Abstract
Plasmodium falciparum merozoites engage the erythrocyte surface through several receptor (host)–ligand (parasite) interactions during a brief exchange that results in parasite invasion of the red blood cell. Tens of thousands of these events occur during the initial cycle of blood-stage infections but advance towards billions as the parasite becomes visible to microscopists attempting to diagnose the underlying cause of illness in febrile patients. Advancing blood-stage infection leads to massive proportions of erythrocytes that rupture during repetitive cycles of asexual reproduction. As the infection leads to illness, non-immune or semi-immune individuals can suffer from life-threatening consequences of severe malarial anemia that play a leading role in pathogenesis. Through natural selection, some erythrocyte membrane polymorphisms are likely to have reduced the invasion success of the P. falciparum merozoite and increased the fitness of the human host population.
In the mid-1970s, initial insights into the molecular nature of the interactions between erythrocyte surface proteins and malaria parasites were gained using Plasmodium knowlesi, a simian parasite amenable to in vitro culture [1]. These studies ultimately led to the discovery that the erythrocyte membrane protein carrying the Duffy blood group was a crucial invasion receptor for Plasmodium knowlesi and the related human parasite Plasmodium vivax [2,3]. These seminal experiments performed by Louis Miller and his colleagues paved the way to the exploration of two key concepts that underlie susceptibility of the red blood cell to malaria infection [4]. First, specific proteins on the erythrocyte surface are co-opted by the merozoite to activate and promote parasite invasion [5,6]. This hypothesis implies that parasite proteins somehow bind erythrocyte receptors and work in partnership as the merozoite pulls itself into the cell and forms the parasitophorous vacuole. Second, polymorphisms that modify the structure or level of expression of erythrocyte membrane proteins alter the efficiency of invasion and, ultimately, susceptibility to malaria. As malaria kills millions of children each year in endemic areas, mutations that diminish the efficiency of the invasion process would confer a selective advantage to the host and might be expected to increase in frequency over time through natural selection. Given the relatively short generation time of malaria compared with humans (asexual stages of 2–4 days and life cycle from 1–4 months for Plasmodium spp. versus 20 years for humans) and an appreciation of the genetic diversity of malaria species, it is reasonable to consider that the parasite has adapted in response to the polymorphic erythrocyte landscape in which it lives. The blueprint for recent investigations of erythrocyte membrane polymorphisms in Papua New Guinea (PNG) is based upon these concepts. Results from these studies provide evidence that the Gerbich-negative (Ge−) blood group phenotype, highly prevalent in malarious regions of PNG, has arisen to confer protection from P. falciparum merozoite invasion [7,8].
Gerbich-negativity and field studies in PNG
The Ge− phenotype is caused by the deletion of exon 3 in the glycophorin C gene (GYPCΔex3; human chromosome 2q14-q21) and is found in Hardy–Weinberg equilibrium in malaria holoendemic regions of PNG [7–13]. Glycophorin C (128 amino acid residues, apparent 35 kDa, integral membrane sialoglycoprotein) [10] is a physiologically important monomer that interacts with the peripheral membrane protein 4.1 to mediate attachment of the sub-membranous cytoskeleton to the erythrocyte membrane. Our recent study conducted in the Wosera region of East Sepik Province revealed an association between GYPC genotypes and ovalocytic erythrocyte morphology [8]. An increased proportion of ovalocytic erythrocytes were observed in blood smears from individuals who were heterozygous or homozygous for GYPCΔex3 compared with individuals who were homozygous GYPC wild-type. It is important to note that levels of ovalocytes were higher in Papua New Guineans than North American control individuals regardless of GYPC genotype. Furthermore, this association was independent of the 27 base-pair deletion in the band 3 gene (SLC4A1Δ27) that deletes nine amino acids from the intracellular NH2-terminal region of this abundant integral membrane protein [14]. Mgone et al. have previously described a strong correlation between SLC4A1Δ27 heterozygosity and Southeast Asian ovalocytosis [15]. As no individuals who are homozygous for SLC4A1Δ27 have yet been identified, it is presumed that the mutation in both copies of the gene is lethal during fetal development [16]. Overall, these findings suggest that multiple erythroid polymorphisms and as-yet unidentified environmental factors influence erythrocyte morphology.
To assess the effect of GYPC genotype on susceptibility to blood-stage infection, we examined the malaria infection status of a population in the Wosera at monthly intervals over a seven-month period. The frequency and intensity of blood-stage P. falciparum or P. vivax infection were not associated with GYPC genotype [8]. This lack of, or equivocal, correlations between erythroid polymorphisms and malaria infection status have been reported for other erythroid polymorphisms. In a previous study in PNG, Allen et al. found that α-globin polymorphisms associated with α+ -thalassemia that have reached genetic fixation in malaria endemic regions of PNG, were protective against severe malaria, but werenot associated with reduced parasitemia [17]. In regard to the balanced SLC4A1Δ27 polymorphism, although two independent studies have observed significant association between this polymorphism and protection from severe malaria [18,19], the polymorphism was not consistently associated with reduced infection prevalence or parasitemia [18,19]. These findings are not unusual to PNG as numerous studies from Africa on the β-globin hemoglobin S (HbS) (sickle cell) [20] and HbC polymorphisms [21,22], human leukocyte antigen (HLA) [20] and glucose-6-phosphate dehydrogenase deficiency variants [23] have reported protection against severe malaria morbidity with equivocal reduction in susceptibility to infection.
Erythrocyte receptors and parasite-binding ligand interactions
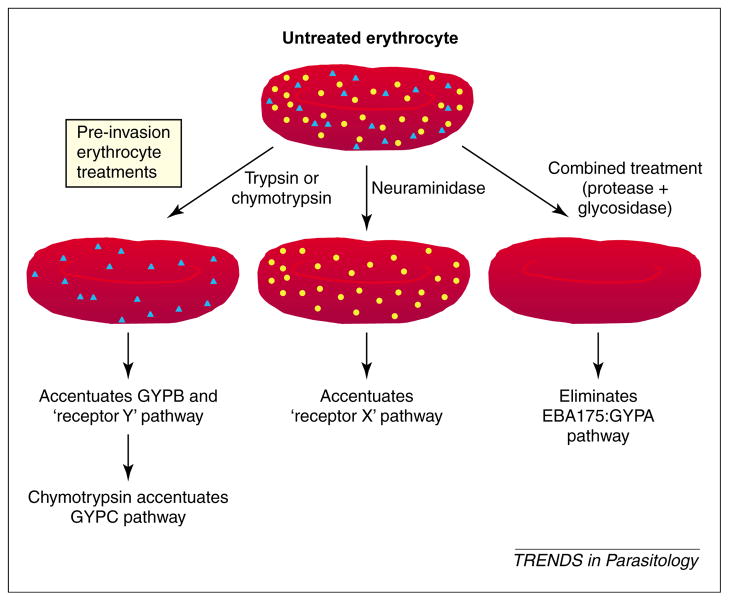
Early results identifying the Duffy blood group antigen as the invasion receptor for P. knowlesi and P. vivax [2,3] implicated the blood group proteins as merozoite targets of human Plasmodium parasite species. Strategies using protease and neuraminidase treatment to cleave and alter polypeptide and sialic acid side-chains, respectively, have identified at least four major erythrocyte receptor pathways leading to P. falciparum invasion [6,24]. These include pathways that use glycophorin A (GYPA; trypsin sensitive, neuraminidase sensitive), glycophorin B (GYPB) and receptor Y (trypsin resistant, neuraminidase sensitive), and receptor X (trypsin sensitive, neuraminidase resistant). Identification of Plasmodium erythrocyte-binding ligands (EBL) or antigens (EBA) is gaining momentum as these proteins exhibit genomic and structural similarities (e.g. single-copy genes, conserved multi-exon gene organization, intron–exon boundaries, and amino- and C-terminal cysteine-rich domains). A recent review by Adams et al. provides an update of this growing list of related molecules [25]. These findings are summarized in Fig. 1.
Fig. 1.
Pathways of erythrocyte invasion. Plasmodium falciparum uses protein and polysaccharide epitopes to engage erythrocytes using a multi-protein invasion complex (reviewed in Ref. [34]). Enzyme treatments modify erythrocytes to accentuate specific generalized pathways by limiting or eliminating access to epitopes that are sensitive to treatment. The pathway denoted by receptor ‘x’ represents erythrocyte invasion that is accentuated by neuraminidase pre-treatment of erythrocytes. The pathway denoted by GYPB and receptor y is accentuated by trypsin or chymotrypsin pre-treatment of erythrocytes. Yellow circles and blue triangles indicate polypeptide epitopes and polysaccharide epitopes, respectively. Abbreviations: EBA, erythrocyte-binding antigen; GYP, glycophorin.
Parasite ligands known to interact with specific host receptors include the P. knowlesi and P. vivax Duffy-binding proteins (Pk- and Pv-DBP) and the Duffy blood group antigen, P. falciparum EBA175 and GYPA. Recently, data mining of the P. falciparum chromosome 13 DNA sequence by three groups identified a new 140 kDa EBL, termed EBA140 (alternatively, BAEBL or EBP-2) [26–28]. Evidence suggesting that the erythrocyte receptor for EBA140 is human GYPC has included traditional strategies usingerythrocytesfromdonorswithbloodgroupdeficiencies and enzyme modifications of erythrocyte cell surfaces [24].
In our more recent study [29], we have provided additional proof that GYPC is the receptor for EBA140. By overlaying parasite proteins shed into culture supernatant onto western blots of erythrocyte membrane proteins, we showed that EBA140 bound to the wild-type GYPC protein, butnottoGYPCoffivePapuaNewGuineanshomozygousfor GYPCΔex3. Evidence that the EBA140–GYPC interaction is physiologically important was revealed by experiments that used P. falciparum strains 3D7 and W2mef, the respective isogenic EBA140 knockout progeny, Ge+ and Ge− negative erythrocytes, antibodies against the EBA140 F2 domain (predicted GYPC-binding region [30]) and chymotrypsin treatment (to limit non-GYPC invasion pathways). Overall, we found that antibodies to EBA140 inhibited merozoite invasion most effectively when invasion of wild-type parasites was restricted to the GYPC pathway, suggesting that, when available, the EBA140–GYPC interaction is important during the process of parasite invasion. That relative invasion efficiencies were not influenced by EBA140 antibody in experiments using Ge− erythrocytes or EBA140-knockout parasites suggests that P. falciparum invades erythrocytes by alternative pathways when GYPC is not available as a receptor.
A recent study by Lobo et al. [31] adds further support to the prediction that the GYPC (receptor)–EBA140 (ligand) interaction is an important invasion pathway for P. falciparum. However, their observations suggesting that the binding region for EBA140 resides in a nine-amino-acid region encoded by exon 2 of the GYPC gene appear to conflict with our studies using erythrocytes from GYPCΔex3 homozygous (Ge−) donors from PNG. Potential explanations for these differences include the possibility that deletions of either GYPC exon 2 or exon 3 would either remove sialic acid epitope(s) necessary for EBA140 binding or alter the presentation of sialic acid epitope(s) in a neighboring region of the GYPC protein. Additional experiments using erythrocytes characterized for various Gerbich phenotypes and underlying GYPC gene polymorphisms, along with additional EBL control interactions, will be helpful in sorting out these differences.
Concluding remarks
Ongoing studies continue to investigate how malaria parasites in PNG interact with the erythrocyte membrane polymorphic proteins that have already been identified. Evidence for EBA140 polymorphism and differences in GYPC binding have already been observed [29–32]. Given the number of erythrocyte membrane polymorphisms observed in PNG so far, the availability of a more global proteomic analysis [33] could provide further insight into how malaria is involved in selection of erythrocyte membrane protein polymorphisms. Finally, given the impact of the SLC4A1Δ27 mutation on protection from severe malaria in PNG, it is clearly important to investigate how GYPCΔex3 influences malaria pathogenesis through case control studies.
Acknowledgments
We thank study volunteers for their willing participation, Lawrence Rare, Manasseh Baea, Moses Biasor, Benson Kiniboro, Aaron Wane, Gerard Casey and Will Kastens for supervising field study teams, assistance in the development of recently complete and ongoing field-based research studies, and collection of samples. We also thank David McNamara and Svetlana Katsnelson for technical assistance, and Brendan Crabb and Alan Cowman for helpful discussions leading to the completion of this manuscript. This work was supported by grants from the National Institutes of Health (AI36478-07, AI46991-01A2, AI49390-01), the National Health and Medical Research Council of Australia, the Howard Hughes Medical Institute and the Wellcome Trust. A.G.M. is a recipient of a Deutsche Forschungsgemeinschaft Research Fellowship.
References
- 1.Butcher GA, et al. Mechanism of host specificity in malarial infection. Nature. 1973;244:40–42. doi: 10.1038/244040a0. [DOI] [PubMed] [Google Scholar]
- 2.Miller LH, et al. Erythrocyte receptors for(Plasmodiumknowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, et al. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 4.Miller LH, et al. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak JA, et al. Invasion of erythrocytes by malaria merozoites. Science. 1975;187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 6.Barnwell JW, Galinski MR. Invasion of vertebrate cells: erythrocytes. In: Sherman IW, editor. Malaria: Parasite Biology, Pathogenesis, and Protection. American SocietyofMicrobiology Press; 1998. pp. 93–120. [Google Scholar]
- 7.Booth PB, McLoughlin K. The Gerbich blood group system, especially in Melanesians. Vox Sang. 1972;22:73–84. doi: 10.1111/j.1423-0410.1972.tb03968.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel SS, et al. The association of the glycophorin C exon 3 deletion with ovalocytosis and malaria susceptibility in the Wosera, Papua New Guinea. Blood. 2001;98:3489–3491. doi: 10.1182/blood.v98.12.3489. [DOI] [PubMed] [Google Scholar]
- 9.Booth PB, et al. Red cell antigen, serum protein and red cell enzyme polymorphisms in Karkar Islanders and inhabitants of the adjacent north coast of New Guinea. Hum Hered. 1982;32:385–403. doi: 10.1159/000153328. [DOI] [PubMed] [Google Scholar]
- 10.Cartron JP, et al. Glycophorin C and related glycoproteins: structure, function, and regulation. Semin Hematol. 1993;30:152–168. [PubMed] [Google Scholar]
- 11.Colin Y, et al. Human erythrocyte glycophorin C. Gene structure and rearrangement in genetic variants. J Biol Chem. 1989;264:3773–3780. [PubMed] [Google Scholar]
- 12.High S, et al. Rearrangements of the red-cell membrane glycophorin C (sialoglycoprotein β) gene. A further study of alterations in the glycophorin C gene. Biochem J. 1989;262:47–54. doi: 10.1042/bj2620047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serjeantson SW, et al. A 3.5 kb deletion in the glycophorin C gene accounts for the Gerbich-negative blood group in Melanesians. Immunol Cell Biol. 1994;72:23–27. doi: 10.1038/icb.1994.4. [DOI] [PubMed] [Google Scholar]
- 14.Jarolim P, et al. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci USA. 1991;88:11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mgone CS, et al. The correlation between microscopical examination and erythrocyte band 3 (AE1) gene deletion in south-east Asian ovalocytosis. Trans R Soc Trop Med Hyg. 1998;92:296–299. doi: 10.1016/s0035-9203(98)91019-7. [DOI] [PubMed] [Google Scholar]
- 16.Mgone CS, et al. Occurrence of the erythrocyte band 3 (AE1) gene deletion in relation to malaria endemicity in Papua New Guinea. Trans R Soc Trop Med Hyg. 1996;90:228–231. doi: 10.1016/s0035-9203(96)90223-0. [DOI] [PubMed] [Google Scholar]
- 17.Allen SJ, et al. α +-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci U S A. 1997;94:14736–14741. doi: 10.1073/pnas.94.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genton B, et al. Ovalocytosis and cerebral malaria. Nature. 1995;378:564–565. doi: 10.1038/378564a0. [DOI] [PubMed] [Google Scholar]
- 19.Allen SJ, et al. Prevention of cerebral malaria in children in Papua New Guinea by Southeast Asian ovalocytosis band 3. Am J Trop Med Hyg. 1999;60:1056–1060. doi: 10.4269/ajtmh.1999.60.1056. [DOI] [PubMed] [Google Scholar]
- 20.Hill AV, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 22.Modiano D, et al. Haemoglobin C protects against clinicalPlasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 23.Ruwende C, Hill A. Glucose-6-phosphate dehydrogenase deficiency and malaria. J Mol Med. 1998;76:581–588. doi: 10.1007/s001090050253. [DOI] [PubMed] [Google Scholar]
- 24.Miller LH, et al. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and. Plasmodium knowlesi J Exp Med. 1977;146:277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams JH, et al. An expanding ebl family of. Plasmodium falciparum Trends Parasitol. 2001;17:297–299. doi: 10.1016/s1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- 26.Mayer DC, et al. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JK, et al. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 28.Narum DL, et al. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol Biochem Parasitol. 2002;119:159–168. doi: 10.1016/s0166-6851(01)00428-5. [DOI] [PubMed] [Google Scholar]
- 29.Maier AG, et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim BK, et al. Receptor and ligand domains for invasion of erythrocytes by. Plasmodium falciparum Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 31.Lobo CA, et al. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl) Blood. doi: 10.1182/blood-2002-10-3076. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Mayer DC, et al. Polymorphism in a Plasmodium falciparum erythrocyte-binding ligand changes its receptor specificity. J Exp Med. 2002;196:1523–1528. doi: 10.1084/jem.20020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low TY, et al. Separation of human erythrocyte membrane associatedproteinswithone-dimensionalandtwo-dimensionalgelelectro-phoresis followed by identification with matrix-assisted laser desorption/ ionization-time of flight mass spectrometry. Proteomics. 2002;2:1229–1239. doi: 10.1002/1615-9861(200209)2:9<1229::AID-PROT1229>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Chitnis CE, Blackman MJ. Host cell invasion by malaria parasites. Parasitol Today. 2000;16:411–415. doi: 10.1016/s0169-4758(00)01756-7. [DOI] [PubMed] [Google Scholar]