Abstract
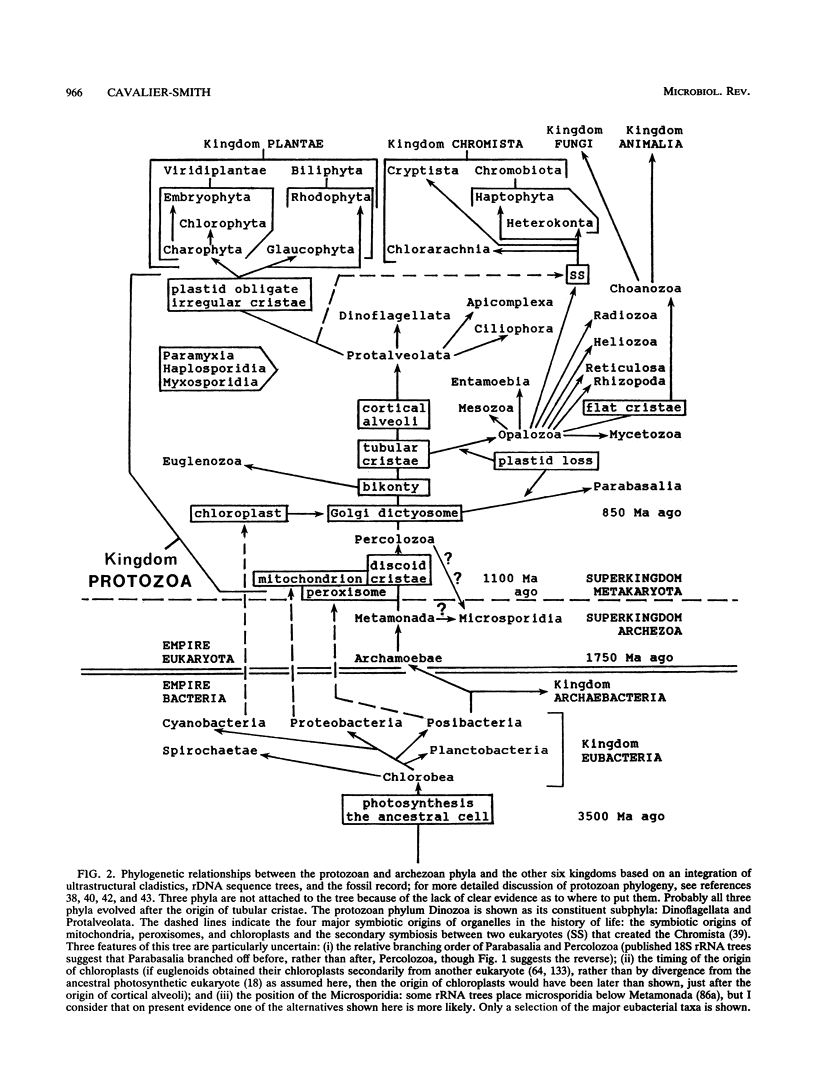
The demarcation of protist kingdoms is reviewed, a complete revised classification down to the level of subclass is provided for the kingdoms Protozoa, Archezoa, and Chromista, and the phylogenetic basis of the revised classification is outlined. Removal of Archezoa because of their ancestral absence of mitochondria, peroxisomes, and Golgi dictyosomes makes the kingdom Protozoa much more homogeneous: they all either have mitochondria and peroxisomes or have secondarily lost them. Predominantly phagotrophic, Protozoa are distinguished from the mainly photosynthetic kingdom Chromista (Chlorarachniophyta, Cryptista, Heterokonta, and Haptophyta) by the absence of epiciliary retronemes (rigid thrust-reversing tubular ciliary hairs) and by the lack of two additional membranes outside their chloroplast envelopes. The kingdom Protozoa has two subkingdoms: Adictyozoa, without Golgi dictyosomes, containing only the phylum Percolozoa (flagellates and amoeboflagellates); and Dictyozoa, made up of 17 phyla with Golgi dictyosomes. Dictyozoa are divided into two branches: (i) Parabasalia, a single phylum with hydrogenosomes and 70S ribosomes but no mitochondria, Golgi dictyosomes associated with striated roots, and a kinetid of four or five cilia; and (ii) Bikonta (16 unicellular or plasmodial phyla with mitochondria and bikinetids and in which Golgi dictyosomes are not associated with striated ciliary roots), which are divided into two infrakingdoms: Euglenozoa (flagellates with discoid mitochondrial cristae and trans-splicing of miniexons for all nuclear genes) and Neozoa (15 phyla of more advanced protozoa with tubular or flat [usually nondiscoid] mitochondrial cristae and cis-spliced spliceosomal introns). Neozoa are divided into seven parvkingdoms: (i) Ciliomyxa (three predominantly ciliated phyla with tubular mitochondrial cristae but no cortical alveoli, i.e., Opalozoa [flagellates with tubular cristae], Mycetozoa [slime molds], and Choanozoa [choanoflagellates, with flattened cristae]); (ii) Alveolata (three phyla with cortical alveoli and tubular mitochondrial cristae, i.e., Dinozoa [Dinoflagellata and Protalveolata], Apicomplexa, and Ciliophora); (iii) Neosarcodina (phyla Rhizopoda [lobose and filose amoebae] and Reticulosa [foraminifera; reticulopodial amoebae], usually with tubular cristae); (iv) Actinopoda (two phyla with axopodia: Heliozoa and Radiozoa [Radiolaria, Acantharia]); (v) Entamoebia (a single phylum of amoebae with no mitochondria, peroxisomes, hydrogenosomes, or cilia and with transient intranuclear centrosomes); (vi) Myxozoa (three endoparasitic phyla with multicellular spores, mitochondria, and no cilia: Myxosporidia, Haplosporidia, and Paramyxia); and (vii) Mesozoa (multicells with tubular mitochondrial cristae, included in Protozoa because, unlike animals, they lack collagenous connective tissue).
Full text
PDF








































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkin A., Schütze K., Dziedzic J. M., Euteneuer U., Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990 Nov 22;348(6299):346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- Bakker-Grunwald T., Wöstmann C. Entamoeba histolytica as a model for the primitive eukaryotic cell. Parasitol Today. 1993 Jan;9(1):27–31. doi: 10.1016/0169-4758(93)90161-8. [DOI] [PubMed] [Google Scholar]
- Belcher J. H., Swale E. M. Luffisphaera gen.nov., an enigmatic scaly micro-organism. Proc R Soc Lond B Biol Sci. 1975 Mar 11;188(1093):495–499. doi: 10.1098/rspb.1975.0033. [DOI] [PubMed] [Google Scholar]
- Bowman B. H., Taylor J. W., Brownlee A. G., Lee J., Lu S. D., White T. J. Molecular evolution of the fungi: relationship of the Basidiomycetes, Ascomycetes, and Chytridiomycetes. Mol Biol Evol. 1992 Mar;9(2):285–296. doi: 10.1093/oxfordjournals.molbev.a040720. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Archamoebae: the ancestral eukaryotes? Biosystems. 1991;25(1-2):25–38. doi: 10.1016/0303-2647(91)90010-i. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Eukaryote kingdoms: seven or nine? Biosystems. 1981;14(3-4):461–481. doi: 10.1016/0303-2647(81)90050-2. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Eukaryotes with no mitochondria. 1987 Mar 26-Apr 1Nature. 326(6111):332–333. doi: 10.1038/326332a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991 May;7(5):145–148. [PubMed] [Google Scholar]
- Cavalier-Smith T. Origin of the cell nucleus. Bioessays. 1988 Aug-Sep;9(2-3):72–78. doi: 10.1002/bies.950090209. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Origins of secondary metabolism. Ciba Found Symp. 1992;171:64–87. doi: 10.1002/9780470514344.ch5. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The evolutionary origin and phylogeny of eukaryote flagella. Symp Soc Exp Biol. 1982;35:465–493. [PubMed] [Google Scholar]
- Cavalier-Smith T. The kingdoms of organisms. Nature. 1986 Dec 4;324(6096):416–417. doi: 10.1038/324416a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The number of symbiotic origins of organelles. Biosystems. 1992;28(1-3):91–108. doi: 10.1016/0303-2647(92)90011-m. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The origin of cells: a symbiosis between genes, catalysts, and membranes. Cold Spring Harb Symp Quant Biol. 1987;52:805–824. doi: 10.1101/sqb.1987.052.01.089. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The origin of eukaryotic and archaebacterial cells. Ann N Y Acad Sci. 1987;503:17–54. doi: 10.1111/j.1749-6632.1987.tb40596.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann N Y Acad Sci. 1987;503:55–71. doi: 10.1111/j.1749-6632.1987.tb40597.x. [DOI] [PubMed] [Google Scholar]
- Christen R., Ratto A., Baroin A., Perasso R., Grell K. G., Adoutte A. An analysis of the origin of metazoans, using comparisons of partial sequences of the 28S RNA, reveals an early emergence of triploblasts. EMBO J. 1991 Mar;10(3):499–503. doi: 10.1002/j.1460-2075.1991.tb07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss J. O. Should there be a separate code of nomenclature for the protists? Biosystems. 1992;28(1-3):1–14. doi: 10.1016/0303-2647(92)90003-h. [DOI] [PubMed] [Google Scholar]
- Corliss J. O. The kingdom Protista and its 45 phyla. Biosystems. 1984;17(2):87–126. doi: 10.1016/0303-2647(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Corliss J. O. What are the taxonomic and evolutionary relationships of the Protozoa to the Protista? Biosystems. 1981;14(3-4):445–449. doi: 10.1016/0303-2647(81)90049-6. [DOI] [PubMed] [Google Scholar]
- Douglas S. E. Eukaryote-eukaryote endosymbioses: insights from studies of a cryptomonad alga. Biosystems. 1992;28(1-3):57–68. doi: 10.1016/0303-2647(92)90008-m. [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Murphy C. A., Spencer D. F., Gray M. W. Cryptomonad algae are evolutionary chimaeras of two phylogenetically distinct unicellular eukaryotes. Nature. 1991 Mar 14;350(6314):148–151. doi: 10.1038/350148a0. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Ferat J. L., Michel F. Group II self-splicing introns in bacteria. Nature. 1993 Jul 22;364(6435):358–361. doi: 10.1038/364358a0. [DOI] [PubMed] [Google Scholar]
- Flavin M., Nerad T. A. Reclinomonas americana N. G., N. Sp., a new freshwater heterotrophic flagellate. J Eukaryot Microbiol. 1993 Mar-Apr;40(2):172–179. doi: 10.1111/j.1550-7408.1993.tb04900.x. [DOI] [PubMed] [Google Scholar]
- Gray M. W. The endosymbiont hypothesis revisited. Int Rev Cytol. 1992;141:233–357. doi: 10.1016/s0074-7696(08)62068-9. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Hashimoto T., Adachi J., Iwabe N., Miyata T. Early branchings in the evolution of eukaryotes: ancient divergence of entamoeba that lacks mitochondria revealed by protein sequence data. J Mol Evol. 1993 Apr;36(4):380–388. doi: 10.1007/BF00182185. [DOI] [PubMed] [Google Scholar]
- Heywood P. Structure, function and terminology of microtubule- and microfilament-containing structures. Cell Biol Int Rep. 1987 Dec;11(12):837–847. doi: 10.1016/0309-1651(87)90118-4. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. J., d'Oliveira C. E., Gorrell T. E., Müller M. Molecular analysis of the hydrogenosomal ferredoxin of the anaerobic protist Trichomonas vaginalis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6097–6101. doi: 10.1073/pnas.87.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Krisans S., Gould S. J., Sommer J. M., Wang C. C., Schliebs W., Kunau W., Brody S., Subramani S. Evolutionary conservation of a microbody targeting signal that targets proteins to peroxisomes, glyoxysomes, and glycosomes. J Cell Biol. 1991 Sep;114(5):893–904. doi: 10.1083/jcb.114.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. E., Kugrens P. Relationship between the flagellates and the ciliates. Microbiol Rev. 1992 Dec;56(4):529–542. doi: 10.1128/mr.56.4.529-542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe D. D., Gunderson J. H., Nerad T. A., Sogin M. L. Small subunit ribosomal RNA+ of Hexamita inflata and the quest for the first branch in the eukaryotic tree. Mol Biochem Parasitol. 1993 May;59(1):41–48. doi: 10.1016/0166-6851(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Lenaers G., Scholin C., Bhaud Y., Saint-Hilaire D., Herzog M. A molecular phylogeny of dinoflagellate protists (pyrrhophyta) inferred from the sequence of 24S rRNA divergent domains D1 and D8. J Mol Evol. 1991 Jan;32(1):53–63. doi: 10.1007/BF02099929. [DOI] [PubMed] [Google Scholar]
- Levine N. D., Corliss J. O., Cox F. E., Deroux G., Grain J., Honigberg B. M., Leedale G. F., Loeblich A. R., 3rd, Lom J., Lynn D. A newly revised classification of the protozoa. J Protozool. 1980 Feb;27(1):37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Maier U. G., Hofmann C. J., Eschbach S., Wolters J., Igloi G. L. Demonstration of nucleomorph-encoded eukaryotic small subunit ribosomal RNA in cryptomonads. Mol Gen Genet. 1991 Nov;230(1-2):155–160. doi: 10.1007/BF00290663. [DOI] [PubMed] [Google Scholar]
- Maier U. G. The four genomes of the alga Pyrenomonas salina (Cryptophyta). Biosystems. 1992;28(1-3):69–73. doi: 10.1016/0303-2647(92)90009-n. [DOI] [PubMed] [Google Scholar]
- Marvin-Sikkema F. D., Kraak M. N., Veenhuis M., Gottschal J. C., Prins R. A. The hydrogenosomal enzyme hydrogenase from the anaerobic fungus Neocallimastix sp. L2 is recognized by antibodies, directed against the C-terminal microbody protein targeting signal SKL. Eur J Cell Biol. 1993 Jun;61(1):86–91. [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Morden C. W., Delwiche C. F., Kuhsel M., Palmer J. D. Gene phylogenies and the endosymbiotic origin of plastids. Biosystems. 1992;28(1-3):75–90. doi: 10.1016/0303-2647(92)90010-v. [DOI] [PubMed] [Google Scholar]
- Muchhal U. S., Schwartzbach S. D. Characterization of a Euglena gene encoding a polyprotein precursor to the light-harvesting chlorophyll a/b-binding protein of photosystem II. Plant Mol Biol. 1992 Jan;18(2):287–299. doi: 10.1007/BF00034956. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of ancestral eukaryotes: a hypothesis based on the biochemistry of amitochondriate parasitic protists. Biosystems. 1992;28(1-3):33–40. doi: 10.1016/0303-2647(92)90005-j. [DOI] [PubMed] [Google Scholar]
- Patterson D. J. The evolution of protozoa. Mem Inst Oswaldo Cruz. 1988 Nov;83 (Suppl 1):580–600. doi: 10.1590/s0074-02761988000500072. [DOI] [PubMed] [Google Scholar]
- Raikov I. B. Unusual extrusive organelles in karyorelictid ciliates: an argument for the ancient origin of this group. Biosystems. 1992;28(1-3):195–201. doi: 10.1016/0303-2647(92)90020-y. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffo M. B. The enigmatic protist Nephromyces. Biosystems. 1981;14(3-4):487–490. doi: 10.1016/0303-2647(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Taylor F. J. Problems in the development of an explicit hypothetical phylogeny of the lower eukaryotes. Biosystems. 1978 Apr;10(1-2):67–89. doi: 10.1016/0303-2647(78)90031-x. [DOI] [PubMed] [Google Scholar]
- Tessier L. H., Keller M., Chan R. L., Fournier R., Weil J. H., Imbault P. Short leader sequences may be transferred from small RNAs to pre-mature mRNAs by trans-splicing in Euglena. EMBO J. 1991 Sep;10(9):2621–2625. doi: 10.1002/j.1460-2075.1991.tb07804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S., Burger-Wiersma T., Giovannoni S. J., Mur L. R., Pace N. R. The relationship of a prochlorophyte Prochlorothrix hollandica to green chloroplasts. Nature. 1989 Jan 26;337(6205):380–382. doi: 10.1038/337380a0. [DOI] [PubMed] [Google Scholar]
- Viscogliosi E., Philippe H., Baroin A., Perasso R., Brugerolle G. Phylogeny of trichomonads based on partial sequences of large subunit rRNA and on cladistic analysis of morphological data. J Eukaryot Microbiol. 1993 Jul-Aug;40(4):411–421. doi: 10.1111/j.1550-7408.1993.tb04935.x. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Woese C. R. Eukaryotic ribosomes that lack a 5.8S RNA. Nature. 1986 Mar 20;320(6059):287–288. doi: 10.1038/320287a0. [DOI] [PubMed] [Google Scholar]
- WHITTAKER R. H. On the broad classification of organisms. Q Rev Biol. 1959 Sep;34:210–226. doi: 10.1086/402733. [DOI] [PubMed] [Google Scholar]
- Wainright P. O., Hinkle G., Sogin M. L., Stickel S. K. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993 Apr 16;260(5106):340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- Whatley J. M., John P., Whatley F. R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):165–187. doi: 10.1098/rspb.1979.0020. [DOI] [PubMed] [Google Scholar]
- Whittaker R. H. New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science. 1969 Jan 10;163(3863):150–160. doi: 10.1126/science.163.3863.150. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. The concept of cellular evolution. J Mol Evol. 1977 Sep 20;10(1):1–6. doi: 10.1007/BF01796132. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Markiw M. E. Biology contravenes taxonomy in the myxozoa: new discoveries show alternation of invertebrate and vertebrate hosts. Science. 1984 Sep 28;225(4669):1449–1452. doi: 10.1126/science.225.4669.1449. [DOI] [PubMed] [Google Scholar]
- Wolters J. The troublesome parasites--molecular and morphological evidence that Apicomplexa belong to the dinoflagellate-ciliate clade. Biosystems. 1991;25(1-2):75–83. doi: 10.1016/0303-2647(91)90014-c. [DOI] [PubMed] [Google Scholar]


