Abstract
Gadolinium-containing phosphonate-coated gold nanoparticles were prepared and then non-covalently coated with an amphiphilic fluorous monomer. The monomer spontaneously self-assembles into a non-covalent monolayer shell around the particle. The binding of the shell utilizes a guanidinium-phosphonate interaction analogous to the one exploited by the Wender molecular transporter system. Particle-shell binding was characterized by a 27% decrease in 19F T1 of the fluorous shell upon exposure to the paramagnetic gadolinium in the particle and a corresponding increase in hydrodynamic diameter from 3 nm to 4 nm. Interestingly, a much smaller modulation of 19F T1 is observed when the shell monomer is treated with a phosphonate-free particle. By contrast, the phosphonate-free particle is a much more relaxive 1H T1 agent for water. Together, these observations show that the fluoroalkylguanidinium shell binds selectively to the phosphonate-covered particle. The system’s relaxivity and selectivity give it potential for use in 19F based nanotheranostic agents.
Keywords: Theranostic, Gold nanoparticle, Surfaces, Relaxivity, Fluorine
1. Introduction
The development of “smart” nanoparticulate medicinal entities is an emerging area at the interface of chemical and clinical sciences. For example, novel drug delivery systems and imaging agents based on drug-loaded polymers or nanoparticles have appeared recently,1 and are making way into the clinic.2 Nanotechnology further offers opportunities to design combined therapeutic plus diagnostic agents, or “theranostic” systems; examples of such include paramagnetic nanoparticles for MRI imaging,3 64Cu-loaded liposomes for PET imaging,4 and others.5
One key factor to controlling the in vivo behavior of these systems is the ability to control their surface properties.6 Along these lines, we are pursuing the development of monomeric organic compounds that engage in predictable self-assembly around nanoparticulate molecular probes and that have the ability to control the bioactivity of the former by modifying their size, surface, and access to the external aqueous milieu.7
We present here a general strategy for applying a poly)ethyleneglycol (PEG)-terminated fluoroalkyl coating to phosphonate-covered nanoparticle agent and illustrate the use of bioorthogonal 19F magnetic resonance relaxivity to track the behavior of the coating monomer as it associates with the particle. The coating monomer (1, Fig. 1) exploits a guanidinium head group, which can interact with particle surface phosphonate groups through a double hydrogen bonding system analogous to the one used by the Wender molecular transporter system for its initial adhesion to cell surfaces.8 Particles utilizing such coreshell interactions have various clinical applications, such as vectors for gene delivery, as investigated in the Wender lab9. Monomer 1 further features a fluoroalkane region that enables the self-assembly of a fluorous, Teflon-like layer that can limit the particle’s exposure to its aqueous surroundings.
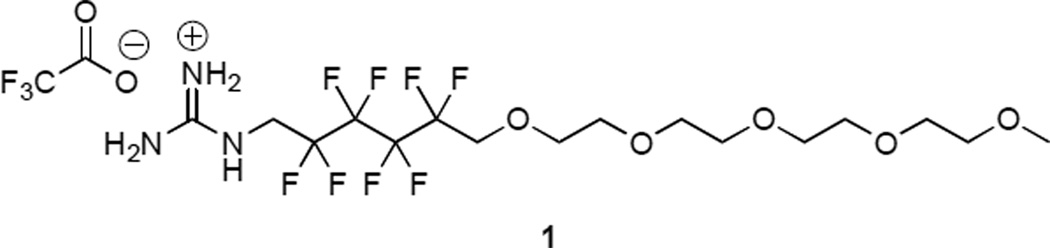
Fig. 1.
Structure of monomer 1.
We have previously characterized the binding of monomer 1 with small-molecule phosphonates,10 and we hypothesize that a particle with a phosphonate surface can utilize the same interaction(s) to bind species 1, thus enabling the self-assembly of a monolayer coating around a particle (Fig. 2). Incorporating a paramagnetic species into such a particle can thus enable the use of monomer 1 as a 19F MRI contrast system for an appropriately filtered, T1-weighted image plane. Although 19F MRI-based nanotheranostic technology is not as well developed as the analogous 1H MRI based systems, 19F MRI based systems are beginning to emerge,11,12 and offer important bioorthogonality unavailable to other imaging modalities.13 We show here potential utility of monomer 1 as a flexible tool for the installation of 19F groups on the surface of a phosphonatecovered nanoparticle and illustrate the corresponding modulation in 19F T1 upon particle-1 binding. We further illustrate 1’s selectivity for a phosphonate surface over a phosphonate free particle and illustrate that the coverage provided by 1 corresponds to a monolayer in aqueous solution.
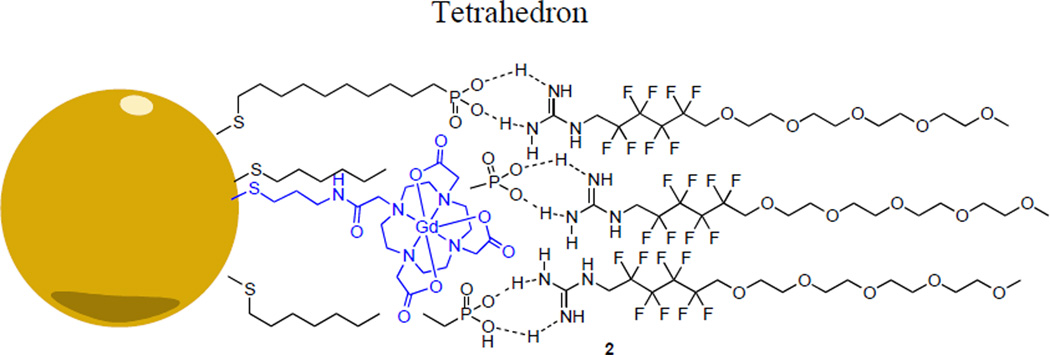
Fig. 2.
Hypothetical interactions between phosphonate core and guanidine shell to yield shell coated phosphonate particle 2
2. Results and discussion
2.1. Design and synthesis of shell monomer 1
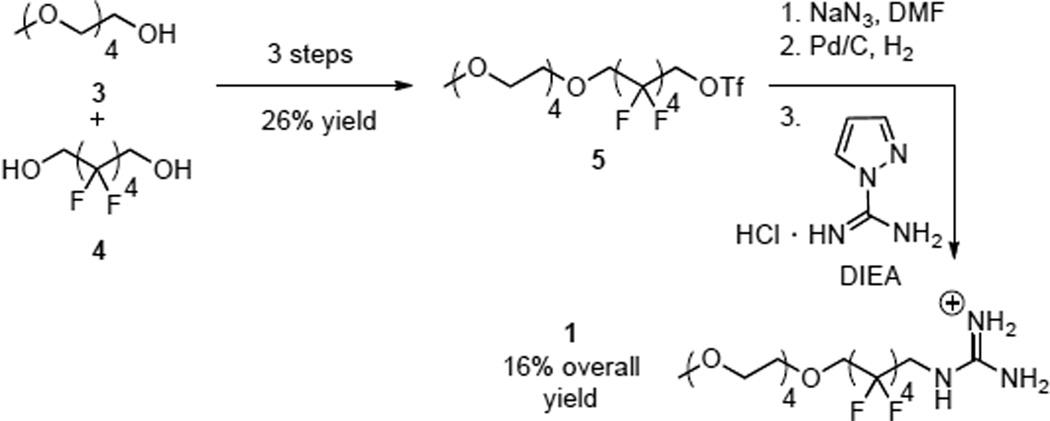
Shell monomer 1 features a guanidinium head group, which is designed to participate in hydrogen bonding with phosphonates on the periphery of a particle. This is attached to a fluorous phase group14 that prevents intercalation of the shell in to the interior of the particle and limits the particle’s exposure to its surroundings. Appropriate fluorous starting materials for this design are readily available side products from teflon synthesis.15 A hydrophilic PEG chain is then appended as a solubilizing group. Scheme 1 summarizes the synthesis of monomer 1, which we have previously reported.10 This route relies on the intermediacy of a bench top-stable alkyl triflate (4), which is a versatile starting material for fluoroalkyl amines.
Scheme 1.
Synthesis of monomer 1.
2.2. NMR properties of monomer 1
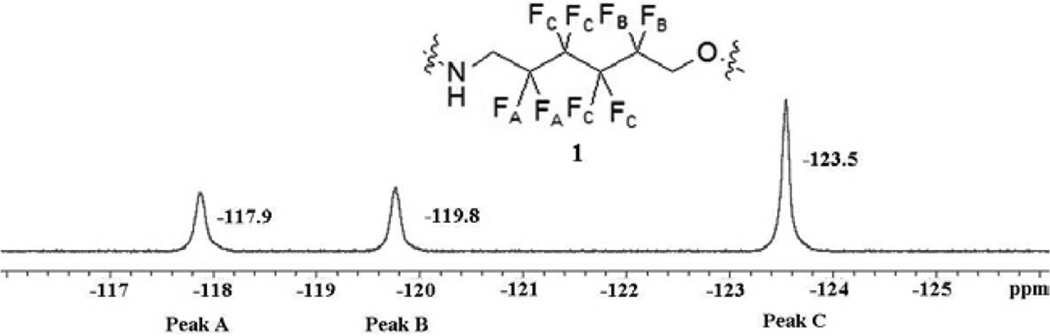
Shell monomer 1 exhibits 3 different peaks in the 19F NMR spectrum, which can be rigorously assigned by a combination of 19F-13C HMBC and 13C-1H HSQC NMR spectra (see supporting information). The most downfield signal (−117.9 ppm, peak A) corresponds to the fluorine CF2 group nearest the guanidine of 1. An upfield (−123.5 ppm, peak C) multiplet corresponds to the two central CF2 groups, and the middle peak of the 19F spectrum (−119.8 ppm, peak B) corresponds to the fluorines closest to the PEG tail: see Supporting Information regarding the 19F signal assignments. The T1 relaxation times were measured in 25 mM pH = 7.6 TRIS-HCl buffer and were found to be 457(8) ms, 436(7) ms, and 497(7) ms for peaks A, B, and C respectively. These data are summarized in Fig. 3
Fig. 3.
NMR Properties of 1.
2.3. Design and synthesis of paramagnetic nanoparticles
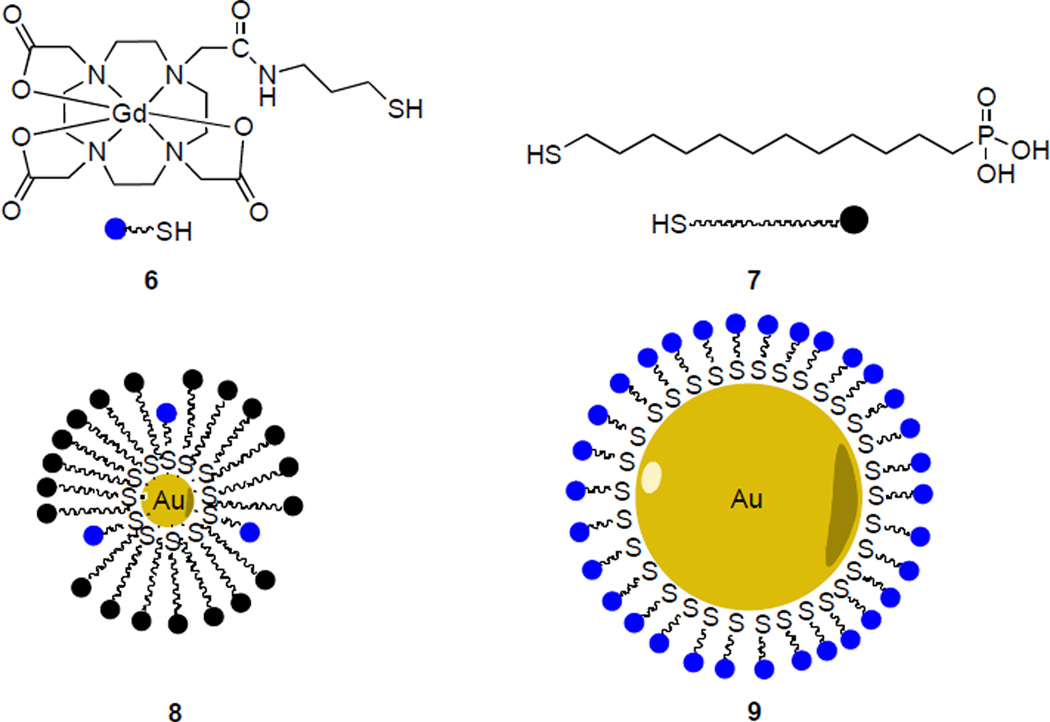
Our design for a template nanoparticle is based on a very simple gold-centered structure, which is decorated with a periphery of thiol-terminated phosphonic acid surfactants (Fig. 4). The selection of this construction is based on its popularity in several scaffolds currently being developed for drug delivery and clinical imaging applications.16 It enables concise size control and flexibility of surface functionalization.17, 18 In this section we present synthetic routes for paramagnetic particles functionalized with both [Gd(DOTA)] and phosphonate peripheries.
Fig. 4.
Composition of nanoparticles.
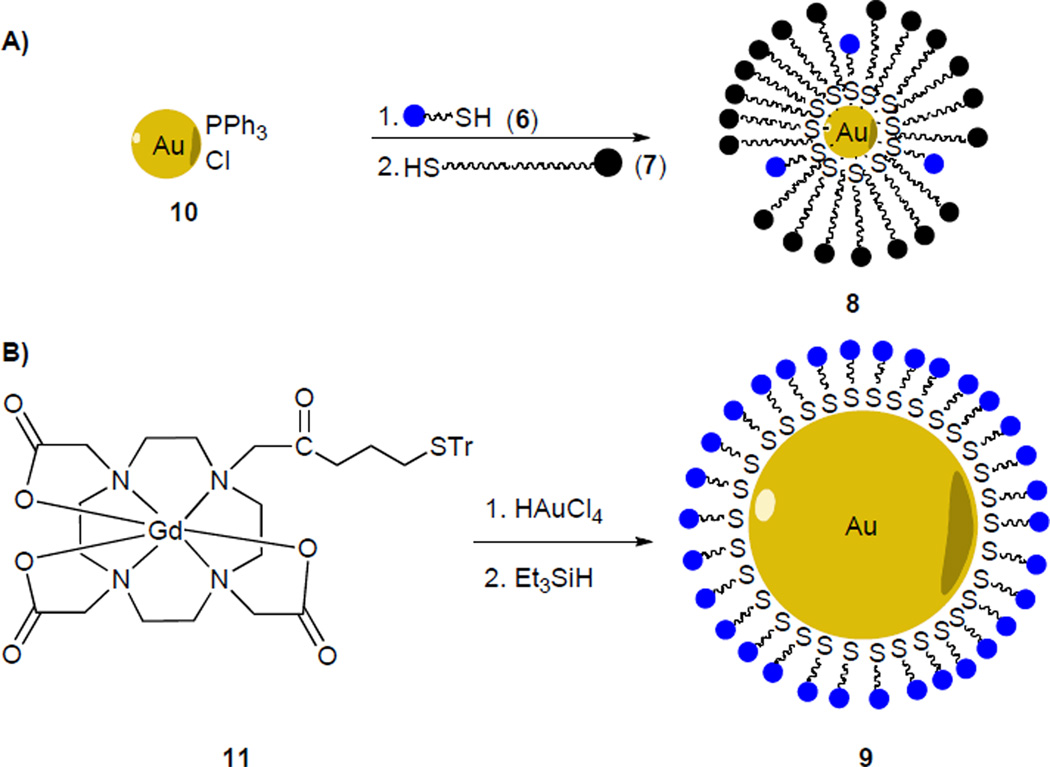
A 1.5 nm phosphine-stabilized gold core (10) was first synthesized based on previously reported conditions.19 Thiols 6 and 7 were assembled onto the gold core via interfacial ligand exchange in a biphasic water/dichloromethane system (Scheme 2A).20 The resulting particles form visible aggregates at neutral pH, but are stable in a pH = 7.6 TRIS-HCl buffer. Monomer 1 spontaneously self assembles onto particles 8 immediately upon introduction to an aqueous solution of 8 to yield hybrid gadolinium-phosphonate particles 2.
Scheme 2.
Synthesis of nanparticles.
Phosphonate-free particles 9 are apparently unstable to aggregation when prepared according to the procedure above, but in-situ deprotection of trityl-protected thiol 11 followed by treatment with HAuCl4 can be used to generate these particles (Scheme 2B).21 This method yields particles that are of 7 nm in hydrodynamic diameter as measured by dynamic light scattering (DLS). 19F longitudinal relaxivity time constants (19F T1) provide evidence for the binding of particle and monomer 1. Upon binding of 1 to the particle core, the fluorine nuclei are brought into close proximity of the particle’s paramagnetic gadolinium centers, where the paramagnetic gadolinium center causes a decrease in the T1 relaxation times of the 19F nuclei.22
2.4. Particle-shell binding
Upon addition of an aqueous solution of particle 8 to 1, the 19F T1 relaxation times for 1 decreased to 332(14) ms, 348(4) ms and 385(4) ms respectively (Table 1). Significant decrease of the observed T1 values upon the addition of paramagnetic particles is consistent with an intimate interaction between 1 and the particle core. To assess the role of the phosphonate functionality on particle 8, phosphonate free particles 9 were also treated with 1. In this experiment, the T1 relaxation times were 434(6) ms, 431(7) ms, 481(5) ms. These data show that 9 exhibits lower changes in T1 than those observed for particle 8. This suggests a more intimate interaction between 1 and 8 versus 1 and 9. Furthermore, exposure of the shell (1) to 1.0 mM GdCl3 (0.25 molar equivalents relative to 1, 158 ppm wt/wt [Gd]) also resulted in a less significant change in 19F T1 than our phosphonate coated particles (8, Table 1), which shows that high [Gd] alone can not account for the observed decrease in 19F T1 observed in the presence of 8.
Table 1.
Decrease of 19F T1 when particle is added to 1.
| Peak A −117.9 ppm |
Peak B −119.8 ppm |
Peak C −123.5 ppm |
|
|---|---|---|---|
| 1 | 457(8) ms | 436(7) ms | 497(7) ms |
| 1 + 8 | 332(14) ms | 348(4) ms | 385(4) ms |
| 1 + 9 | 434(6) ms | 431(7) ms | 481(5) ms |
| 1 + GdCl3 | 429(13) ms | 396(8) ms | 466(6) ms |
1H T1 time constants of H2O for aqueous solutions of particles 8 and 9 reveal that, although 8 is a more effective 19F T1 contrast agent, 9 is a more efficient 1H T1 contrast agent. We measured the H2O 1H T1 relaxation of the same particle solutions used for acquisition of the foregoing 19F T1 data. Particle 8 showed a 1H T1 of 1.18(5) s, and particle 9 exhibited a T1 of 0.495(3) s. The significantly lower H2O 1H T1 of 9 shows that it is a more relaxive 1H contrast agent, whereas particle 8 is a more relaxive 19F T1 contrast agent. This remarkable contrast between 1H and 19F highlights the importance of a guanidinium-phosphate interaction in binding 1 to the particles: whereas phosphonaterich 8 holds 1 in the proximity of the paramagnetic gadolinium, phosphonate-free 9 does so less well, relative to a water standard. Moreover, absolute gadolinium concentration measurements by ICP (ppm wt/wt) indicate [Gd] = 52 ppm in the experimental solution of 8 and [Gd] = 37 ppm in the experimental solution of 9. Although these values are similar, they are inconsistent with the relatively short 1H T1 of the 9 solution, which can be attributed to slower tumbling of the larger particle.
2.5. Particle sizes
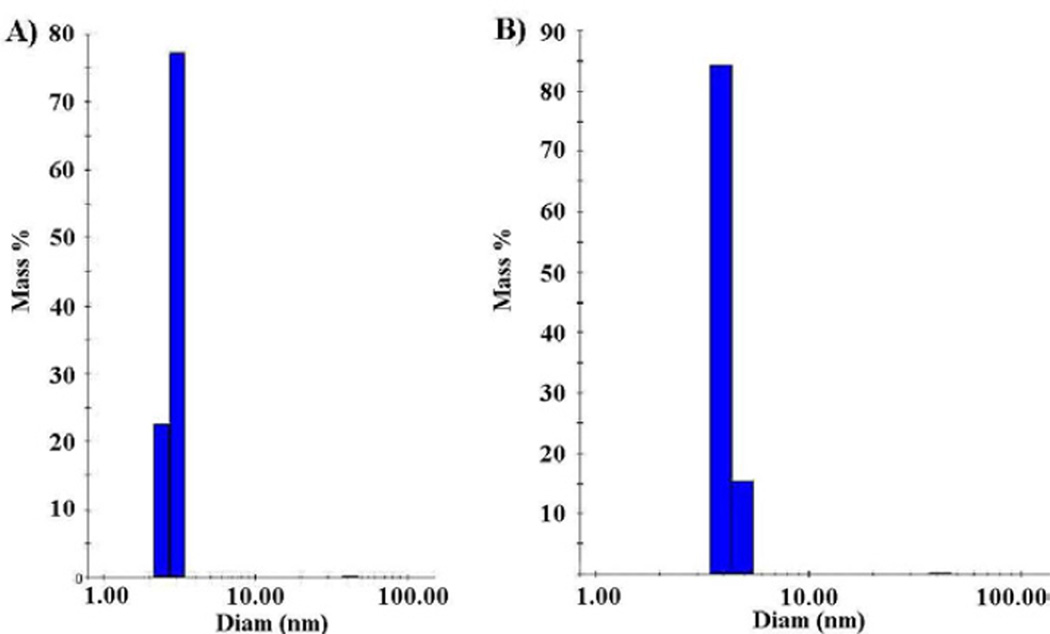
The foregoing relaxivity-based shell binding data are supported by dynamic light scattering (DLS) measurements. DLS data for particle 8 indicate monodisperse particles of hydrodynamic diameter of 3.0 nm (Fig. 5A). Addition of shell to the particle increased the size of the particle to 4.0 nm (Fig. 5B). The increase in diameter of 1 nm upon association of 1 is appropriate for the addition of two equivalents of 1, along with associated water, along the diameter. Further, these data also show that the particles are not aggregating upon changing their surface from phosphonate to PEG.
Fig. 5.
(A) DLS of particle 8 (average hydrodynamic diameter = 3.0 nm) (B) DLS of particle 2 (after the addition of monomer 1, hydrodynamic diameter = 4.0 nm).
3. Conclusion
In conclusion we have characterized the non-covalent coating of a gadolinium-containing gold nanoparticle with a fluorous shell. The coating utilizes a phosphonate-guanidine interaction and is characterized by both a decrease in 19F T1 and an appropriate increase in the particle’s hydrodynamic radius. We further use 1H and 19F NMR relaxivity data to show that phosphonate groups on the surface of a particle increase the particle’s affinity for fluoroguanidine 1 relative to water. Because these observations describe a general procedure for the selective self-assembly for fluoroaklyl groups around the surface of a phosphonate-covered material, we believe that they have potential applications in biomaterials and 19F MRI-based nanotheranostic systems.
4. Experimental section
General procedures
Deuterated NMR solvents were purchased from Cambridge Isotopes Labs. All NMR spectra were obtained on a Varian 500 MHz spectrometer at 25 °C. DLS were acquired on a Wyatt Dynapro Titan instrument. UV-Vis spectra were acquired on a Shimadzu UV-1800 spectrometer. Phosphine-stabilized gold nanoparticles (9) with diameter 1.5 nm were synthesized according to previously reported conditions,23 and characterized by a match of their UV-Vis spectrum. TRIS buffer was purchased from Angus Chemicals. ICP-MS data were acquired by the University of Illinois at Urbana-Champaign School of Chemical Sciences Microanalysis Laboratory.
4.1. Synthesis of gadolinium-thiol 6
Gadolinium thiol 6 was synthesized based on previously reported conditions.23 Mass data for 6 is consistent with a known sample. MALDI for C19H32GdN5O7S: Calculated 632.13 g/mol; found 631.94 g/mol. Measured isotopic distribution matches calculated prediction.
4.2. Synthesis of particle 8
Phosphine stabilized gold nanoparticles 10 (1.0 mg) were dissolved in dichloromethane (1 mL), and TRIS-HCl buffer (1 mL of 25 mM pH = 7.6) was added to form a biphasic mixture. While stirring at room temperature, gadolinium thiol 6 (40 µL of a 10 mM aqueous solution) was added, and the solution was stirred for 4 hours. Thiol phosphonic acid 724 was then added and the reaction was stirred until the brown color partitioned completely into the aqueous layer. The aqueous layer was diluted with TRIS-HCl buffer (1.0 mL of a 25 mM solution at pH = 7.6) and extracted with dichloromethane25 (5 mL, 5 times), then filtered through a 0.45 µm syringe filter to yield an aqueous solution of particle 8.
4.3. Synthesis of particle 9
Trityl-protected gadolinium complex 11 (33.4 mg, 38 µmol) was suspended in tetrahydrofuran (0.4 mL). Chloroauric acid26 (HAuCl4, 13.0 mg, 38 µmol) was added to the solution at room temperature and the reaction was stirred for 1 hour. Triethyl silane27 (Et3SiH, 4.2 mg, 38 µmol) was then added, and the solution stirred for 18 hours at room temperature. The solution was filtered and ethanol28 (10 mL) was added to initiate precipitatation. The precipitate was washed with ethanol (5 times, 10 mL), and the filter paper was sonicated in H2O (10 mL) to dissolve precipitate. The resulting aqueous solution was then filtered through a 0.45 mm syringe filter to yield particles 9 as an aqueous solution. DLS measurements on the resulting solution showed a monodisperse nanoparticulate material with hydrodynamic diameter of 7 nm.
4.4. Measurement of 19F T1 of 1-coated particles
A portion of 1 (3 µL of a 100 mM solution) was lyophilized to an oil in a 1/2 dram glass vial. A 125 µL aliquot of aqueous particle solution was delivered to the vial containing lypholized 1, stirred, then placed in a 3 mm diameter coaxial NMR tube insert and 19F T1 time constants were acquired using the parameters previously stated.
Supplementary Material
Acknowledgments
This work is sponsored by the Donald E. and Delia B. Baxter Foundation, the USC Ming Hsieh Institute, and the Robert E. and May R. Wright Foundation. We are grateful to the National Science Foundation (DBI-0821671, CHE-0840366), the National Institutes of Health (1 S10 RR25432), and the University of Southern California for their sponsorship of NMR spectrometers. We are grateful to Anna Dawsey for synthesis of the gold core, Xinping Wu for the synthesis of the shell monomer, Christopher Beier for assistance in acquiring UV-Vis spectra, Dr. Shuxing Li and the USC nanobiophysics core for assistance in acquiring DLS data, and Dr. Allan Kershaw for assistance in acquiring NMR data.
Footnotes
Supplementary Material
Graphical 1-D and 2-D NMR spectra, NMR T1 inversion recovery spectra and fits, optical absorption spectra, and DLS histograms. Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/xxx
References and Notes
- 1.Barreto JA, O'Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Mat. Views. 2011;23:H18. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 2.(a) Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Cancer Res. 1994;54:987. [PubMed] [Google Scholar]; (b) Lasic DD. Nature. 1996;380:561. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]; (c) Malam Y, Loizidou M, Seifalian AM. Trends Pharmacol. Sci. 2009;30:592. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.a) Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. Biomaterials. 2010;31:3016. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang F, Huang X, Zhu L, Guo N, Niu G, Swierczewska M, Lee S, Xu H, Wang AY, Mohamedali KA, Rosenblum MG, Lu G, Chen X. Biomaterials. 2012;33:5414. doi: 10.1016/j.biomaterials.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Medarova Z, Rashkovetsky L, Pantazopoulos P, Moore A. Cancer Res. 2009;69:1182. doi: 10.1158/0008-5472.CAN-08-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Guthi JS, Yang S, Huang G, Li S, Khemtong C, Kessinger CW, Peyton M, Minna JD, Brown KC, Gao J. Mol. Pharmaceutics. 2009;7:32. doi: 10.1021/mp9001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson AL, Binderup T, Rasmussen P, Henriksen JR, Elma DR, Kjaer A, Andresen TL. Biomaterials. 2001;32:2334. doi: 10.1016/j.biomaterials.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Rowe MD, Thamm DH, Kraft SL, Boyes SG. Biomacromolecules. 2009;10:983. doi: 10.1021/bm900043e. [DOI] [PubMed] [Google Scholar]
- 6.a) Chen T, Xu S, Zhao T, Zhu L, Wei D, Li Y, Zhang H, Zhao C. ACS Appl. Mater. Interfaces. 2012;4:5766. doi: 10.1021/am301223n. [DOI] [PubMed] [Google Scholar]; b) Marega R, Karmani L, Flamant L, Nageswaran PG, Valembois V, Masereel B, Feron O, Borght TV, Lucas S, Michiels C, Gallez B, Bonifazi D. J. Mater. Chem. 2012;22:21305. [Google Scholar]; c) Bisker G, Yeheskely-Hayon D, Minai L, Yelin D. J. Controlled Release. 2012;162:303. doi: 10.1016/j.jconrel.2012.06.030. [DOI] [PubMed] [Google Scholar]; d) Bhattacharyya S, Khan JA, Curran GL, Robertson JD, Bhattacharyya R, Mukherjee P. Adv. Mater. 2011;23:5034. doi: 10.1002/adma.201102287. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Angew. Chem. Int. Ed. 2010;49:3280. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Chang AY, Williams TJ, Boz E. Ultrasound-Activated Nanoparticles as Imaging Agnets and Drug Delivery Vehicles PCT. WO2011079317 A3. Int. Appl. 2011; b) Williams TJ, Chang AY. Removable Protective Shell for Imaging Agents and Bioactive Substances. Appl. WO 2012178184 A2. PCT Int. 2012
- 8.(a) Frankel AD, Pabo CO. Cell. 1988;55:1189. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13003. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. Nature Med. 2000;6:1253. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]; (d) Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. J. Am. Chem. Soc. 2004;126:9506. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 9.Siprashvili Z, Scholl FA, Oliver SF, Adams A, Contag CH, Wender PA, Khavari PA. Human Gene Therapy. 2003;14:1225. doi: 10.1089/104303403767740768. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Boz E, Sirkis AM, Chang AY, Williams TJ. J. Fluorine Chem. 2012;135:292. [Google Scholar]
- 11.a) Janjic JM, Srinivas M, Kadayakkara DKK, Ahrens ET. J. Am. Chem. Soc. 2008;130:2832. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]; b) Du W, Nystrom AM, Zhang L, Powell KT, Li Y, Cheng C, Wickline SA, Wooley KL. Biomacromolecules. 2008;9:2826. doi: 10.1021/bm800595b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diou O, Tsapis N, Fattal E. Expert Opin. Drug Deliv. 2012;9:1475. doi: 10.1517/17425247.2012.736486. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Cabelloa J, Barnetta BP, Bottomleya PA, Bulte JWM. NMR Biomed. 2011;24:114. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallach DFH, Mathur R. In: Handbook of Nonmedical Applications of Liposomes. Lasic DD, Barenholz DDLY, Barenholz Y, editors. CRC Press; 1996. pp. 115–123. [Google Scholar]
- 15.(a) Kotov SV, Ivanov GD, Kostov GK. J. Fluorine Chem. 1988;41:293. [Google Scholar]; (b) Tortelli V, Tonelli C. J. Fluorine Chem. 1990;47:199. [Google Scholar]; (c) Takakura T, Yamabe M, Kato M. J. Fluorine Chem. 1988;41:173. [Google Scholar]
- 16.a) Kumar A, Zhang X, Liang X. Biotechnol. Adv. 2012 doi: 10.1016/j.biotechadv.2012.10.002. in press. [DOI] [PubMed] [Google Scholar]; b) Rana S, Bajaj A, Mout R, Rotello VM. Adv. Drug Delivery Rev. 2012;64:200. doi: 10.1016/j.addr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rippel RA, Seifalian AM. J. Nanosci. Nanotechnol. 2011;11:3740. doi: 10.1166/jnn.2011.4170. [DOI] [PubMed] [Google Scholar]; d) Kim C, Ghosh P, Rotello VM. Nanoscale. 2009;1:61. doi: 10.1039/b9nr00112c. [DOI] [PubMed] [Google Scholar]; e) Kumawat L, Jain A. Int. J. Pharm. Sci. Res. 2012;3:52. [Google Scholar]
- 17.a) Gangwar RK, Dhumale VA, Kumari D, Nakate UT, Gosavi SW, Sharma RB, Kale SN, Datar S. Mat. Sci. Eng. C. 2012;32:2659. [Google Scholar]; b) Kodiyan A, Silva EA, Kim J, Aizenberg M, Mooney DJ. ACS Nano. 2012;6:4796. doi: 10.1021/nn205073n. [DOI] [PubMed] [Google Scholar]
- 18.a) Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. Nanoscale Res. Lett. 2011;6:283. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Akiyama Y, Mori T, Katayama Y, Niidome T. J. Controlled Release. 2009;139:81. doi: 10.1016/j.jconrel.2009.06.006. [DOI] [PubMed] [Google Scholar]; c) Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 19.Weare WW, Reed SM, Warner MG, Hutchison JE. J. Am. Chem. Soc. 2000;122:12890. [Google Scholar]
- 20.Warner MG, Reed SM, Hutchison JE. Chem. Mater. 2000;12:3316. [Google Scholar]
- 21.Wallner A, Jafri SHM, Blom T, Gogoll A, Leifer K, Baumgartner J, Ottosson H. Langmuir. 2011;27:9057. doi: 10.1021/la2019007. [DOI] [PubMed] [Google Scholar]
- 22.Mizukami S, Takikawa R, Sugihara F, Hori Y, Tochio H, Walchli M, Shirakawa M, Kiuchi K. J. Am. Chem. Soc. 2007;130:794. doi: 10.1021/ja077058z. [DOI] [PubMed] [Google Scholar]
- 23.Raghunand N, Jagadish B, Trouard TP, Galons JP, Gillies RJ, Mash EA. Magn. Reson. Med. 2006;55:1272. doi: 10.1002/mrm.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(12-mercaptododecyl)phosphonic acid was purchased from SiKÉMIA.
- 25.Dichloromethane was purchased from Macron Chemicals.
- 26.Chloroauric acid was purchased from STREM Chemicals.
- 27.Triethylsilane was purchased from Alfa Aesar.
- 28.Ethanol was purchased from Koptec.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.