Abstract
Baclofen is a GABAB receptor agonist commonly used to relief spasticity related to motor disorders. The effects of baclofen on voluntary motor output are limited and not yet understood. Using noninvasive transcranial magnetic and electrical stimulation techniques, we examined electrophysiological measures probably involving GABAB (long-interval intracortical inhibition and the cortical silent period) and GABAA (short-interval intracortical inhibition) receptors, which are inhibitory effects mediated by subcortical and cortical mechanisms. We demonstrate increased active long-interval intracortical inhibition and prolonged cortical silent period during voluntary activity of an intrinsic finger muscle in humans with chronic incomplete cervical spinal cord injury (SCI) compared with age-matched controls, whereas resting long-interval intracortical inhibition was unchanged. However, long-term (∼6 years) use of baclofen decreased active long-interval intracortical inhibition to similar levels as controls but did not affect the duration of the cortical silent period. We found a correlation between signs of spasticity and long-interval intracortical inhibition in patients with SCI. Short-interval intracortical inhibition was decreased during voluntary contraction compared with rest but there was no effect of SCI or baclofen use. Together, these results demonstrate that baclofen selectively maintains use-dependent modulation of largely subcortical but not cortical GABAB neuronal pathways after human SCI. Thus, cortical GABAB circuits may be less sensitive to baclofen than spinal GABAB circuits. This may contribute to the limited effects of baclofen on voluntary motor output in subjects with motor disorders affected by spasticity.
Introduction
Baclofen is a GABAB receptor agonist commonly used to reduce the symptoms of spasticity after spinal cord injury (SCI) and other motor disorders (Aydin et al., 2005; Roy and Edgerton, 2012). Its effects on synaptic transmission have been attributed to decreasing neurotransmitter release from primary afferent terminals (Curtis et al., 1997) and to increasing the sodium current to a larger extent than reducing the calcium inflow by postsynaptic effects in motoneurons (Li et al., 2004).
Studies in humans with SCI have shown a decrease in cortical (Shimizu et al., 2000; Saturno et al., 2008; Roy et al., 2011) and subcortical (Calancie et al., 1993; Faist et al., 1994; Aymard et al., 2000) GABAergic inhibition, which may be part of a compensatory effect to the loss of descending and ascending motor and sensory pathways. Although these studies were done in a resting condition, it is thought that baclofen reduces the symptoms of spasticity by increasing GABAergic inhibition (Orsnes et al., 2000; Kumru and Kofler, 2012). Baclofen decreases the synaptic effectiveness of afferent fibers (Jiménez et al., 1991; Quevedo et al., 1992) and has inhibitory or excitatory effects in motoneurons depending on the dose (Li et al., 2004).
The effects of long-term use of baclofen on transmission in specific GABAergic neuronal circuits during voluntary activity remain unknown. In uninjured individuals, transmission in cortical and subcortical inhibitory pathways mediated by GABAB receptors during voluntary activity contributes to modulate excitability of corticospinal and spinal motoneurons involved in the intended movement (Pierrot-Deseilligny and Burke, 2005; Reis et al., 2008). GABAB receptors are abundantly present in the cerebral cortex and dorsal horn of the spinal cord (Price et al., 1984, 1987; Misgeld et al., 1995; Yang et al., 2001); therefore, baclofen may affect GABAB inhibition in both cortex and spinal cord. Some lines of evidence suggest, however, a more selective effect of baclofen on subcortical pathways. In animals, administration of baclofen affect to a lesser degree synaptic efficacy of descending motor pathways compared with sensory afferent fibers (Jiménez et al., 1991; Quevedo et al., 1992). In humans, the effects of baclofen on spasticity are stronger after intrathecal compared with oral administration (Penn et al., 1989; Azouvi et al., 1996) in patients with complete and partial SCI (Burke et al., 1971) with limited effects in voluntary motor function (Burke et al., 1971; Latash et al., 1989; Domingo et al., 2012). Thus, we hypothesized that long-term use of baclofen in patients with SCI will maintain transmission in subcortical but not cortical GABAB neuronal circuits during voluntary activity compared with uninjured controls. We also predicted that the effects of baclofen will be specific neuronal pathways mediated by GABAB receptors.
To test our hypothesis, we used transcranial magnetic and electrical stimulation to examine excitability in cortical and subcortical electrophysiological pathways probably mediated by GABAB and GABAA receptors. Baclofen effects on clonus present during voluntary activity and muscle spasms were measured. We demonstrate that SCI results in increased subcortical and cortical GABAB inhibition during voluntary activity compared with uninjured controls, whereas long-term (∼6 years) use of baclofen maintains use-dependent modulation of subcortical but not cortical GABAB neuronal pathways.
Materials and Methods
Subjects.
Sixteen patients with cervical SCI and 18 age-matched right-handed controls (SCI: mean age = 46.8 ± 12.8 years, 2 female; Table 1; controls: mean age = 39.4 ± 15.4yr, 9 female; p = 0.14) participated in the study. All subjects gave informed consent to experimental procedures, which were approved by the local ethics committee at the University of Pittsburgh. Patients had a chronic (≥1 year), cervical (C4–C8) injury with remaining sensory innervation of the C6 dermatome for light touch and pin-prick tests using the American Spinal Injury Association (ASIA) classification. Fourteen patients had a traumatic injury whereas two had a degenerative disease (Patients 13 and 16; Table 1). Three patients were classified as ASIA A due to lack of sacral sparing (Marino et al., 2003) but were able to perform voluntary contraction with their index finger, and 13 were classified as ASIA C or D. Eight patients took baclofen [SCI (Baclofen)] as part of their daily drug therapy for 6.5 ± 5.4 years (Table 1, Baclofen dose) and eight never took baclofen since their diagnosis [SCI (No-Baclofen)]. All patients were able to exert an isometric maximal voluntary contraction (MVC) by moving their index finger into abduction against resistance. Electromyographic (EMG) activity exerted during MVCs was larger in controls than in patients [controls = 646.4 ± 33.6 μV, SCI (Baclofen) = 300.5 ± 21.9 μV, SCI (No-Baclofen) = 398 ± 22.7 μV, F = 4.6, p = 0.01]. Thus, testing was completed by matching voluntary activity as a percentage of MVC across groups.
Table 1.
SCI participant demographics
| Pt | Level | Age, years | Gender | Aetiology | Injury, years | ASIA | Motor score (FDI,/5) | Light touch (12) | Pin-prick (12) | Baclofen dose (mg/d) | Years taking baclofen | Spasm frequency score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI (Baclofen) | 1 | C7 | 51 | M | T | 11 | C | 5/5 | 2 | 2 | 60 | 2 | 3 |
| 2 | C6 | 30 | M | T | 6 | A | 5/5 | 1 | 1 | 60 | 6 | 4 | |
| 3 | C7 | 57 | F | T | 13 | D | 3/5 | 2 | 2 | 120 | 13 | 2 | |
| 4 | C5/6 | 39 | M | T | 10 | C | 1/5 | 1 | 1 | 500* | 10 | 3 | |
| 6 | C4 | 59 | M | T | 7 | D | 3/5 | 1 | 2 | 160 | 15 | 4 | |
| 7 | C7 | 51 | M | T | 1 | D | 4/5 | 2 | 2 | 80 | 2.5 | 4 | |
| 8 | C5 | 38 | M | T | 1 | D | 4/5 | 2 | 1 | 120 | 1 | 2 | |
| SCI (No-Baclofen) | 9 | C7 | 41 | F | T | 20 | A | 3/5 | 1 | 1 | — | — | 4 |
| 10 | C4 | 58 | M | T | 3 | D | 5/5 | 2 | 2 | — | — | 2 | |
| 11 | C5 | 50 | M | T | 1 | D | 5/5 | 2 | 2 | — | — | 1 | |
| 12 | C5/6 | 34 | M | T | 4 | D | 4/5 | 2 | 2 | — | — | 2 | |
| 13 | C6 | 59 | M | NT | 17 | D | 5/5 | 2 | 2 | — | — | 1 | |
| 14 | C7 | 45 | M | T | 12 | D | 5/5 | 2 | 2 | — | — | 3 | |
| 15 | C4 | 66 | M | T | 3 | D | 5/5 | 1 | 2 | — | — | 2 | |
| 16 | C4 | 64 | M | NT | 6 | D | 5/5 | 2 | 2 | — | — | 2 | |
*Milliquarts via surgically implanted baclofen pump.
M, Male; F, female; T, traumatic; NT, non traumatic; Light touch and pinprick: 1 = impaired, 2 = intact; Spasm frequency score: 0 = no spasms, 1 = one or fewer spasms per day, 2 = between 1 and 5 spasms per day, 3 = 5 to <10 spasms per day, and 4 = 10 or more spasms per day.
EMG recordings.
EMG was recorded from the first dorsal interosseous muscle (FDI) of the right side in controls and from the less affected hand in participants with SCI through surface electrodes (Ag–AgCl, 10 mm diameter) arranged in a monopolar configuration. One electrode was secured to the skin over the belly of the FDI with a reference electrode positioned over the proximal interphalangeal joint of the index finger. The signals were amplified (Neurolog System, NL844, NL820, Digitimer), filtered (30–1000 Hz, Neurolog System NL844, NL136, Digitimer), and sampled at 2 kHz for off-line analysis using Signal 4.09 software (CED 1401, Cambridge Electronic Design).
Experimental paradigm.
Subjects were seated in chair with the tested arm flexed at the elbow at 90°, forearm pronated, wrist restrained by straps, and with the index finger resting against a custom lever (Fig. 1A). At the start of the experiment participants performed three brief MVCs for 3–5 s into index finger abduction, separated by 30 s. The maximal forces were used to set targets for subsequent submaximal contractions. Testing was completed at rest and when individuals performed 25% of MVC into index finger abduction. During voluntary contraction, integrated EMG signal (Neurolog System, NL703, Digitimer) was displayed continuously on an oscilloscope and verbal feedback was provided to the subjects (Fig. 1A, top traces) to assure that physiological measurements in the FDI were acquired during the same level of background EMG activity at all times. A familiarization trial was completed at the beginning of each experiment to ensure that subjects were able to complete the task. A total of 5.0 ± 5.2% trials in which the mean rectified EMG was >2 SD of the mean resting EMG, measured 200 ms before the stimulus artifact, were excluded from further analysis (Bunday and Perez, 2012).
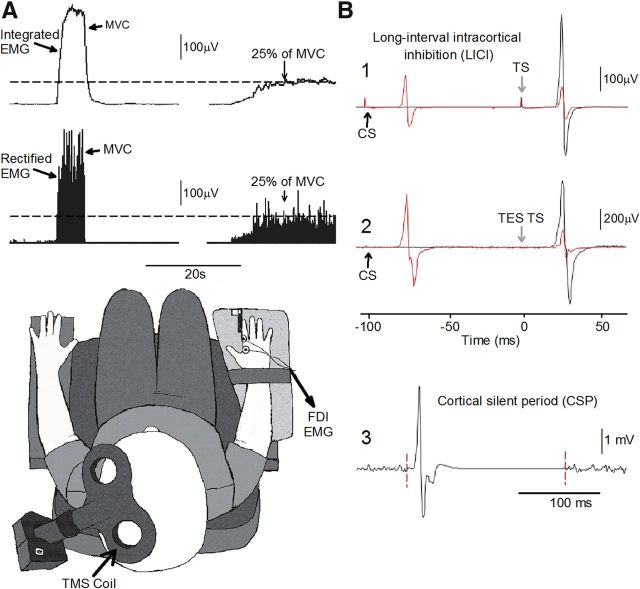
Figure 1.
Experimental setup. A, Raw EMG traces showing (top left traces) with the index finger into abduction by activating the FDI muscle and the visual display presented to all subjects (top right traces) during testing. Subjects were instructed by an oscilloscope to maintain at rest and to perform 25% of MVC with the index finger into abduction. Schematic of the experimental setup showing the posture of both hands and TMS coil during testing (illustration). Note that control subjects completed the test with the right dominant hand and patients with SCI used their less affected hand. B, Raw MEP traces elicited by TMS and TES stimulation recorded from the FDI muscle in a representative subject during all conditions tested. MEPs elicited by the TS (black traces) and CS (red traces) are indicated by arrows during testing of LICI using TMS (B1) and TES (B2). Note that during testing of LICI the CS was given 100 ms before the TS (B1, B2). An example of the CSP (B3) elicited by using TMS during 25% of MVC is presented. The CSP was measured between the stimulus artifact (left dotted line) and the return of background EMG (right dotted line).
Transcranial magnetic stimulation.
Transcranial magnetic stimuli were delivered from a Magstim 200 stimulator (Magstim) through a figure-eight coil (loop diameter, 7 cm; type no. 16342) with a monophasic current waveform. Transcranial magnetic stimulation (TMS) was delivered to the optimal scalp position for activation of the left or right FDI muscle. The scalp position for FDI was determined with the coil held tangential to the scalp and the handle pointing backward and 45° away from the midline. With this coil position the induced current flowed in a posterior-medial direction and probably produced D and early I wave activation of corticospinal neurons (Sakai et al., 1997). During testing, the TMS coil was held to the head of the subject with a custom coil holder with the head held with straps against a headrest to restrict movements. TMS measurements included resting motor threshold (RMT) and active motor threshold (AMT), maximal motor-evoked potential (MEP-max) size, long-interval intracortical inhibition (LICI), cortical silent period (CSP), and short-interval intracortical inhibition (SICI).
MEPs.
RMT [controls = 49.3 ± 8.0%, SCI (Baclofen) = 68 ± 21.8%, SCI (No-Baclofen) = 55.1 ± 7.7%, F = 6.6, p < 0.01] was defined as the minimal stimulus intensity required to induce MEPs >50 μV peak-to-peak amplitude in 5 of 10 consecutive trials in the relaxed FDI muscle and AMT [controls = 40.3 ± 5.6%, SCI (Baclofen) = 56.5 ± 18.0%, SCI (No-Baclofen) = 56.9 ± 22.3%, F = 5.1, p < 0.01] was defined as the minimal stimulus intensity able to evoke MEPs >200 μV peak-to-peak amplitude in at least 5 of 10 consecutive trials during 25% of MVCs with the FDI muscle (Rothwell et al., 1999). The MEP-max was defined in all participants at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increase. The MEP-max was different across groups [controls = 4.2 ± 2.5 mV, SCI (Baclofen) = 2.1 ± 2.5 mV, SCI (No-Baclofen) = 1.8 ± 2.1 μV, F = 3.8, p = 0.03]. Post hoc testing showed no differences in RMT (p = 0.2), AMT (p = 0.9), and MEP-max (p = 0.7) between patient groups.
LICI.
LICI was tested using previously described methods (Valls-Solé et al., 1992; Wassermann et al., 1996). A conditioning stimulus (CS) was delivered by TMS at an intensity that elicited ∼40% of inhibition at rest in all groups [controls = 59.6 ± 11.3% of maximal stimulator output (MSO), n = 18; SCI (Baclofen) = 78.9 ± 20% of MSO, n = 8; SCI (No-Baclofen) = 72.2 ± 10.6% of MSO, n = 8; F = 6.2, p < 0.01]. Post hoc testing showed no differences in the CS intensity between patient groups (p = 0.7). The test stimulus (TS) was elicited by using TMS and set at an intensity to produce an MEP of ∼50% of the MEP-max at rest [controls = 63.3 ± 8.4% of MSO, n = 18; SCI (Baclofen) = 83.0 ± 17.2% of MSO, n = 8; SCI (No-Baclofen) = 72.5 ± 10.4% of MSO, n = 8; F = 8.5, p = 0.001]. Post hoc testing showed no differences in the TS intensity between patient groups (p = 0.08). The same CS and TS intensity was used at rest and during 25% of MVC. The CS was delivered 100 ms before the TS (Fig. 1B1). The CS and TS intensity was different across groups; therefore, LICI was also tested in controls by adjusting the intensity of the CS and TS to match the intensity used in patients. Because the size of the MEP elicited by the CS and the TS increased when testing was completed during 25% of MVC, LICI was also tested by adjusting the size of the test MEP and the MEP elicited by the CS in all groups (condition referred as to 25% of MVCADJ; Table 2). LICI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP [(conditioned MEP × 100)/(test MEP)]. Twenty test MEPs and 20 conditioned MEPs were measured in each condition. Measurements were repeated two to three times on each condition and averaged.
Table 2.
LICI: MEP amplitudes
| Control | SCI (Baclofen) | SCI (No-Baclofen) | p values | |
|---|---|---|---|---|
| LICI (CS and TS elicited by TMS) |
||||
| Rest | ||||
| CS (mV) | 0.5 ± 0.5 | 0.2 ± 0.4 | 0.3 ± 0.3 | p = 0.35 |
| TS (mV) | 1.3 ± 1.1 | 0.9 ± 1.8 | 0.6 ± 0.8 | p = 0.37 |
| 25% of MVC | ||||
| CS (mV) | 2.6 ± 2.3 | 0.6 ± 0.8 | 0.8 ± 1.1 | p = 0.02 |
| TS (mV) | 4.3 ± 2.9 | 1.5 ± 2.3 | 2.4 ± 2.0 | p = 0.04 |
| 25% of MVCADJ | ||||
| CS (mV) | 1.1 ± 0.9 | 0.7 ± 1.2 | 0.4 ± 0.5 | p = 0.62 |
| TS (mV) | 1.2 ± 0.9 | 1.1 ± 1.9 | 0.7 ± 0.7 | p = 0.21 |
| LICI (CS elicited by TMS and TS elicited by TES) |
||||
| Rest | ||||
| CS (mV) | 0.3 ± 0.3 | 0.4 ± 0.5 | 0.3 ± 0.4 | p = 0.14 |
| TS (mV) | 0.9 ± 0.6 | 0.9 ± 0.9 | 0.7 ± 0.6 | p = 0.71 |
| 25% of MVC | ||||
| CS (mV) TS (mV) | 3.3 ± 1.9 | 1.1 ± 0.6 | 1.4 ± 2.4 | p = 0.23 |
| TS (mV) | 5.9 ± 2.7 | 1.1 ± 0.8 | 2.5 ± 3.5 | p = 0.03 |
Mean (±SD) size of MEPs elicited by the TS and CS during testing of LICI using TMS and TES stimulation. FDI MEP size is reported at rest, 25% of MVC, and 25% of MVCADJ in all groups. p values represent ANOVA tests performed across groups on each condition. Note that size of the MEP elicited by the TS, using TMS, was used was similar at rest and in the 25% of MVCADJ condition across groups but increased during 25% of MVC. Also, note that that size of the MEP elicited by the TS using TES was increased during 25% of MVC compared to rest.
LICI was also tested using a CS elicited by TMS (at the same intensity described above) and a TS elicited by transcranial electrical stimulation (TES; Fig. 1B2; Table 2). The TS evoked by a high-voltage electrical current (200 μs duration, Digitimer DS7AH) that passed between 9 mm brass electrodes fixed to the scalp with electrode conductive gel. The cathode was located at the vertex and the anode 7 cm laterally (Rothwell, 1997). The stimulation intensity was set to elicit an MEP of 3–5% of the maximal motor response (M-max) at rest tested by supramaximal stimulation of the ulnar nerve at the wrist and recorded in the FDI muscle [controls = 248.3 ± 158.1 mA, n = 10; SCI (Baclofen) = 306.7 ± 45.1 mA, n = 3; SCI (No-Baclofen) = 264.5 ± 45.1 mA, n = 3]. The latency of MEPs elicited by TMS and TES were different in all groups [controls: TMS = 22.3 ± 1.9 ms, TES = 20.2 ± 1.8 ms, p < 0.01; SCI (Baclofen): TMS = 26.8 ± 1.6 ms, TES = 25.1 ± 1.8, p < 0.01; SCI (No-Baclofen): TMS = 27.1 ± 2.2 ms, TES = 25.3 ± 2.4, p = 0.04] indicating that TES activated corticospinal axons bypassing the motor cortex. LICI was calculated using the same formula described above. Ten test MEPs and 10 conditioned MEPs were measured in each condition. Measurements were repeated two times at rest and during 25% of MVC.
CSP.
We measured the duration of the CSP during 25% of MVC, whereas the size of MEPs elicited by TMS was maintained similar across groups [p = 0.26; controls, n = 18; SCI (Baclofen), n = 8; SCI (No-Baclofen), n = 8]. The duration of the CSP was measured by calculating the mean amplitude of the rectified EMG activity >100 ms prior the TMS stimulus artifact and by detecting when the EMG returned to 50% of prestimulus values from the stimulus artifact for a period of 10 s (Butler et al., 2012; Fig. 1B3). In addition, the CSP was measured using a custom script to determine the mean rectified EMG activity averaged 100 ms before the artifact and the end of the silent period when the mean rectified EMG activity was 2 SD of the baseline. These measurements were confirmed by visual inspection. The same result in all groups was found when the data were analyzed with either criterion. Twenty MEPs were tested in each group. The CSP was also measured after MEPs elicited by TES during 25% of MVC [controls, n = 10; SCI (Baclofen), n = 3; SCI (No-Baclofen), n = 3]. Ten MEPs were tested in each group.
SICI.
SICI was tested using a previously described method (Kujirai et al., 1993). A CS was delivered by TMS at subthreshold intensity that elicited ∼40% of inhibition at rest in all groups [controls = 34.5 ± 4.5, n = 10; SCI (Baclofen) = 44.6 ± 11.9%, n = 6; SCI (No-Baclofen) = 43.2 ± 14.0, n = 6; F = 2.5, p = 0.11]. The TS intensity was adjusted to produce an MEP of ∼50% of the MEP-max [controls = 67.1 ± 7.5, n = 10; SCI (Baclofen) = 81.3 ± 19.6%, n = 6; SCI (No-Baclofen) = 68.7 ± 17.8, n = 6; F = 1.9, p = 0.17]. The same CS and TS intensity was used at rest and during 25% of MVC. The CS was delivered 2.5 ms before the TS. Because the intensity used for the CS and TS was similar across groups, no control studies were conducted. Because MEP size increased during voluntary contraction SICI was also tested by adjusting the size of the test MEP to match the MEP amplitude produced during rest (condition referred to as 25% of MVCADJ). SICI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP [(conditioned MEP × 100)/(test MEP)]. Twenty test MEPs and 20 conditioned MEPs were tested in each condition. Measurements were repeated two to three times on each condition and averaged.
Clonus EMG analysis.
Clonus EMG activity has been reported during voluntary activity in patients with SCI (Beres-Jones et al., 2003; Wallace et al., 2012). We detected clonus in the FDI muscle in some of the trials during voluntary contraction in five of eight patients with SCI not taking baclofen and in one of eight patients taking baclofen. Using a custom script EMG burst duration, duration of EMG period between bursts or interburst (a period of a decrease or a relative silence following the burst of EMG), mean rectified EMG activity during the interburst, and burst frequency were measured in individual trials. The custom script rectified and smoothed EMG data in the FDI muscle using a time constant of 8 ms. EMG data were analyzed for 1.5 s after the CSP in individual frames. Within this period, the burst onset was defined as the time when the mean rectified EMG reached a value of 2 SD above the mean rectified EMG for at least 25 ms. The burst offset was defined as the time when EMG activity remained below these values at least 25 ms. Markers representing the burst duration were created within a memory buffer channel. The interburst duration was calculated from the end of one burst to the start of the next consecutive burst. Mean burst frequency was calculated by counting the number of burst in each frame from the beginning of the first burst and the end of the last burst detects during the 1.5 s period of EMG analysis. Based on previous criteria (Beres-Jones et al., 2003; Wallace et al., 2012), we defined clonic EMG to be at least two consecutive EMG bursts (duration from 25 to 130 ms) with a silent period between them (duration from 25 to 280 ms). Measurements were confirmed by visual inspection.
Data analysis.
Normal distribution was tested using the Shapiro–Wilk's test and homogeneity of variances using the Levene median test. Repeated-measures ANOVAs were performed to determine the effect of group [controls, SCI (Baclofen), and SCI (No-Baclofen)] and conditions [rest, 25% of MVC, and 25% of MVCADJ] on LICI, CSP, SICI, and the size of the MEP elicited by the CS and TS (during LICI measurements), and the size of the MEP during CSP measurements. The same analysis was completed to compare RMT, AMT, MSO intensities for CS and TS, MEP latencies, MEP-max, mean rectified EMG, and MVCs across groups. Post hoc Holm–Sidak test was used to test for significant comparisons. Unpaired t tests were used to compare the MEP amplitudes across groups, and paired t tests were used to compare LICI between rest and 25% of MVC during the intensity control experiments, and LICI tested by TES. Pearson correlation analysis was used as needed. Significance was set at p < 0.05. Group data are presented as the means ± SD in the text.
Results
LICI
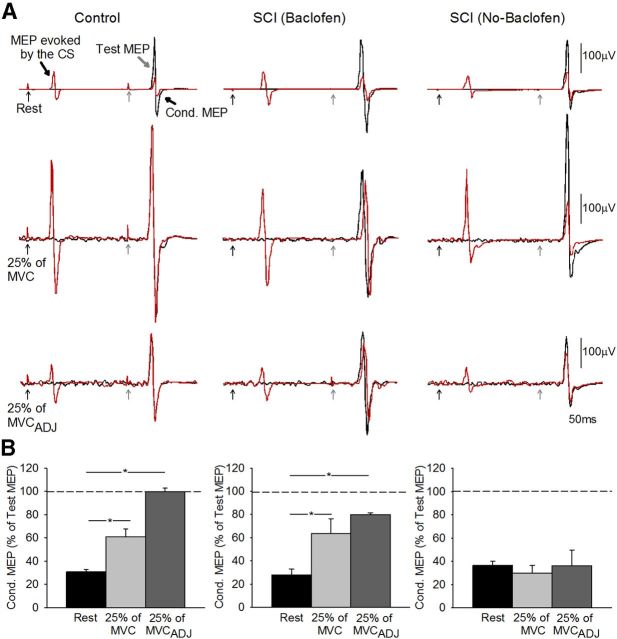
Figure 2A illustrates raw data from LICI measured in the FDI muscle in a control subject (left) and in a patient with SCI taking (middle) and not taking baclofen (right). Note that LICI decrease during voluntary contraction (25% of MVC and 25%MVCADJ) compared with rest in the control subject and in the SCI patient taking baclofen but not in the patient that never took baclofen.
Figure 2.
LICI using TMS. A, LICI tested in the resting FDI in a representative control subject (control, top left traces) and in a patient with SCI taking [SCI (Baclofen), top middle traces] and not taking [SCI (No-Baclofen), top right traces] baclofen when the conditioning and test stimulus were given by TMS. The test MEP (black traces) and conditioned MEP (Cond MEP, red traces) are indicated by black arrows. Traces show the average 20 test MEP and 20 Cond. MEP. B, Group data (controls, n = 18, bottom left; SCI Baclofen, n = 8, bottom left; SCI No-Baclofen, n = 8, bottom right). The abscissa shows all conditions tested (rest, black bars; 25% of MVC, light gray bars; 25% of MVCADJ, dark gray bars). The ordinate shows the magnitude of the conditioned MEP expressed as a percentage of the test MEP. The horizontal dashed line represents the size of the test MEP. Note that LICI decreased during index finger abduction compared with rest in controls and in patients taking baclofen but remains unchanged during voluntary contraction and rest in patients not taking baclofen. Error bars indicate SEs; *p < 0.05.
Repeated-measures ANOVA showed a significant effect of group (F = 7.29, p < 0.01), conditions (F = 28.5, p < 0.001), and in their interaction (F = 6.1, p < 0.001) on LICI. Post hoc testing showed that LICI was decreased during 25% of MVC compared with rest in controls (rest = 30.6 ± 9.2% and 25% of MVC = 61.0 ± 27.3%, p < 0.001; Fig. 2B, left) and in patients taking baclofen (rest = 27.9 ± 14.1% and 25% of MVC = 63.3 ± 36.4%, p < 0.001; Fig. 2B, middle) but not in those not taking baclofen (rest = 36.3 ± 10.3% and 25% of MVC = 29.7 ± 18.6%, p = 0.52; Fig. 2B, right). During voluntary contraction mean background rectified EMG activity in the FDI remained similar across conditions (p = 0.10) and groups (p = 0.32).
Because MEP size increased during voluntary contraction in all groups (p < 0.001) LICI was also tested by adjusting the size of the MEPs evoked by the TS during 25% of MVC to match resting values (25% of MVCADJ). Similar to our previous results, during 25% of MVCADJ LICI was decreased compared with rest in controls (p < 0.001) and in patients taking baclofen (p < 0.001) but not in those patients not taking baclofen (p = 0.42). When the CS and TS intensity were increased in controls to match the intensity used in patients we found that LICI was decreased during 25% of MVC (61.7 ± 37.9%) compared with rest (29.6 ± 20.6%, p < 0.03). When LICI was tested by comparing the conditioned MEP during voluntary activity to the test MEP elicited at rest, we found that LICI was decreased to a similar extent in all groups [controls = 207.1 ± 102.6; SCI (Baclofen) = 181.1 ± 134.3%; SCI (No-Baclofen) = 160.9 ± 151.3, F = 0.4, p = 0.6].
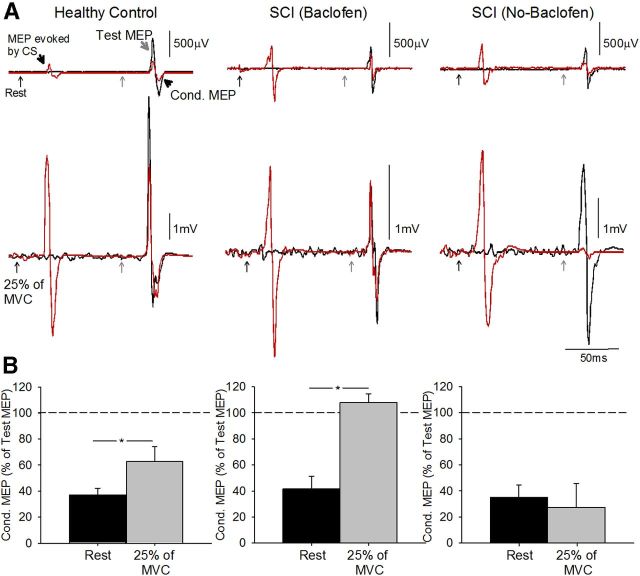
Figure 3A illustrates examples of LICI tested using TES for the TS across conditions in representative subjects. Note that LICI was decreased during 25% of MVC in the control subject and in the patient taking baclofen but not in the participant not taking baclofen. Similarly, in all subjects, LICI was decreased during 25% of MVC compared with rest in the control group (rest = 39.2 ± 15.6% and 25% of MVC = 66.3 ± 32.1%, p = 0.02) and in patients taking baclofen (rest = 41.2 ± 24.4% and 25% of MVC = 107.8 ± 18.1%, p = 0.01). In contrast, LICI remained the same in both conditions in patients not taking baclofen (rest = 40.2 ± 16.3% and 25% of MVC = 26.5 ± 30.8%, p = 0.57). Mean background rectified EMG activity in the FDI remained similar across groups (p = 0.23). Overall, together these results together show that the magnitude of LICI was decreased during voluntary activity compared with rest in controls and patients taking baclofen but not patients who never took baclofen regardless if LICI was tested using a TS elicited by TMS or TES.
Figure 3.
LICI using TES. A, LICI tested in the resting FDI in representative subjects when the conditioning stimulus was given by TMS and test stimulus was given by TES [control, top left traces; SCI (Baclofen), top middle traces; SCI (No-Baclofen), top right traces]. The test MEP (black traces) and conditioned MEP (red traces) are indicated by black arrows. Traces show the average 10 test MEP and 10 Cond. MEP. B, Group data (controls, n = 10, bottom left; SCI Baclofen, n = 3, bottom left; SCI No-Baclofen, n = 3, bottom right. The abscissa shows all conditions tested (rest, black bars; 25% of MVC, light gray bars). The ordinate shows the magnitude of the conditioned MEP expressed as a percentage of the test MEP. The horizontal dashed line represents the size of the test MEP. Note that LICI decreased during index finger abduction compared with rest in controls and in patients taking baclofen but remains unchanged in participant's not taking baclofen. Error bars indicate SEs; *p < 0.05.
CSP
Figure 4 illustrates examples of the CSP elicited by TMS and TES during 25% of MVC in representative participants. Note that the duration of the CSP was increased in patients compared with a control subject when the CSP was elicited by TMS but not by TES. Repeated-measures ANOVA showed a significant effect of group (F = 7.3, p < 0.01) on the duration of the CSP. Post hoc testing showed that the CSP duration was increased during 25% of MVC in patients taking (213.5 ± 50.1 ms, p < 0.01) and not taking baclofen (224.3 ± 55.7 ms, p = 0.01) compared with controls (169.3 ± 19.1 ms). No differences were observed between patient groups (p = 0.3). The duration of the CSP elicited by TES was similar across groups [controls = 141.1 ± 33.5 ms; SCI (Baclofen) = 135.2 ± 51.9 ms; SCI (No-Baclofen) = 142.8 ± 44.8 ms, p = 0.9]. Overall, these results show that the duration of the CSP tested by TMS was longer in patients that controls, regardless of their intake of baclofen, but the duration of CSP tested by TES was similar across groups.
Figure 4.
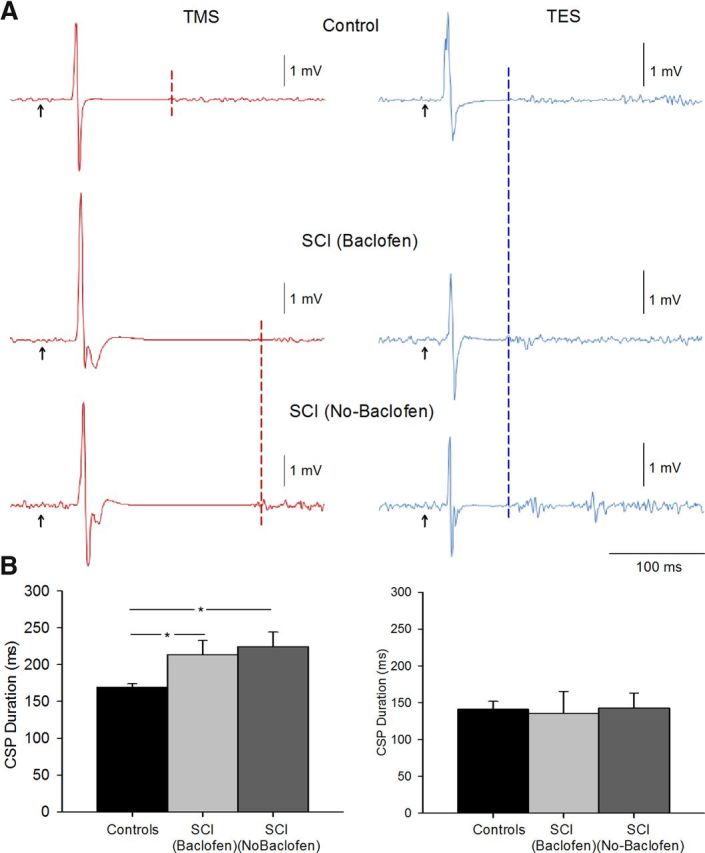
CSP. A, Raw MEP traces in representative subjects showing the CSP duration (indicated by dashed lines) after the MEP during 25% of MVC [control, top traces; SCI (Baclofen), middle traces; SCI (No-Baclofen), bottom traces] baclofen. Traces show the average 20 MEPs tested by TMS and 10 MEPs tested by TES. B, Group data tested by TMS [controls, n = 18, black bar; SCI (Baclofen), n = 8, light gray bar; SCI (No-Baclofen), n = 8, dark gray bar], and TES [controls, n = 10, black bar; SCI (Baclofen), n = 3, light gray bar; SCI (No-Baclofen), n = 3, dark gray bar]. The abscissa shows all groups tested and the ordinate shows the duration of the CSP. The duration of the CSP elicited by TMS was longer in patients compared with controls. Note that the duration of the CSP elicited by TES during voluntary contraction was similar across groups. Error bars indicate SEs; *p < 0.05.
SICI
Figure 5 illustrates representative examples of SICI measured in the FDI muscle across conditions tested. Note that SICI was decreased during voluntary contraction compared with rest in all subjects. Repeated-measures ANOVA showed a significant effect of conditions (F = 56.8, p < 0.001), but not group (F = 0.9, p = 0.43) nor in their interaction (F = 1.8, p = 0.15) on SICI. Post hoc testing showed that SICI was decreased during 25% of MVC compared with rest in all groups [controls: rest = 37.8 ± 10.9%, 25% of MVC = 91.6 ± 10.1%, p < 0.01; SCI (Baclofen): rest = 45.2 ± 19.0%, 25% of MVC = 78.4 ± 5.1%, p < 0.001; SCI (No-Baclofen): rest = 31.1 ± 10.7%, 25% of MVC = 69.6 ± 22.1%, p < 0.001]. Similarly, SICI was decreased during 25% of MVCADJ compared with rest in controls (p < 0.001) and in patients taking (p < 0.001) and not taking (p < 0.001) baclofen. Mean background rectified EMG activity in the FDI remained similar across voluntary contractions (p = 0.21) and groups (p = 0.78).
Figure 5.
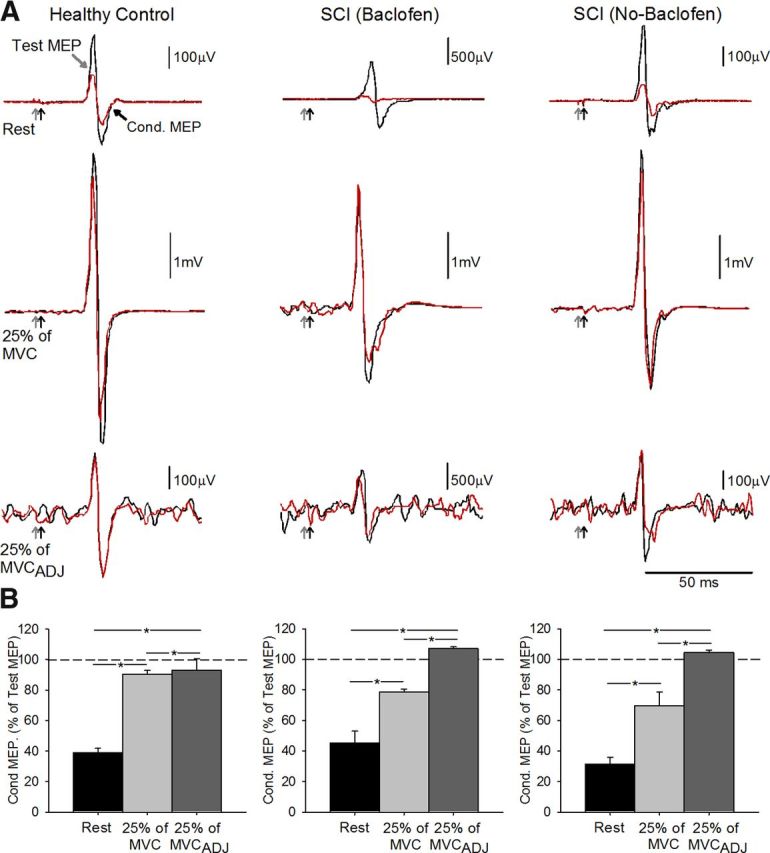
SICI. A, SICI recorded from the resting FDI in a representative control subject (top left traces) and in a patient taking (top middle traces) and not taking (top right traces) baclofen. The test MEP (black traces) and conditioned MEP (red traces) are indicated by black arrows. Traces show the average 20 test MEP and 20 Cond. MEP. B, Group data [controls, n = 10, bottom left; SCI Baclofen, n = 6, bottom left; SCI No-Baclofen, n = 6, bottom right]. The abscissa shows all conditions tested (rest, black bars; 25% of MVC, light gray bars; 25% of MVCADJ, dark gray bars). The ordinate shows the magnitude of the conditioned MEP expressed as a percentage of the test MEP. The horizontal dashed line represents the size of the test MEP. Note that SICI decreased during index finger abduction compared with rest in all groups tested. Error bars indicate SEs; *p < 0.05.
Clonus EMG
Mean burst frequency in the FDI muscle was 6.9 ± 1.0 Hz (range, 5.7–8.8 Hz) in patients not taking baclofen and 7.3 ± 0.6 Hz (range, 6.3–8.6 Hz) in the patient taking baclofen. The burst duration was 49.6 ± 20.8 ms (range, 31.1–113.3 ms) and interburst duration was 103.0 ± 23.7 ms (range, 63.0–138.6 ms) in patients not taking baclofen. Similarly, burst duration was 34.7 ± 6.3 ms (range, 27.4–52.2 ms) and interburst duration was 103.4 ± 37.7 ms (range, 72.6–139.8 ms) in the patient taking baclofen. A negative correlation was found between interburst duration and the magnitude of changes (percentage change from rest to active) in LICI (r = −0.72, p < 0.01; Fig. 6B) but not the CSP (r = 0.09, p = 0.75; Fig. 6C) in patients not taking baclofen. The magnitude of changes (percentage change from rest to active) in LICI (r = −0.72, p < 0.01; Fig. 6B) was negatively correlated with the spasm score (r = −0.76, p = 0.02) in patients not taking baclofen.
Figure 6.
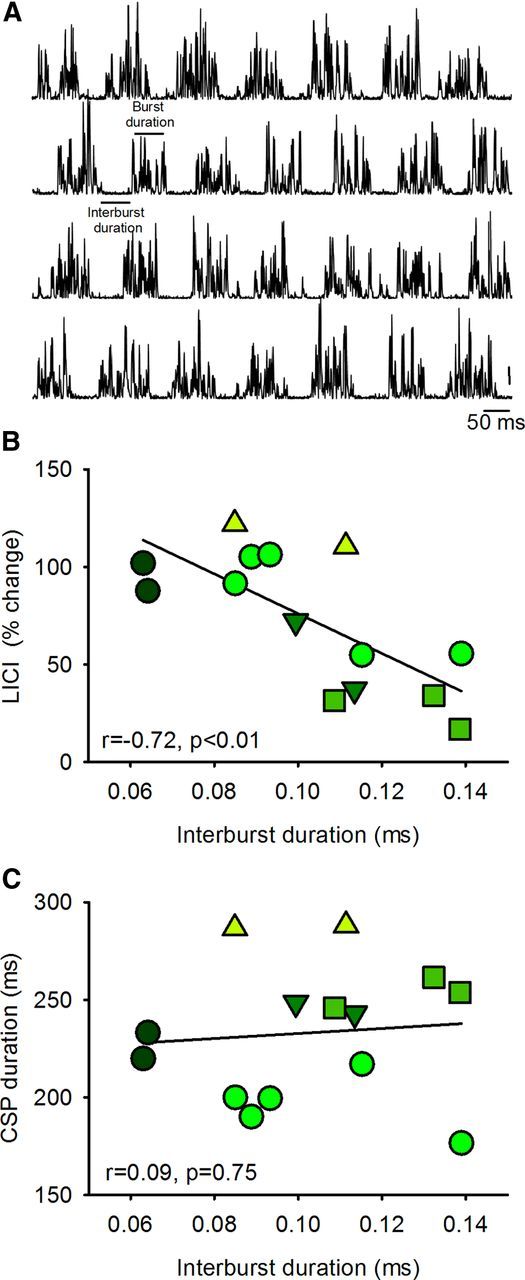
Clonus during voluntary contraction. A, Raw EMG traces recorded from the FDI muscle in a patient not taking baclofen during 25% of MVC. Traces show the rectified EMG in representative trials. Graphs show a correlation analysis between the average duration of periods of decreased EMG activity between bursts of clonus (interburst duration) and the magnitude of LICI (B), and CSP duration (C). In all graphs, the abscissa shows the duration of periods of decreased EMG activity between burst of clonus during 25% of MVC. The ordinate shows the magnitude of LICI (percentage change from rest to active) (B) and the CSP duration (C). Each symbol represents a different patient and repetition a symbol indicates multiple measurements in the same patient. Note that there was an inverse correlation between interburst duration and LICI but not the CSP. Thus, patients who showed more pronounced LICI during voluntary contraction also showed more prolonged periods of EMG silence during clonus in the FDI muscle.
Discussion
The present study investigated the effect of long-term use of baclofen on GABAB-mediated inhibition during voluntary activity after chronic incomplete SCI. We examined electrophysiological measures probably involving GABAB (LICI and CSP) and GABAA (SICI) receptors. These physiological inhibitory effects are mediated by subcortical and cortical mechanisms. We found that patients with SCI showed increased LICI during voluntary muscle contraction and prolonged CSP duration compared with uninjured controls. Long-term (∼6 years) use of baclofen maintained active LICI to similar levels as controls and did not affect the duration of the CSP. The interburst duration during clonus and the number of muscle spasms were inversely correlated with LICI, suggesting an association between signs of spasticity and GABAB-mediated inhibition. SICI was decreased during voluntary contraction compared with rest in all groups. Our results indicate that baclofen selectively maintains use-dependent modulation of largely subcortical but not cortical GABAB neuronal pathways after human SCI.
Baclofen maintains subcortical but not cortical GABAB inhibition after SCI
Despite the fact that GABAB receptors in the mammalian CNS are extensively distributed in the cerebral cortex and spinal cord dorsal horn (Price et al., 1984, 1987; Misgeld et al., 1995; Yang et al., 2001), our results indicate that baclofen selectively maintains modulation of largely subcortical but not cortical GABAB inhibition during voluntary activity after SCI. We demonstrate that LICI was decreased during small levels of isometric voluntary contraction compared with rest in controls (Wassermann et al., 1996; Hammond and Vallence, 2007; McNeil et al., 2011) and in patients taking baclofen but not in participants who never took baclofen. Recent studies showed that subcortical pathways are involved in LICI (McNeil et al., 2009, 2011) in addition to cortical mechanisms (Nakamura et al., 1997; Chen et al., 1999; Di Lazzaro et al., 2002). Our data are in agreement because we found that LICI was modulated to a similar extent when the TS was elicited by TMS or TES. Because TES activates axons of pyramidal tract cells in the subcortical white matter (Burke et al., 1993; Di Lazzaro et al., 1998) it is most likely that the changes we observed here involved subcortical influences. Pharmacological studies indicate a role of GABAB receptors in mediating LICI (Werhahn et al., 1999; McDonnell et al., 2006); therefore, it is possible that the prolonged use of baclofen contributed to modulate LICI in patients taking baclofen to similar level as controls. Baclofen can bind to presynaptic GABAB receptors leading to a decrease in the release of GABA by negative feedback (Deisz, 1999) altering GABAergic synaptic transmission according to physiological needs (Ohliger-Frerking et al., 2003). Importantly, LICI remained increased during voluntary activity in patients who never took baclofen. Our results are in line with animal studies showing that GABAergic inhibitory events occurring at the spinal cord level are increased after SCI (Tillakaratne et al., 2000; Diaz-Ruiz et al., 2007; Sadlaoud et al., 2010). Moreover, we found little evidence that motor cortical inhibition could have contributed to the increased LICI during voluntary contraction in these patients. First, the size of the conditioned MEP tested during LICI during voluntary activity was larger than the unconditioned MEP elicited at rest in all groups, suggesting that additional motor cortical elements that can be activated by TMS are facilitated. Second, we found that motor cortical SICI, a probably GABAA-mediated effect, decreases during voluntary activity in all groups. Although, LICI and SICI inhibitory effects are probably mediated by GABAergic connections involving GABAB and GABAA receptors the involvement of different receptor subtypes does not exclude the possibility that a common neuronal population mediate these inhibitory effects.
Studies in humans have shown that GABAergic inhibition is decreased after SCI (Calancie et al., 1993; Faist et al., 1994; Aymard et al., 2000). At first sight these results might seem in contradiction to our findings and raise the question of how GABAergic inhibition is affected after SCI. It is important to consider that previous studies tested measurements at rest, did not separate patients according to the use of baclofen, and tested another measurement of GABAergic inhibition by examining presynaptic inhibition of Ia afferents. Animal (Stuart and Redman, 1992) and human (Orsnes et al., 2000) studies on spasticity have shown that baclofen has no effect on classical presynaptic inhibition. Presynaptic inhibition of Ia afferents, accompanied by primary afferent depolarization, is caused by axo-axonal GABAA synapses and activation of GABAA receptors via GABAergic interneurons (Rudomin and Schmidt, 1999). At present, the precise role of GABAB receptors in mediating presynaptic inhibition is not clear. Thus, changes in GABAergic inhibition after injury need to be considered in a task-dependent context with attention to the type of GABA receptors involved and the medication take by patients.
We also found that CSP durations were similar in patients regardless of baclofen use and were longer than controls. The first part of the CSP may be mediated by spinal contributions, whereas the later part results from suppression of neural output by interneurons at the cortical level (Fuhr et al., 1991; Chen et al., 1999; Tergau et al., 1999). Our results show that the duration of the CSP tested with TES was similar across groups, suggesting that differences observed between patients and controls (when the CSP was tested by TMS) involve cortical mechanisms. This agrees with previous results showing that GABAergic inhibition tested during voluntary activity is increased in patients with SCI compared with controls (Freund et al., 2011; Bunday and Perez, 2012). GABAB receptors play a role in the inhibition tested during the CSP (Ziemann et al., 1996; Siebner et al., 1998); thus, our findings suggest that GABAB-mediated effects by cortical circuits are less sensitive to baclofen than GABAB-mediated subcortical circuits. This agrees with animal studies showing that baclofen affect synaptic efficacy of descending motor axons, including pyramidal neurons (Kato et al., 1978), to a lesser extent than sensory afferents (Jiménez et al., 1991; Quevedo et al., 1992). This may in part be related to the larger density of GABAB receptors found in terminals of afferent axons compared with the terminals of descending axons (Jiménez et al., 1991).
LICI and CSP measures can be influenced by stimulation parameters and the strength of voluntary contraction (Reis et al., 2008; McNeil et al., 2011). In our study, background EMG activity was maintained similar across groups and stimulation parameters were controlled; therefore, it is unlikely that these aspects contributed to our findings. Although in our study patients were not randomized due to the nature of the study, the groups showed similar sensory and motor scores, as well as maximal voluntary EMG outcomes, making it less likely that difference in these aspects affected our results. Taking these considerations together, our results indicate that the effects of long-term use of baclofen are largely mediated at subcortical rather than cortical levels; although the precise duration and dose of baclofen-use needed for these changes to occur remains to be tested.
Functional considerations
Approximately 70% of individuals with SCI develop symptoms of spasticity. As in previous reports we found clonus in patients with SCI during voluntary activity (Palmer et al., 1998; Beres-Jones et al., 2003; Wallace et al., 2012). It is not surprising that clonus was present only in one patient taking baclofen since this medication decreases these symptoms (Latash et al., 1989; Penn et al., 1989). The interburst duration during clonus, but not the frequency or burst duration, was inversely correlated with LICI but not the CSP, suggesting that this period of decreased EMG activity might by affected by increased inhibition in subcortical GABAB-mediated neuronal pathways. As patients with SCI with lesser muscles spams showed pronounced LICI, we speculate that the increase in LICI might represent a compensatory mechanism to attempt to decrease spastic symptoms during voluntary activity after SCI.
A critical question is if these electrophysiological changes present during voluntary activity may have an impact on voluntary motor function. Previous studies showed that baclofen has limited effects on voluntary motor output (Burke et al., 1971; Latash et al., 1989; Domingo et al., 2012), decreases contractile properties of motor units of partially paralyzed muscles (Thomas et al., 2010), and has side effects, such as drowsiness and drug tolerance (Rösche, 2002). We argue that the lack of effects of long-term use of baclofen on cortical GABAB inhibition might contribute to the limited effects of baclofen on voluntary motor outcomes. Indeed, in humans, intake of the GABAB receptor agonist baclofen in controls decreases long-term potentiation, such as motor cortical plasticity (McDonnell et al., 2007), motor learning processes (Willerslev-Olsen et al., 2011), and voluntary force (Hornby et al., 2004). This is consistent with the view that attenuation in GABAergic signaling contributes to increase recovery of motor function after SCI (Tillakaratne et al., 2002). Our results, as with others studies, raised some issues that may caution the use of baclofen as an antispastic medication in individuals with motor disorders; a combination of baclofen with other medications (D'Amico et al., 2013) or approaches challenging motor cortical circuits might increase the efficacy of baclofen in the control of spasticity and voluntary movements after SCI, highlighting the need for future research in this area.
Footnotes
This work was funded by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (Grant R01-NS-076589-1 to M.A.P.).
The authors declare no competing financial interests.
References
- Aydin G, Tomruk S, Keleş I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil. 2005;84:584–592. doi: 10.1097/01.phm.0000171173.86312.69. [DOI] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Pénicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996;77:35–39. doi: 10.1016/S0003-9993(96)90217-8. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res. 2003;149:222–236. doi: 10.1007/s00221-002-1349-5. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol. 2012;107:2901–2911. doi: 10.1152/jn.00850.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Andrews CJ, Knowles L. The action of a GABA derivative in human spasticity. J Neurol Sci. 1971;14:199–208. doi: 10.1016/0022-510X(71)90089-X. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Petersen NC, Herbert RD, Gandevia SC, Taylor JL. Origin of the low-level EMG during the silent period following transcranial magnetic stimulation. Clin Neurophysiol. 2012;123:1409–1414. doi: 10.1016/j.clinph.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation: evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Gynther BD, Lacey G, Beattie DT. Baclofen: reduction of presynaptic calcium influx in the cat spinal cord in vivo. Exp Brain Res. 1997;113:520–533. doi: 10.1007/PL00005604. [DOI] [PubMed] [Google Scholar]
- D'Amico JM, Li Y, Bennett DJ, Gorassini MA. Reduction of spinal sensory transmission by facilitation of 5HT1 receptors in non-injured and spinal cord injured humans. J Neurophysiol. 2013;109:1485–1493. doi: 10.1152/jn.00822.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA. GABA(B) receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/S0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Salgado-Ceballos H, Montes S, Maldonado V, Tristan L, Alcaraz-Zubeldia M, Ríos C. Acute alterations of glutamate, glutamine, GABA, and other amino acids after spinal cord contusion in rats. Neurochem Res. 2007;32:57–63. doi: 10.1007/s11064-006-9225-5. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998;109:397–401. doi: 10.1016/S0924-980X(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/S1388-2457(02)00264-X. [DOI] [PubMed] [Google Scholar]
- Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J Neurotrauma. 2012;29:865–879. doi: 10.1089/neu.2011.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics: differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Freund P, Rothwell J, Craggs M, Thompson AJ, Bestmann S. Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury. Eur J Neurosci. 2011;34:1839–1846. doi: 10.1111/j.1460-9568.2011.07895.x. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-L. [DOI] [PubMed] [Google Scholar]
- Hammond G, Vallence AM. Modulation of long-interval intracortical inhibition and the silent period by voluntary contraction. Brain Res. 2007;1158:63–70. doi: 10.1016/j.brainres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Heckman CJ, Harvey RL, Rymer WZ. Changes in voluntary torque and electromyographic activity following oral baclofen. Muscle Nerve. 2004;30:784–795. doi: 10.1002/mus.20176. [DOI] [PubMed] [Google Scholar]
- Jiménez I, Rudomin P, Enriquez M. Differential effects of (-)-baclofen on Ia and descending monosynaptic EPSPs. Exp Brain Res. 1991;85:103–113. doi: 10.1007/BF00229991. [DOI] [PubMed] [Google Scholar]
- Kato M, Waldmann U, Murakami S. Effects of baclofen on spinal neurones of cats. Neuropharmacology. 1978;17:827–833. doi: 10.1016/0028-3908(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Kofler M. Effect of spinal cord injury and of intrathecal baclofen on brainstem reflexes. Clin Neurophysiol. 2012;123:45–53. doi: 10.1016/j.clinph.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Latash ML, Penn RD, Corcos DM, Gottlieb GL. Short-term effects of intrathecal baclofen in spasticity. Exp Neurol. 1989;103:165–172. doi: 10.1016/0014-4886(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. 2004;92:2694–2703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26:50–56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res. 2011;209:287–297. doi: 10.1007/s00221-011-2552-z. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-K. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohliger-Frerking P, Wiebe SP, Stäubli U, Frerking M. GABA(B) receptor-mediated presynaptic inhibition has history-dependent effects on synaptic transmission during physiologically relevant spike trains. J Neurosci. 2003;23:4809–4814. doi: 10.1523/JNEUROSCI.23-12-04809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsnes G, Crone C, Krarup C, Petersen N, Nielsen J. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin Neurophysiol. 2000;111:1372–1379. doi: 10.1016/S1388-2457(00)00352-7. [DOI] [PubMed] [Google Scholar]
- Palmer DT, Horn LJ, Harmon RL. Botulinum toxin treatment of lumbrical spasticity: a brief report. Am J Phys Med Rehabil. 1998;77:348–350. doi: 10.1097/00002060-199807000-00020. [DOI] [PubMed] [Google Scholar]
- Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, Kroin JS. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–1521. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord. New York: Cambridge UP; 2005. Propriospinal relay for descending motor commands; pp. 452–510. [Google Scholar]
- Price GW, Wilkin GP, Turnbull MJ, Bowery NG. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984;307:71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- Price GW, Kelly JS, Bowery NG. The location of GABAB receptors binding sites in mammalian spinal cord. Synapse. 1987;1:530–538. doi: 10.1002/syn.890010605. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Jiménez I, Rudomin P. Differential action of (-)-baclofen on the primary afferent depolarization produced by segmental and descending inputs. Exp Brain Res. 1992;91:29–45. doi: 10.1016/S0079-6123(08)62313-4. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösche J. Treatment of spasticity. Spinal Cord. 2002;40:261–262. doi: 10.1038/sj.sc.3101313. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/S0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol. 2011;122:1387–1395. doi: 10.1016/j.clinph.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Roy RR, Edgerton VR. Neurobiological perspective of spasticity as occurs after a spinal cord injury. Exp Neurol. 2012;235:116–1122. doi: 10.1016/j.expneurol.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sadlaoud K, Tazerart S, Brocard C, Jean-Xavier C, Portalier P, Brocard F, Vinay L, Bras H. Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J Neurosci. 2010;30:3358–3369. doi: 10.1523/JNEUROSCI.6310-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Saturno E, Bonato C, Miniussi C, Lazzaro V, Callea L. Motor cortex changes in spinal cord injury: a TMS study. Neurol Res. 2008;30:1084–1085. doi: 10.1179/174313208X332968. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hino T, Komori T, Hirai S. Loss of the muscle silent period evoked by transcranial magnetic stimulation of the motor cortex in patients with cervical cord lesions. Neurosci Lett. 2000;286:199–202. doi: 10.1016/S0304-3940(00)01125-3. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(SICI)1097-4598(199809)21:9<1209::AID-MUS15>3.0.CO%3B2-M. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J Physiol. 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Häger-Ross CK, Klein CS. Effects of baclofen on motor units paralysed by chronic cervical spinal cord injury. Brain. 2010;133:117–125. doi: 10.1093/brain/awp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO%3B2-F. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Ross BH, Thomas CK. Characteristics of lower extremity clonus after human cervical spinal cord injury. J Neurotrauma. 2012;29:915–924. doi: 10.1089/neu.2010.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lundbye-Jensen J, Petersen TH, Nielsen JB. The effect of baclofen and diazepam on motor skill acquisition in healthy subjects. Exp Brain Res. 2011;213:465–474. doi: 10.1007/s00221-011-2798-5. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang D, Li YQ. Distribution and depression of the GABAB receptor in the spinal dorsal horn of adult rat. Brain Res Bull. 2001;55:479–485. doi: 10.1016/S0361-9230(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]