This other Eden, demi-paradise,This fortress built by Nature for herself Against infection
—William Shakespeare, Richard II. Act II, Scene 1
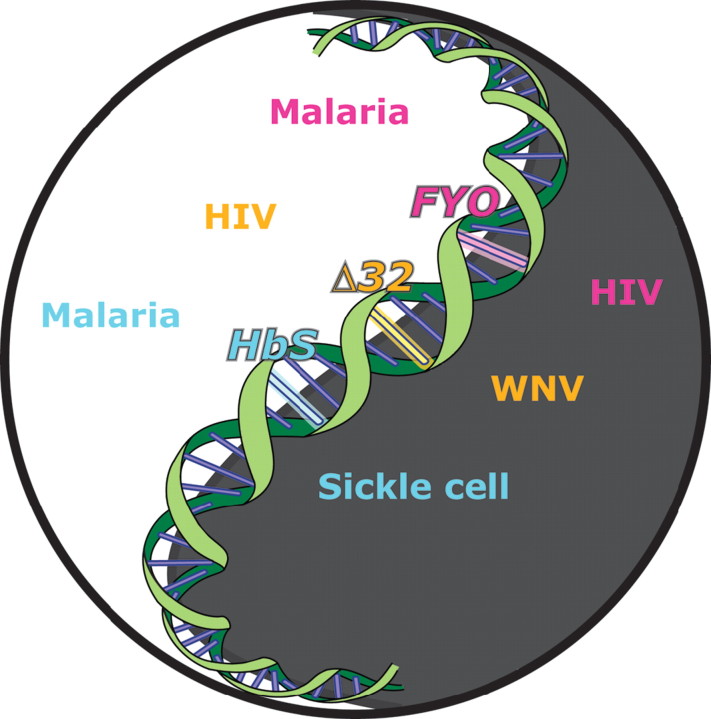
Was Shakespeare, that keen observer of human behavior, also an insightful infectious disease epidemiologist? Had he been prompted to pen these thoughts because he had astutely observed that some individuals resisted acquiring some infections or did poorly once infected compared with others? Validating Shakespeare's crystal ball, 250 years later, it was first postulated that these fortresses are genetic traits, with J. B. S. Haldane and A. C. Allison suggesting that malaria was an evolutionary force that selected for malaria-resistant genes [1]. However, there is a tradeoff. As illustrated by the results reported by Murphy and colleagues in this issue of the Journal[2] and the vignettes described below,it has become apparent that these genetic variants are oftentimes akin to a double-edged sword, serving as a fortress against one infection while conferring susceptibility to another [3][figure 1]. For example,genetic traits that result in hemoglobin and/or red blood cell disorders (eg,sickle cell disease and thalessemia) protect against malaria. The African-specific allele that results in the null state for Duffy antigen receptor for chemokines (DARC) on erythrocytes protects against Plasmodium vivax malaria. However, the role of DARC null state in infectious diseases is likely to be much more complex, because it may correlate with a blunted inflammatory response to endotoxins [4,5], serve as a genetic basis for the ethnic leukopenia that is observed commonly in persons of African ancestry [6], increase the risk of acquiring human immunodeficiency virus (HIV) infection [7], and confer a survival advantage to leukopenic HIV-positive African Americans [8].
Figure 1.
The yin-yang of infectious disease alleles. The concept of yin and yang illustrates the detrimental (black region) and beneficial (white region) effects of different infectious disease-influencing alleles in the human genome. Illustrated are the CCR5Δ32 allele, Duffy antigen receptor for chemokines (DARC)-null allele (FYO), and the sickle cell allele (HbS). HIV, human immunodeficiency virus; WNV, West Nile virus.
Murphy and colleagues now highlight another genetic tradeoff [2]: the null state of CC chemokine receptor 5 (CCR5) is associated with early symptom development and more pronounced clinical manifestations after infection with West Nile virus (WNV), whereas this same genetic state is known to confer strong protection against risk of acquiring HIV infection [9].The CCR5 null state, which is due to homozygosity for the European-specific 32 base pair (bp) coding deletion mutation (Δ32), propelled the HIV field forward in the mid-1990s [9], spawned an explosion of studies that explored the association of CCR5Δ32/Δ32 with a myriad of infectious and noninfectious diseases, and led to the development of CCR5 blockers for the treatment of HIV disease.
In retrospect, defining the link between CCR5 surface expression and HIV pathogenesis appears to be Act I of a 3-act Shakespearean play on the role of CCR5.Act II is punctuated by scenes that reveal the double-edged nature of the phenotypes associated with the possession of the CCR5Δ32/Δ32 genotype. From a historical perspective, it is noteworthy that the Murphy laboratory has played a major role in both acts thus far. In Act I, his laboratory was among the first to clone and functionally characterize CCR5 [10] and, along with the Berger laboratory,demonstrate that CCR5 is the major coreceptor required for cell entry of HIV-1 [11]. In Act II, although CCR5-null mice were found to have immune perturbations following inflammatory challenges [12,13], the plot really heated up when the Murphy laboratory challenged these mice with WNV [14], a mosquito-borne neurotropic flavivirus. From this point onward,the story resembles Macbeth.
Murphy's group showed that after challenge with WNV, CCR5-null mice had markedly increased viral titers in the central nervous system and had increased mortality [14] compared with that of wildtype mice, thus suggesting that CCR5 expression was necessary to mount a strong host defense against WNV. Subsequently,they demonstrated that there was a strong epidemiologic association between homozygosity for CCR5Δ32 and WNV in humans [15,16].
However, because they were unable to distinguish in their previous studies whether the observations were associated with susceptibility to acquiring WNV or associated with the severity of clinical presentation, they conducted the present study [2]. Lim et al [2] now show that the prevalence of CCR5Δ32/Δ32 was comparable in case patients with WNV infection and control participants, which suggests that this genotype is not a susceptibility factor for acquiring WNV infection. However, among the case patients, those patients who were homozygous for CCR5Δ32 experienced significantly more symptoms, on average, than did those patients who were heterozygous for CCR5Δ32 or who had wild-type CCR5 genotype. These data indicate that the CCR5 null state is a risk factor for more pronounced early clinical manifestations after infection with WNV [2].
A noteworthy aspect of the present report by Lim et al [2] is the study design.The case patients and control participants were derived from ∼35 million blood donors who were screened for WNV [2]. This contrasts with their prior studies,which examined subjects who sought medical attention for symptomatic disease and were compared with otherwise healthy subjects [15,16]. Also minimizing selection bias, both case patients and control participants in this report were administered the same standardized symptom questionnaire before disclosure of their true WNV infection status [2], and this study feature facilitated evaluation of the association of the CCR5 null state with the number and severity of early symptoms of WNV infection.
What will Act III reveal? Readers are referred to some possibilities posited in recent opinion pieces [17–19]. We focus on 4 points. First, the present study raises a pathogenic conundrum: why does the CCR5 null state confer risk for a more aggressive disease but not associate with risk of acquiring WNV infection (ie, viral entry)? One possibility is that CCR5-mediated signaling events generate critical immune responses that contain the spread of infection but are irrelevant for the initial entry of WNV. In this regard, there are abundant in vitro data linking CCR5 and its ligands to T cell immunity, and 2 recent studies provide corroborative in vivo data: first, that both humans and mice lacking CCR5 surface expression display reduced delayed-type hypersensitivity skin test responses (an in vivo correlate of T cell function and interleukin 2 [IL-2] production [20,21]), and second, that CCR5 expression regulates T cell proliferation, as well as IL-2 and CD25 expression during T lymphocyte activation [22]. Notably, T cells from CCR5-null mice secrete lower amounts of IL-2 than do wild-type mice; a similar phenotype is observed in CCR5Δ32 homozygotes, as well as after Ab-mediated blockade of CCR5 in human T cells genetically intact for CCR5 expression [22]. These studies underscore that CCR5 expression may influence clinical outcomes after viral infection by affecting parameters (eg, T cell immunity) that are independent of viral entry [21,23]. This may have relevance to antiviral immune responses to flaviviruses, including WNV, because CD4+ T cells have a critical function in the control and resolution of primaryWNVinfection; a strong Th1 T cell response, as characterized by interferon and IL-2 production, results in reduction of neurological sequelae [18, 24, 25]. Thus, one possibility is that the phenotype of “low CCR5 expression-low IL-2 levels” may contribute to WNV pathogenesis.Hence, we anticipate that Act III will define the precise mechanisms by which CCR5 influences antiviral responses to flaviviruses as well as to lentiviruses.
Second, the possible consequences of infection with WNV or other flaviviruses in HIV-positive patients who are receiving CCR5 blockers remains unknown, because very little is understood regarding the long-term effects of CCR5 blockers on immune functions in vivo. A previous study found that Maraviroc, a CCR5 antagonist, did not influence IL-2 and CD25 levels, whereas germ-line inactivation of CCR5 and Ab-mediated blockade of CCR5 did influence IL-2 and CD25 levels [22]. This may have been due to differences in the receptor configuration and resulting functionality of Ab-bound and inhibitor-bound forms of CCR5 [26,27].Hence, it is conceivable that the effects on immune function secondary to germ-line absence of CCR5 in humans and mice versus chemical antagonism of CCR5, such as after administration of Maraviroc, are dissimilar. Given that distinct biological responses of CCR5 might be determined through different receptor conformations [28,29], presumably the signaling pathways triggered in cells exposed to Maraviroc versus cells genetically lacking CCR5 may be distinct. Highlighting this possibility is the recent observation that CCR5 forms hetero-oligomeric complexes with at least 2 other chemokine receptors (CCR2 and CXCR4), and specific antagonists of 1 set of receptors (eg, CCR2 and CCR5) lead to functional cross-inhibition of the other (ie, CXCR4) [30]. This has relevance to the full evaluation of the health consequences of CCR5 blockers, because these data suggest that antagonists of 1 chemokine receptor may regulate the functional properties of another to which they do not directly bind [30]. Thus, Act III may reveal that the immune consequences of CCR5 blockers may not be identical to those found in CCR5-null people.
Third, the studies by the Murphy group pose a dilemma. Is there a threshold of CCR5 expression, albeit low, that promotes WNV disease? At least in the context of HIV infection, there appears to be a threshold of CCR5 surface expression that is permissive for cell entry, such that small changes in CCR5 density are associated with large increases in HIV infectivity and efficacy of CCR5 blockers [31–37]. The converse may be operative in WNV infection, in that CCR5 expression levels below a certain (low) threshold may enhance the risk of a more aggressive WNV clinical presentation. Whether such a threshold of CCR5 expression exists is a testable hypothesis because subjects bearing one or lacking the CCR5Δ32 allele display a wide range of CCR5 surface expression levels [38], and this variability may be partly due to CCR5 promoter polymorphisms that influence expression [39–41]. Additionally, one may also need to consider other factors that result in low CCR5 expression levels. For example, the copy number of CCL3L1, a potent CCR5 agonist, correlates inversely with CCR5 expression [42]. Hence, Act III may clarify this dilemma.
Finally, we anticipate that Act III will continue to be punctuated by additional examples that show the tradeoffs associated with the CCR5 null state. This is already happening: a recent study showed that CCR5Δ32 homozygosity is associated with increased susceptibility to tick-borne encephalitis virus [43]. These tradeoffs are a reminder of the constant tug of war between host and pathogen and also of the need to be vigilant, because what we find in one context might differ in another.
Acknowledgments
We thank Una Aluyen for excellent assistance with the graphics.
Footnotes
Financial support: The Veterans Administration Center on AIDS and HIV infection of the South Texas Veterans Health Care System, a MERIT award (R37046326) from the National Institutes of Health, Doris Duke Distinguished Clinical Scientist Award, and Burroughs Wellcome Clinical Scientist Award in Translational Research (to S.K.A.).
References
- 1.Lederberg J. JBS Haldane (1949) on infectious disease and evolution. Genetics. 1999;153:1–3. doi: 10.1093/genetics/153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim JK, McDermott DH, Lisco A, et al. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection, but not for viral transmission. J Infect Dis. 2010;201:178–85. doi: 10.1086/649426. (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiehm ER. Disease versus disease: how one disease may ameliorate another. Pediatrics. 2006;117:184–191. doi: 10.1542/peds.2004-2773. [DOI] [PubMed] [Google Scholar]
- 4.Mayr FB, Spiel AO, Leitner JM, et al. Duffy antigen modifies the chemokine response in human endotoxemia. Crit Care Med. 2008;36:159–165. doi: 10.1097/01.CCM.0000297875.55969.DB. [DOI] [PubMed] [Google Scholar]
- 5.Mayr FB, Spiel AO, Leitner JM, et al. Racial differences in endotoxin-induced tissue factor-triggered coagulation. J Thromb Haemost. 2009;7:634–640. doi: 10.1111/j.1538-7836.2009.03307.x. [DOI] [PubMed] [Google Scholar]
- 6.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5:e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Neil S, Kulkarni H, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe. 2008;4:52–62. doi: 10.1016/j.chom.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni H, Marconi VC, He W, et al. The Duffy-null state is associated with a survival advantage in leukopenic HIV-infected persons of African ancestry. Blood. 2009;114:2783–2792. doi: 10.1182/blood-2009-04-215186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry,tropism,and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 10.Combadiere C, Ahuja SK, Tiffany HL, Murphy PM. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1α, MIP-1β, and RANTES. J Leukoc Biol. 1996;60:147–152. doi: 10.1002/jlb.60.1.147. [DOI] [PubMed] [Google Scholar]
- 11.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophagetropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 12.Huffnagle GB, McNeil LK, McDonald RA, et al. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J Immunol. 1999;163:4642–4646. [PubMed] [Google Scholar]
- 13.Sato N, Kuziel WA, Melby PC, et al. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1α-, or CCR2-deficient mice. J Immunol. 1999;163:5519–5525. [PubMed] [Google Scholar]
- 14.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival inWest Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim JK, Louie CY, Glaser C, et al. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262–265. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 17.Lim JK, Glass WG, McDermott DH, Murphy PM. CCR5: no longer a “good for nothing”gene-chemokine control of West Nile virus infection. Trends Immunol. 2006;27:308–312. doi: 10.1016/j.it.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Diamond MS, Klein RS. A genetic basis for human susceptibility to West Nile virus. Trends Microbiol. 2006;14:287–289. doi: 10.1016/j.tim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Klein RS. A moving target: the multiple roles of CCR5 in infectious diseases. J Infect Dis. 2008;197:183–186. doi: 10.1086/524692. [DOI] [PubMed] [Google Scholar]
- 20.Dolan MJ, Clerici M, Blatt SP, et al. In vitro T cell function,delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Dolan MJ, Kulkarni H, Camargo JF, et al. CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV-AIDS pathogenesis via viral entry-independent mechanisms. Nat Immunol. 2007;8:1324–1336. doi: 10.1038/ni1521. [DOI] [PubMed] [Google Scholar]
- 22.Camargo JF, Quinones MP, Mummidi S, et al. CCR5 expression levels influence NFAT translocation, IL-2 production,and subsequent signaling events during T lymphocyte activation. J Immunol. 2009;182:171–182. doi: 10.4049/jimmunol.182.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt P, Carrington M. Host genetic determinants of HIV pathogenesis: an immunologic perspective. Curr Opin HIV AIDS. 2008;3:342–348. doi: 10.1097/COH.0b013e3282fbaa92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance ofWest Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent,orally bioavailable,and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Blanpain C, Vanderwinden JM, Cihak J, et al. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol Biol Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagane B, Ballet S, Planchenault T, et al. Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol Pharmacol. 2005;67:1966–1976. doi: 10.1124/mol.104.009779. [DOI] [PubMed] [Google Scholar]
- 30.Sohy D, Yano H, de Nadai P, et al. Heterooligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective”-antagonists. J Biol Chem. 2009;284:31270–31279. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhmann SE, Platt EJ, Kozak SL, Kabat D. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J Virol. 2000;74:7005–7015. doi: 10.1128/jvi.74.15.7005-7015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS. 2001;15:1627–1634. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 34.Reynes J, Baillat V, Portales P, Clot J, Corbeau P. Relationship between CCR5 density and viral load after discontinuation of antiretroviral therapy. JAMA. 2004;291:46. doi: 10.1001/jama.291.1.46. [DOI] [PubMed] [Google Scholar]
- 35.Lelievre JD, Petit F, Perrin L, et al. The density of coreceptors at the surface of CD4+ T cells contributes to the extent of human immunodeficiency virus type 1 viral replication-mediated T cell death. AIDS Res Hum Retroviruses. 2004;20:1230–1243. doi: 10.1089/aid.2004.20.1230. [DOI] [PubMed] [Google Scholar]
- 36.Heredia A, Latinovic O, Gallo RC. Reduction of CCR5 with low-dose rapamycin enhances the antiviral activity of vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci USA. 2008;105:20476–20481. doi: 10.1073/pnas.0810843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heredia A, Gilliam B, DeVico A, et al. CCR5 density levels on primary CD4 T cells impact the replication and enfuvirtide susceptibility of R5 HIV-1. AIDS. 2007;21:1317–1322. doi: 10.1097/QAD.0b013e32815278ea. [DOI] [PubMed] [Google Scholar]
- 38.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mummidi S, Bamshad M, Ahuja SS. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J Biol Chem. 2000;275:18946–18961. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- 40.Thomas SM, Tse DB, Ketner DS, et al. CCR5 expression and duration of high risk sexual activity among HIV-seronegative men who have sex with men. AIDS. 2006;20:1879–1883. doi: 10.1097/01.aids.0000244207.49123.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salkowitz JR, Bruse SE, Meyerson H, et al. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin Immunol. 2003;108:234–240. doi: 10.1016/s1521-6616(03)00147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez E, Kulkarni H, Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 43.Kindberg E, Mickiene A, Ax C, et al. Adeletion in the chemokine receptor 5 S(CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008;197:266–269. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]