Abstract
Geographic overlap between malaria and the occurrence of mutant hemoglobin and erythrocyte surface proteins has indicated that polymorphisms in human genes have been selected by severe malaria1,2. Deletion of exon 3 in the glycophorin C gene (called GYPCΔex3 here) has been found in Melanesians; this alteration changes the serologic phenotype of the Gerbich (Ge) blood group system, resulting in Ge negativity3,4. The GYPCΔex3 allele reaches a high frequency (46.5%) in coastal areas of Papua New Guinea where malaria is hyperendemic5. The Plasmodium falciparum erythrocyte-binding antigen 140 (EBA140, also known as BAEBL)6-8 binds with high affinity to the surface of human erythrocytes. Here we show that the receptor for EBA140 is glycophorin C (GYPC) and that this interaction mediates a principal P. falciparum invasion pathway into human erythrocytes. EBA140 does not bind to GYPC in Ge-negative erythrocytes, nor can P. falciparum invade such cells using this invasion pathway. This provides compelling evidence that Ge negativity has arisen in Melanesian populations through natural selection by severe malaria.
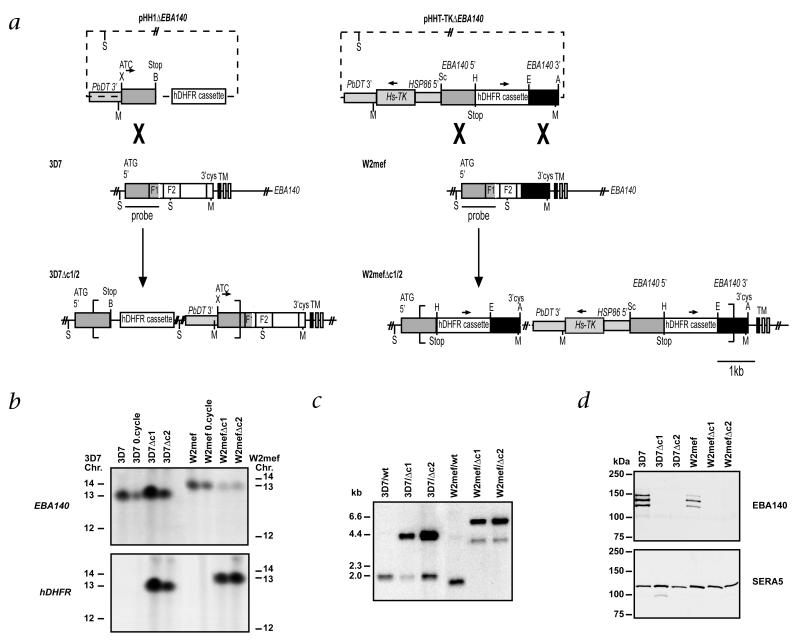
EBA140 from P. falciparum binds directly to human erythrocytes6-9. EBA140 is homologous to EBA175, which binds to GYPA on the erythrocyte and functions in invasion. Both proteins are members of a family that may provide a broader erythrocyte receptor range and evasion of host immune responses. It is likely that diversification of the EBA family has provided the parasite with an advantage by broadening its ability to invade erythrocytes using multiple receptor ligands10. To determine the function of EBA140, we generated parasites in which the gene for EBA140 had been disrupted (called ΔEBA140 here). We transfected plasmids pHH1ΔEBA140 and pHHT-TKΔEBA140 into 3D7 and W2mef parasites respectively (Fig. 1a). These plasmids inserted into the gene encoding EBA140 by homologous recombination, as shown by analysis of chromosomes (Fig. 1b) and genomic DNA (Fig. 1c). Western blot analysis (Fig. 1d) using antibodies against EBA140 showed three protein bands in 3D7 and W2mef parasites (ref. 6). We found no EBA140 bands for 3D7Δc1, 3D7Δc2, W2mefΔc1 and W2mefΔc2 parasites, in which the gene encoding EBA140 had been disrupted.
Fig. 1.
Disruption of the gene encoding EBA140 in P. falciparum. a, Top, transfection plasmids pHH1ΔEBA140 and pHHT-TKΔEBA140 with selection cassette containing human DHFR and the Pb-DT3′ sequence; the pHHT-TKΔEBA140 plasmid contains the thymidine kinase cassette (Hs-TK). Middle, structure of the endogenous EBA140 gene for 3D7 and W2mef (F1 and F2 domains indicated). Bottom, integration into 3D7 EBA140 occurs by a single homologous recombination event; into W2mef, by a double homologous recombination event (more than one copy inserted so Hs-TK is retained). S, ScaI; Sc, SacII; B, BglII; X, XhoI; H, HpaI; E, EcoRI; A, AvrII; M, MfeI. b, Analysis of chromosomes from 3D7- and W2mef-transfected parasites. Chromosomes from 3D7 and W2mef; 3D7 and W2mef after one cycle of selection for integration (0.cycle); and cloned lines 3D7Δc1, 3D7Δc2 W2mefΔc1 and W2mefΔc2 were separated by pulsed-field gel electrophoresis and probed with genes encoding EBA140 or human DHFR (hDHFR). The EBA140 probe hybridizes to chromosome 13 in 3D7 and W2mef as well as in the transfected lines, indicating integration into this chromosome. This is confirmed by hybridization of the hDHFR probe, which detects chromosome 13 in the transfected cloned lines 3D7Δc1, 3D7Δc2 and W2mefΔc1 and W2mefΔc2. The ‘0.cycle’ parasites also hybridizes to the hDHFR probe, but to episomal plasmid that ran off the gel here. Left and right margins, chromosomal positions (Chr.). c, Southern blot analysis of genomic DNA confirming disruption of gene encoding EBA140 in 3D7 and W2mef. Genomic DNA from 3D7, Wmef and the cloned transfected lines 3D7Δc1, 3D7Δc2, W2mefΔc1 and W2mefΔc2 was digested with MfeI and ScaI and hybridized with the 5′ region of the gene encoding EBA140. d, Western blot analysis of supernatants from 3D7, W2mef and the transfected clones 3D7Δc1, 3D7Δc2, W2mefΔc1 and W2mefΔc2, using antibodies against EBA140. No bands are seen in the transfected clones, confirming that disruption of the gene encoding EBA140 results in loss of expression of EBA140. SERA5, another secreted protein (loading control). Left margins (c and d), molecular size markers.
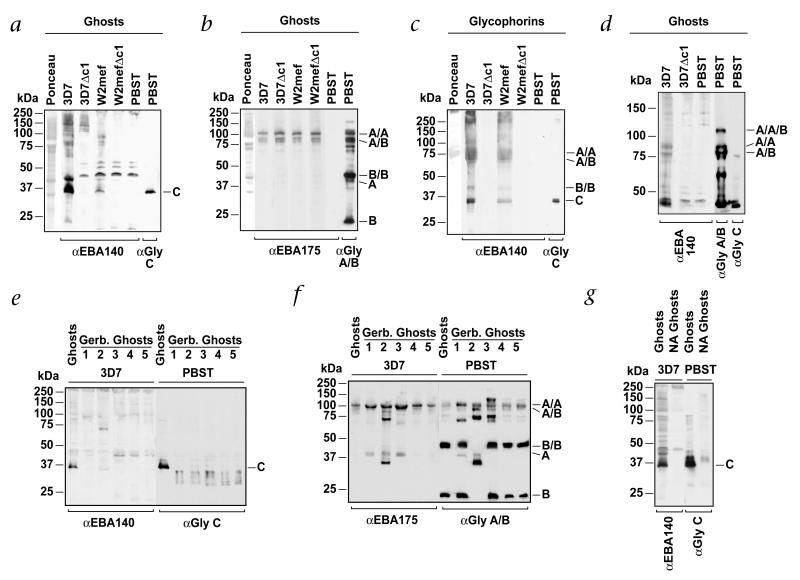
To identify the receptor(s) for EBA140 on human erythrocytes, we used overlay experiments in which erythrocyte proteins were incubated with culture supernatants from 3D7, W2mef and ΔEBA140 parasites (Fig. 2a). The supernatants contained a mixture of proteins released from merozoites, including EBA140 and EBA175 (refs. 11,12). In overlay experiments, EBA140 from 3D7 and W2mef parasites bound mainly to a protein of approximately 35 kDa; the specificity of this interaction was demonstrated by loss of binding in experiments with supernatants from ΔEBA140 parasites. We used identical experiments to assess EBA175 binding (Fig. 2b). As expected, EBA175 bound to the GYPA homodimer and GYPA–GYPB heterodimer13. EBA140 bound to a protein of the same size as GYPC, as shown by use of antibodies against GYPC (Fig. 2a). These results indicate EBA140 binds mainly to GYPC.
Fig. 2.
EBA140 binds to GYPC on human erythrocytes. a, EBA140 binds to GYPC and other proteins in erythrocyte ghost preparations. Normal erythrocyte ghost cells were separated by SDS–PAGE, blotted and incubated with merozoite supernatants from 3D7, 3D7Δc1, W2mef and W2mefΔc1 (above blots). Far left lane, Ponceau staining of total erythrocytes proteins. PBST (far right lanes), ghost erythrocytes probed with antibodies against EBA140 or GYPC. b, EBA175 binds to GYPA homodimers and GYPA–GYPB heterodimers. Erythrocyte ghost proteins were incubated with supernatants from 3D7, W2mef, 3D7Δc1 and W2mefΔc1. Far left lane, Ponceau staining. PBST (far right lanes), probed directly with antibodies against EBA175 or GYPA–GYPB without prior incubation with supernatant. c, EBA140 binds to purified glycophorin proteins. Purified glycophorins separated by SDS–PAGE were incubated with supernatants from 3D7, W2mef, 3D7Δc1 and W2mefΔc1. Far left lane, Ponceau staining. PBST (far right lanes), probed with antibodies against EBA140 or GYPC. Right, GYPB and GYPC monomers. The GYPA dimer migrates at 75 kDa here. This glycophorin preparation consists mainly of GYPA but also has some GYPB and small amounts of GYPC. d, EBA140 binds to proteins the same size as GYPA and GYPA/B dimers. Samples similar to those in a and b were separated by 6% acrylamide gel electrophoresis and incubated with supernatants (above gel) to more distinctly separate the larger-molecular-weight bands to which EBA140 binds. PBST (far right lanes), probed with antibodies against EBA140, GYPA/GYPB or GYPC. e, EBA140 does not bind to mutant GYPC. Proteins from ghost erythrocytes obtained from normal or Ge-negative individuals (GYPCΔex3 homozygotes5) were incubated with supernatant from 3D7 (above blot). Binding of EBA140 was detected with antibodies against EBA140. Left, an identical membrane probed with antibodies against GYPC. f, EBA175 binds to GYPA dimers in Ge-negative erythrocytes. A membrane identical to that in e was incubated with supernatant from 3D7 followed by detection of bound EBA175 with antibodies against EBA175. Right, incubation with antibodies against GYPA–GYPB. Ge-negative erythrocytes (lane 2) also have a mutant GYPB. g, Binding of EBA140 to erythrocytes is sialic acid-dependent. Proteins from normal ghost erythrocytes or those treated with neuraminidase (NA) were incubated with supernatants from 3D7 (left) and bound EBA140 identified with antibodies against EBA140. PBST (far right lanes), GYPC detected using antibodies against GYPC. Left margins, molecular size markers. Right margins, A/A, GYPA homodimer; A/B, GYPA/B heterodimer; B/B, GYPB homodimer; A, GYPA monomer; B, GYPD monomer; C, GYPC. α, antibody against; Gly, glycophorin.
We further assessed the parasite ligand-host receptor interactions using purified GYPA, GYPB, GYPC and GYPD with supernatants from 3D7 and W2mef parasites (Fig. 2c). EBA140 from 3D7 and W2mef parasites bound mainly to GYPC, although binding to glycophorins of higher molecular weights was evident. The specificity of these interactions was demonstrated by lack of binding when supernatants from ΔEBA140 parasites were used. The sizes of the larger proteins to which EBA140 bound corresponded to the sizes of the GYPB monomer, GYPA homodimer and GYPA-GYPB heterodimer (Fig. 2d), indicating EBA140 interacts with these proteins. These results are consistent with previous data showing that EBA140 binds to trypsin-treated erythrocytes from which GYPC has been removed7,8. Consistent with this interpretation, we did not find binding to the higher-molecular-weight proteins for the ΔEBA140 parasite supernatants (Fig. 2d). Moreover, after neuraminidase removal of sialic acid residues from erythrocyte proteins, binding of EBA140 to GYPC and the higher-molecular-weight proteins was eliminated (Fig. 2g).
To demonstrate direct binding between EBA140 and GYPC, we used erythrocytes from five Papua New Guineans who were homozygous for GYPCΔex3 and expressed a smaller GYPC protein corresponding to the Ge-negative phenotype5 (Fig. 2e). Overlay experiments using supernatant from 3D7 parasites and erythrocytes from normal or GYPCΔex3 homozygous individuals showed binding of EBA140 mainly to GYPC; however, in Ge-negative erythrocytes, we found no GYPC binding. This was in contrast to overlays that showed EBA175–GYPA binding for all samples (Fig. 2f). These results confirm that EBA140 does not interact with altered GYPC protein in Ge-negative erythrocytes and that interaction with GYPA and GYPB is likely to be responsible for the residual binding of EBA140 to Ge-negative erythrocytes noted before8.
Furthermore, we found that binding of EBA140 to GYPC was greater for 3D7 parasite supernatant than that from W2mef parasites, despite the similar expression of these proteins (Fig. 2a). In contrast, 3D7 and W2mef EBA175 bound equally well to GYPA (Fig. 2b). Comparison of the EBA140 sequences for 3D7 (ref. 6) and W2mef parasites7 showed the deduced protein sequences were identical except for three amino acids in the F1 domain (3D7 compared with W2mef: I185V, N239S, K261T). The F2 domain of EBA175 is the main region required for binding to GYPA; nevertheless, the F1 domain is also important12,14. These results indicate EBA140 F1 domain polymorphisms may be involved in determining the affinity of binding to GYPC and other binding partners.
Invasion of human erythrocytes by P. falciparum can occur through at least three different receptors: GYPA, GYPB and ‘X’ (ref. 15). EBA175 mediates invasion through GYPA (refs. 12,14); this protein has similarity to EBA140 (refs. 6,7). To determine if 3D7Δc1, 3D7Δc2, W2mefΔc1 and W2mefΔc2 parasites, which lack expression of EBA140, had altered invasive abilities, we tested efficiencies of invasion into erythrocytes treated with neuraminidase, trypsin or chymotrypsin. GYPC on the erythrocyte surface is removed by trypsin, but not chymotrypsin, and neuraminidase removes sialic acid residues12. We found no significant difference between 3D7, W2mef and transfectant lines in their ability to invade enzyme-treated erythrocytes or untreated cells (data not shown). This indicated that either EBA140 does not participate in merozoite invasion of erythrocytes or loss of function is compensated by other ligands. Disruption of the gene encoding EBA175 has shown that parasites can compensate the loss of function of this ligand by increased use of other invasion pathways16. Analysis of invasion of these parasites into normal and Ge-negative erythrocytes showed that 3D7 and W2mef parasites invaded the latter less efficiently (61 ± 3.5% and 62 ± 5.4%, respectively). This has been described before for a rare GYPC mutation and the GYPCΔex3 deletion, for which invasion efficiencies of 57% and 81%, respectively, were found7,17.
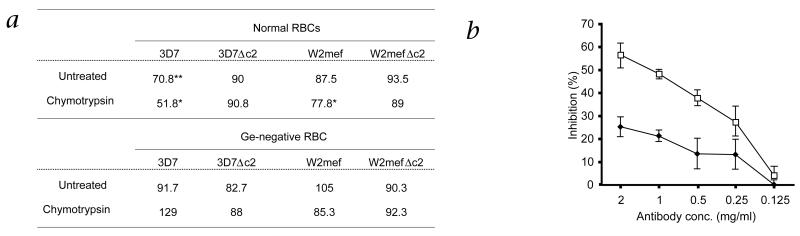
To determine if P. falciparum can invade erythrocytes through GYPC using EBA140, we compared the ability of parasites to invade normal and Ge-negative erythrocytes in the presence of antibodies against EBA140 (Fig. 3). Compared with results in untreated erythrocytes, antibodies against EBA140 inhibited invasion of 3D7 parasites (70.8%). The degree of inhibition by antibody against EBA140 was increased for chymotrypsin-treated erythrocytes (51.8%) (Fig. 3a). GYPC is resistant to chymotrypsin treatment. Treatment with this protease limits the receptor repertoire on the surface of chymotrypsin-treated erythrocytes, increasing the dependence of invasion on GYPC. To assess the specificity of inhibition of EBA140–GYPC interaction, we did similar experiments with 3D7Δc2 parasites lacking EBA140. Inhibition of invasion by antibodies against EBA140 was abolished, confirming the idea that inhibition of erythrocyte invasion for EBA140-competent 3D7 parasites results from interference with the function of this parasite ligand. Although the overall effect of treatment with antibody against EBA140 was less profound in experiments using W2mef and W2mefΔc2, antibody still reduced invasion by the parental strain compared with that of the ΔEBA140 strain. Given the reduced W2mef parasite EBA140 affinity for GPYC noted before (Fig. 2), it is likely that this strain is predisposed to erythrocyte invasion pathways that do not involve EBA140.
Fig. 3.
Antibodies against the EBA140 F2 domain inhibit the EBA140–GYPC invasion pathway of P. falciparum. a, Data represent percentage of parasite invasion in the presence of EBA140 F2 antibodies relative to invasion in the presence of nonspecific antibodies from pre-immune serum. The inhibition of 3D7 wild-type parasites is more profound when red blood cells (RBCs) are treated with chymotrypsin. Chymotrypsin will cleave GYPB but not GYPC; this limits the receptor repertoire on the surface of the chymotrypsin-treated erythrocytes, enabling the dissection of invasion through GYPC versus other receptors. Conversely, inhibition by antibody against EBA140 is abolished when invasion of ΔEBA140 parasites is tested because of the lack of expression of EBA140. No inhibition is detectable when Ge-negative RBCs are used because they lack a GYPC receptor capable of binding to EBA140. Data represent the average of at least three experiments done in triplicate. *, P < 0.05 and **, P < 0.01, wild-type compared with EBA140-null parasite lines (paired t-test). b, Inhibition of invasion of 3D7 wild-type parasites by the EBA140 F2 antibody is concentration dependent. Data represent percent of inhibition of 3D7 wild-type invasion (◆) after addition of antibody against EBA140 F2 compared with that in the presence of antibodies of pre-immune serum (antibody concentration, horizontal axis). The inhibitory effect of the EBA140 F2 antibody on invasion is more profound for erythrocytes pre-treated with chymotrypsin (□). Error bars indicate confidence levels (α = 0.1) of five independent experiments done in triplicate.
We found that antibodies binding to EBA140 in merozoite invasion assays were not acting by steric hindrance but rather were directly inhibiting ligand function. We compared merozoite invasion of wild-type parasites expressing EBA140 in normal and Ge-negative erythrocytes in the presence of antibodies against EBA140 (Fig. 3a). Antibodies against EBA140 inhibited wild-type merozoite invasion in untreated and chymotrypsin-treated erythrocytes, and this effect could be ‘titrated’ to below 250 μg/ml, which is comparable to results obtained with antibodies against MSP-119 and AMA1, two other P. falciparum merozoite proteins involved in erythrocyte invasion18,19 (Fig. 3b). The EBA140 antibodies did not inhibit invasion of 3D7 or W2mef parasites into Ge-negative erythrocytes, whereas merozoite invasion was inhibited for normal erythrocytes. Antibodies against EBA140 bound to EBA140 expressed in both 3D7 and W2mef parasites; however, this had no effect on invasion into Ge-negative erythrocytes that express an altered GYPC receptor (Fig. 3a). This is because merozoite invasion using EBA140–GYPC interaction is not functional in Ge-negative erythrocytes. These experiments show that antibodies against EBA140 inhibit the ligand function of this protein in merozoite invasion.
We further evaluated the effects of antibodies against EBA140 on merozoite invasion of Ge-negative erythrocytes. We found no inhibition of erythrocyte invasion for 3D7, W2mef or ΔEBA140 parasites, indicating that the mutant GYPC does not function in invasion by the EBA140 ligand (Fig. 3). The absence of residual inhibition of invasion into Gerbich erythrocytes by wild-type parasites indicates that the ‘promiscuous’ binding of EBA140 ligand to glycophorins other than GYPC is not of relevance during the invasion process. These results indicate that GYPCΔex3 erythrocytes are invaded less efficiently by P. falciparum because of the inability of the parasites to use EBA140–GYPC pathway.
Unexpectedly, the allele underlying Ge negativity3,4 has increased to a frequency of 46.5% in coastal populations of Papua New Guinea where malaria is endemic5. The prevalence of infection with P. falciparum has been examined in relation to Ge status determined by DNA-based genotyping: One study found a lower smear-positive rate for P. falciparum; however, another study did not find a difference in Ge-negative individuals5. Other erythrocyte polymorphisms such as hemoglobin S also occur at high frequency, and individuals with this allele are as likely to be infected with P. falciparum as those who lack the sickle cell genotype. They are, however, less likely to have a high parasite density and are much less likely to develop cerebral or severe malaria20-22. Given that glycophorin C is the receptor used by EBA140 and wild-type parasites invade Ge-negative cells less efficiently than normal erythrocytes, it will be useful to determine whether there has been selection for this allele to provide protection against severe malaria.
Our findings show that the P. falciparum ligand EBA140 binds to GYPC and this interaction mediates a previously unknown invasion pathway into human erythrocytes. Antibodies against EBA140 inhibit the EBA140–GYPC pathway, resulting in a reduction in merozoite invasion. We also found a similar reduction in invasion of P. falciparum into Ge-negative erythrocytes; our data indicate this is due to the inability of merozoites to use the EBA140–GYPC invasion pathway. Lack of this invasion pathway may account for much of the observed decreased invasion of Gerbich erythrocytes; other changes to the properties of the mutant erythrocytes would be a minor component. Decreased parasite invasion rates into erythrocytes of Ge-negative individuals would result in decreased parasitemias, which might have a substantial effect on the severity of disease. This provides a molecular mechanism of natural selection for the GYPCΔex3 allele in human populations. Overall, our results provide evidence supporting the idea that selection of Ge-negativity in Melanesians confers protection against the most pernicious form of malaria.
Methods
Plasmid construction
The pHH1ΔEBA140 plasmid was derived from pHH1 (ref. 23) and contained a 1-kb DNA fragment from the 5′ end of the gene encoding EBA140 amplified using oligonucleotide primers aw25 and aw26. The pHH-TKΔEBA140 plasmid was derived from pHH-TK (ref.24) by insertion of 1-kb 5′ and 3′ segments of the gene encoding EBA140 using the oligonucleotide primer combinations aw9 plus aw12 and aw38 plus 39, respectively. The pHH-TK plasmid is a derivative of the pHH1 vector containing the cassette for the expression of Herpes simplex thymidine kinase, which converts the normally harmless nucleoside analog ganciclovir into a toxic form24; it also contains the human dihydrofolate reductase gene (DHFR). This vector was used to ‘encourage’ gene deletion through a double crossover recombination event.
Oligonucleotides used include: aw9, 5′-ATCCCGCGGCCAATAAATTATATATAATGAAAGG-3′; aw12, 5′-AGTGTTAACGGCACATTCTTTACTTATGTT-3′; aw25, 5′-ATCCTCGAGATCAAAGGATATTTTAATATATATTTTTTAATTCC-3′; aw26, 5′-GATAGATCTTTACCATCAAGAAGTTTTCATTCC-3′; aw38, 5′-ATCGAATTCTGTAGAAAAGTTAAGTGGTGATG-3′; aw39, 5′GATCCTAGGTTAAAAACATTTATATTCTGGAC-3′ (restriction sites introduced for cloning are underlined; italics indicate changes to the endogenous sequence).
Plasmodium strains, culture conditions and parasite transfection
P. falciparum asexual stages were maintained in human 0+ erythrocytes. 3D7 is a cloned line derived from NF54 and was obtained from D. Walliker (Edinburgh University). W2mef is a cloned line derived from the Indochina III-CDC strain. Transfection with 80 μg of purified plasmid DNA (Qiagen, Hilden, Germany) and selection for stable transfectants was done as described before25,26.
Chromosome and DNA analysis
Genomic DNA and whole chromosomes were prepared from trophozoites. To detect genomic integration of the plasmid into chromosome 13, chromosomes were separated by pulsed-field gel electrophoresis as described before27.
SDS–PAGE and immunoblot analysis
Parasite pellets and supernatants were collected from double-synchronized parasites after 40 h (late schizonts). Proteins were separated by 6% SDS–PAGE. Western blotting on to nitrocellulose (0.45 μm; Schleicher and Schuell, Dassel, Germany) was done according to standard protocols, and blots were processed for antigen detection with a chemiluminescence system (ECL; Amersham, Arlington Heights, Illinois). Antigens were detected with rabbit antibodies against EBA140 (against a region before the 3′ cysteine domain)6, rabbit antibodies against SERA5, mouse monoclonal antibodies against GYPA and GYPB (E5) and against GYPC (E3; E5 and E3 from Sigma, St Louis, Missouri)28. Secondary antibodies were horseradish peroxidase-coupled sheep antibodies against rabbit or mouse immunoglobulin (Chemicon, Temecula, California).
Overlay assays
The overlay protein-binding assay used supernatants obtained after schizont rupture from synchronized and neuraminidase- or trypsin-treated parasites (to prevent reinvasion) at 5-10% parasitemia. Supernatants were centrifuged at 1,000g (GS-6KR; Beckman, Fullerton, California) and stored at −20 °C. Erythrocyte ‘ghosts’ were prepared by hypotonic lysis as described before29. Ghost proteins (30 μg/lane, corresponding to ~5 × 107 cells) or purified MN-glycophorins (20 μg/lane) (Sigma, St. Louis, Missouri) were separated by 10% SDS–PAGE and transferred onto nitrocellulose. The membrane was stained with Ponceau stain, cut into strips and blocked with 10% milk powder in phosphate buffered saline/0.1% Tween-20 (PBST) (0.5%) for 1 h. After being washed with PBST three times, 5 min each wash, the strips were incubated overnight at 4 °C with parasite culture supernatant. The strips were washed five times, 5 min each wash, before antigen detection proceeded as outlined above.
Erythrocyte invasion assay
Uninfected erythrocytes were treated with enzymes as described before6. Erythrocytes infected with ring stages of tightly synchronized parental and knockout parasite lines were digested with neuraminidase (66.7 mU/ml) or trypsin (1 mg/ml). Experiments were done in triplicate in flat-bottomed microtiter plates in hypoxanthine-free medium. Enzyme-treated or untreated (mock) erythrocytes at 4% hematocrit were inoculated with 0.5% parasitemia in a total volume of 100 μl/well. To allow for reinvasion, parasites were incubated for 48 h before 3H-hypoxanthine (Amersham, Buckinghamshire, United Kingdom) was added at a final concentration of 1 μCi/well. After an additional 16 h, the cells were subjected to a freeze–thaw cycle to facilitate collection onto glass fiber filters with the aid of a cell collector (Packard, Mississauga, Ontario, Canada). Incorporated 3H-hypoxanthine was measured in a scintillation counter. The percentage of invasion was calculated in comparison to invasion of the same parasite line into untreated erythrocytes, which was set at 100%.
Antibody inhibition assay
The antibody inhibition assay was done as the erythrocyte invasion assay except that the hematocrit was kept at 2% and initial parasitemia was 0.25%. At 24 h before the addition of 3H-hypoxanthine, protein A–purified immunoglobulin G antibodies of rabbit pre-immune antiserum or rabbit antiserum raised against a His-tagged fusion protein of the F2 domain of 3D7 EBA140 were added at final concentrations between 0.125–1 mg/mls Invasion in the presence of specific EBA140 F2 antibodies was compared with invasion in the presence of nonspecific antibodies from pre-immune serum (100% control).
Acknowledgments
We thank study volunteers for their willing participation, and M. Bockarie and G. Casey (Papua New Guinea Institute of Medical Research) for collecting the blood. We thank J. Thompson and T. Triglia for assistance, and A. Batchelor, S. Miller and B. Crabb for gifts of antibodies. We thank R. Thomson for independent statistical assistance. We acknowledge the Red Cross Blood Service (Melbourne, Australia) for supply of human erythrocytes and serum. This work is supported by grants from the National Health and Medical Research Council of Australia and the National Institutes of Health USA (AI36478-07, AI46919-01A2, AI49390-01). A.F.C. is supported by an International Research Scholarship from the Howard Hughes Medical Institute. M.T.D. is supported by a Wellcome Trust Advanced Training Fellowship (Tropical Medicine) and A.G.M. is a recipient of a Deutsche Forschungsgemeinschaft Research-Fellowship.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Haldane JBS. The rate of mutation in human genes. In: bonnier GA, editor. Proceedings of the VII International Congress on Genetics. 1949. pp. 267–273. [Google Scholar]
- 2.Miller LH, Good MF, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 3.Booth PB, McLoughlin K. The Gerbich blood group system, especially in Melanesians. Vox Sang. 1972;22:73–84. doi: 10.1111/j.1423-0410.1972.tb03968.x. [DOI] [PubMed] [Google Scholar]
- 4.Serjeantson SW, White BS, Bhatia K, Trent RJ. A 3.5 kb deletion in the gly-cophorin C gene accounts for the Gerbich-negative blood group in Melanesians. Immunol. Cell Biol. 1994;72:23–27. doi: 10.1038/icb.1994.4. [DOI] [PubMed] [Google Scholar]
- 5.Patel SS, et al. The association of the glycophorin C exon 3 deletion with ovalocytosis and malaria susceptibility in the Wosera, Papua New Guinea. Blood. 2001;98:3489–3491. doi: 10.1182/blood.v98.12.3489. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JK, Triglia T, Reed MB, Cowman AF. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Micro. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 7.Mayer DC, Kaneko O, Hudson-Taylor DE, Reid ME, Miller LH. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. USA. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narum DL, Fuhrmann SR, Luu T, Sim BK. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 2002;119:159–168. doi: 10.1016/s0166-6851(01)00428-5. [DOI] [PubMed] [Google Scholar]
- 9.Jungery M, Pasvol G, Newbold CI, Weatherall DJ. A lectin-like receptor is involved in invasion of erythrocytes by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1983;80:1018–1022. doi: 10.1073/pnas.80.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 11.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 12.Sim BKL, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 13.Issitt PD, Anstee DJ. Applied Blood Group Serology. Montgomery Scientific Publications; Durham: 1998. [Google Scholar]
- 14.Sim BK, et al. Plasmodium falciparum: Further characterization of a functionally active region of the merozoite invasion ligand EBA-175. Exp. Parasitol. 1994;78:259–268. doi: 10.1006/expr.1994.1027. [DOI] [PubMed] [Google Scholar]
- 15.Dolan SA, et al. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 16.Reed MB, et al. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid independent pathway of invasion. Proc. Natl. Acad. Sci. USA. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasvol G, Anstee D, Tanner MJ. Glycophorin C and the invasion of red cells by Plasmodium falciparum. Lancet. 1984;1:907–908. doi: 10.1016/s0140-6736(84)91366-7. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell RA, Saul A, Cowman AF, Crabb BS. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nature Med. 2000;6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- 19.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen SJ, et al. Morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in children with sickle cell trait in The Gambia. Trans. R. Soc. Trop. Med. Hyg. 1992;86:494–498. doi: 10.1016/0035-9203(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 21.Hill AVS, et al. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 22.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–703. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 23.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 24.Duraisingh MT, Triglia T, Cowman AF. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int. J. Parasitol. 2002;32:81–89. doi: 10.1016/s0020-7519(01)00345-9. [DOI] [PubMed] [Google Scholar]
- 25.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triglia T, Wang P, Sims PFG, Hyde JE, Cowman AF. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 1998;17:3807–3815. doi: 10.1093/emboj/17.14.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio JP, Thompson JK, Cowman AF. The var genes of Plasmodium falciparum are located in the subtelomeric region of most chromosomes. EMBO J. 1996;15:4069–4077. [PMC free article] [PubMed] [Google Scholar]
- 28.Telen MJ, Bolk TA. Human red cell antigens. IV. The abnormal sialoglycoprotein of Gerbich-negative red cells. Transfusion. 1987;27:309–314. doi: 10.1046/j.1537-2995.1987.27487264736.x. [DOI] [PubMed] [Google Scholar]
- 29.Fairbanks G, Steck TL, Wallach DFH. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]