;266(2):824–829. [PubMed] [Google Scholar]
- Snavely M. D., Miller C. G., Maguire M. E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991 Jan 15;266(2):815–823. [PubMed] [Google Scholar]
- Staab J. F., Earhart C. F. EntG activity of Escherichia coli enterobactin synthetase. J Bacteriol. 1990 Nov;172(11):6403–6410. doi: 10.1128/jb.172.11.6403-6410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to boron and chromium compounds in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):637–640. doi: 10.1128/aac.13.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Tabillion R., Kaltwasser H. Energieabhängige 63Ni-Aufnahme bei Alcaligenes eutrophus Stamm H1 und H16. Arch Microbiol. 1977 May 13;113(1-2):145–151. doi: 10.1007/BF00428595. [DOI] [PubMed] [Google Scholar]
- Taglicht D., Padan E., Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J Biol Chem. 1991 Jun 15;266(17):11289–11294. [PubMed] [Google Scholar]
- Tetaz T. J., Luke R. K. Plasmid-controlled resistance to copper in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1263–1268. doi: 10.1128/jb.154.3.1263-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa L. S., Rosen B. P. Molecular characterization of an anion pump. The ArsB protein is the membrane anchor for the ArsA protein. J Biol Chem. 1990 Jan 5;265(1):190–194. [PubMed] [Google Scholar]
- Tisa L. S., Rosen B. P. Transport systems encoded by bacterial plasmids. J Bioenerg Biomembr. 1990 Aug;22(4):493–507. doi: 10.1007/BF00762959. [DOI] [PubMed] [Google Scholar]
- Torriani A. From cell membrane to nucleotides: the phosphate regulon in Escherichia coli. Bioessays. 1990 Aug;12(8):371–376. doi: 10.1002/bies.950120804. [DOI] [PubMed] [Google Scholar]
- Trevors J. T. Copper resistance in bacteria. Microbiol Sci. 1987 Jan;4(1):29–31. [PubMed] [Google Scholar]
- Tsai K. J., Yoon K. P., Lynn A. R. ATP-dependent cadmium transport by the cadA cadmium resistance determinant in everted membrane vesicles of Bacillus subtilis. J Bacteriol. 1992 Jan;174(1):116–121. doi: 10.1128/jb.174.1.116-121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove B., Staudenmaier H., Braun V. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol. 1990 Dec;172(12):6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderhaug M. O., Litwack E. D., Epstein W. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J Bacteriol. 1989 Feb;171(2):1192–1195. doi: 10.1128/jb.171.2.1192-1195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T., Distefano M. D., Moore M. J., Shewchuk L. M., Verdine G. L. Molecular basis of bacterial resistance to organomercurial and inorganic mercuric salts. FASEB J. 1988 Feb;2(2):124–130. doi: 10.1096/fasebj.2.2.3277886. [DOI] [PubMed] [Google Scholar]
- Walter E. G., Taylor D. E. Plasmid-mediated resistance to tellurite: expressed and cryptic. Plasmid. 1992 Jan;27(1):52–64. doi: 10.1016/0147-619x(92)90006-v. [DOI] [PubMed] [Google Scholar]
- Wang Y., Moore M., Levinson H. S., Silver S., Walsh C., Mahler I. Nucleotide sequence of a chromosomal mercury resistance determinant from a Bacillus sp. with broad-spectrum mercury resistance. J Bacteriol. 1989 Jan;171(1):83–92. doi: 10.1128/jb.171.1.83-92.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
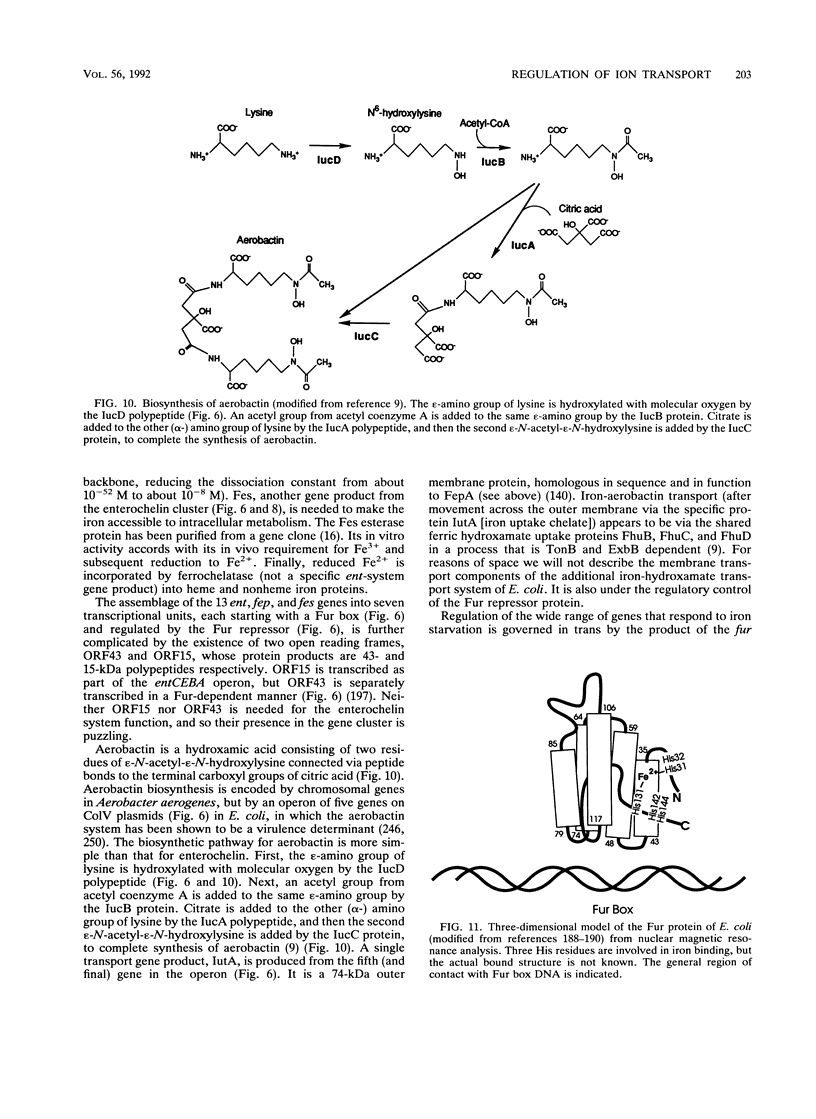
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S., Neilands J. B., Bittner M. L., Hemming B. C., Haymore B. L., Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. S., Batut J., Klose K. E., Keener J., Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991 Oct 4;67(1):155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram L., Eitinger T., Friedrich B. Construction and properties of a triprotein containing the high-affinity nickel transporter of Alcaligenes eutrophus. FEBS Lett. 1991 May 20;283(1):109–112. doi: 10.1016/0014-5793(91)80565-k. [DOI] [PubMed] [Google Scholar]
- Wu J., Rosen B. P. The ArsR protein is a trans-acting regulatory protein. Mol Microbiol. 1991 Jun;5(6):1331–1336. doi: 10.1111/j.1365-2958.1991.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Yamada M., Makino K., Shinagawa H., Nakata A. Regulation of the phosphate regulon of Escherichia coli: properties of phoR deletion mutants and subcellular localization of PhoR protein. Mol Gen Genet. 1990 Feb;220(3):366–372. doi: 10.1007/BF00391740. [DOI] [PubMed] [Google Scholar]
- Yoon K. P., Misra T. K., Silver S. Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1991 Dec;173(23):7643–7649. doi: 10.1128/jb.173.23.7643-7649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. P., Silver S. A second gene in the Staphylococcus aureus cadA cadmium resistance determinant of plasmid pI258. J Bacteriol. 1991 Dec;173(23):7636–7642. doi: 10.1128/jb.173.23.7636-7642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


