Abstract
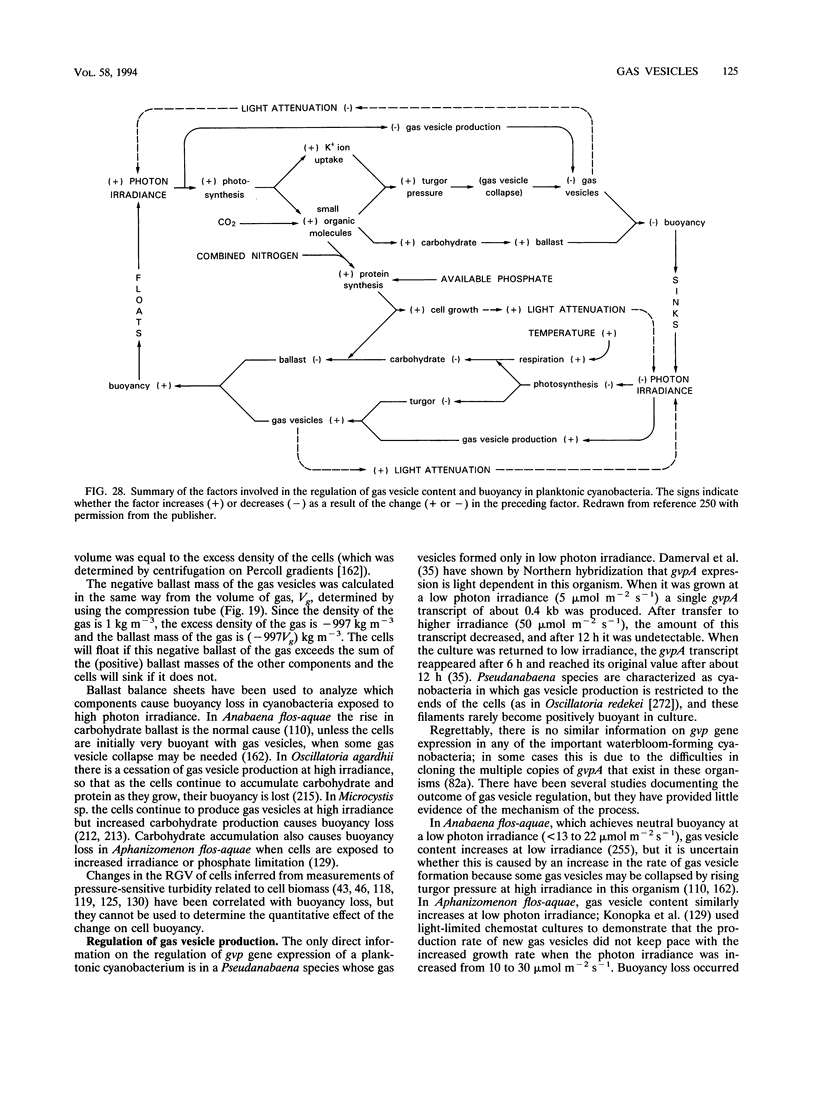
The gas vesicle is a hollow structure made of protein. It usually has the form of a cylindrical tube closed by conical end caps. Gas vesicles occur in five phyla of the Bacteria and two groups of the Archaea, but they are mostly restricted to planktonic microorganisms, in which they provide buoyancy. By regulating their relative gas vesicle content aquatic microbes are able to perform vertical migrations. In slowly growing organisms such movements are made more efficiently than by swimming with flagella. The gas vesicle is impermeable to liquid water, but it is highly permeable to gases and is normally filled with air. It is a rigid structure of low compressibility, but it collapses flat under a certain critical pressure and buoyancy is then lost. Gas vesicles in different organisms vary in width, from 45 to > 200 nm; in accordance with engineering principles the narrower ones are stronger (have higher critical pressures) than wide ones, but they contain less gas space per wall volume and are therefore less efficient at providing buoyancy. A survey of gas-vacuolate cyanobacteria reveals that there has been natural selection for gas vesicles of the maximum width permitted by the pressure encountered in the natural environment, which is mainly determined by cell turgor pressure and water depth. Gas vesicle width is genetically determined, perhaps through the amino acid sequence of one of the constituent proteins. Up to 14 genes have been implicated in gas vesicle production, but so far the products of only two have been shown to be present in the gas vesicle: GvpA makes the ribs that form the structure, and GvpC binds to the outside of the ribs and stiffens the structure against collapse. The evolution of the gas vesicle is discussed in relation to the homologies of these proteins.
Full text
PDF










































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. G., Carr N. G. The developmental biology of heterocyst and akinete formation in cyanobacteria. Crit Rev Microbiol. 1981;9(1):45–100. doi: 10.3109/10408418109104486. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P. Magnetotactic bacteria. Annu Rev Microbiol. 1982;36:217–238. doi: 10.1146/annurev.mi.36.100182.001245. [DOI] [PubMed] [Google Scholar]
- Blaseio U., Pfeifer F. Transformation of Halobacterium halobium: development of vectors and investigation of gas vesicle synthesis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6772–6776. doi: 10.1073/pnas.87.17.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Walsby A. E. Crystalline structure of the gas vesicle wall from Anabaena flos-aquae. J Mol Biol. 1976 Aug 5;105(2):183–199. doi: 10.1016/0022-2836(76)90106-6. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Wober W. Structure of the wall of Halobacterium halobium gas vesicles. J Mol Biol. 1976 Sep 25;106(3):871–878. doi: 10.1016/0022-2836(76)90270-9. [DOI] [PubMed] [Google Scholar]
- Bomar D., Giovannoni S., Stackebrandt E. A unique type of eubacterial 5S rRNA in members of the order Planctomycetales. J Mol Evol. 1988;27(2):121–125. doi: 10.1007/BF02138371. [DOI] [PubMed] [Google Scholar]
- Bowen C. C., Jensen T. E. Blue-Green Algae: Fine Structure of the Gas Vacuoles. Science. 1965 Mar 19;147(3664):1460–1462. doi: 10.1126/science.147.3664.1460. [DOI] [PubMed] [Google Scholar]
- Buchholz B. E., Hayes P. K., Walsby A. E. The distribution of the outer gas vesicle protein, GvpC, on the Anabaena gas vesicle, and its ratio to GvpA. J Gen Microbiol. 1993 Oct;139(10):2353–2363. doi: 10.1099/00221287-139-10-2353. [DOI] [PubMed] [Google Scholar]
- Buckland B., Walsby A. E. A study of the strength and stability of gas vesicles isolated from a blue-green alga. Arch Mikrobiol. 1971;79(4):327–337. doi: 10.1007/BF00424908. [DOI] [PubMed] [Google Scholar]
- Caldwell D. E., Tiedje J. M. A morphological study of anaerobic bacteria from the hypolimnia of two Michigan lakes. Can J Microbiol. 1975 Mar;21(3):362–376. doi: 10.1139/m75-051. [DOI] [PubMed] [Google Scholar]
- Caldwell D. E., Tiedje J. M. The structure of anaerobic bacterial communities in the hypolimnia of several Michigan lakes. Can J Microbiol. 1975 Mar;21(3):377–385. doi: 10.1139/m75-052. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Pfennig N. Comparative study of the structure of gas vacuoles. J Bacteriol. 1969 Nov;100(2):1049–1061. doi: 10.1128/jb.100.2.1049-1061.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszàr K., Houmard J., Damerval T., Tandeau de Marsac N. Transcriptional analysis of the cyanobacterial gvpABC operon in differentiated cells: occurrence of an antisense RNA complementary to three overlapping transcripts. Gene. 1987;60(1):29–37. doi: 10.1016/0378-1119(87)90210-1. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Damerval T., Castets A. M., Guglielmi G., Houmard J., Tandeau de Marsac N. Occurrence and distribution of gas vesicle genes among cyanobacteria. J Bacteriol. 1989 Mar;171(3):1445–1452. doi: 10.1128/jb.171.3.1445-1452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval T., Castets A. M., Houmard J., Tandeau de Marsac N. Gas vesicle synthesis in the cyanobacterium Pseudanabaena sp.: occurrence of a single photoregulated gene. Mol Microbiol. 1991 Mar;5(3):657–664. doi: 10.1111/j.1365-2958.1991.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Damerval T., Guglielmi G., Houmard J., De Marsac N. T. Hormogonium Differentiation in the Cyanobacterium Calothrix: A Photoregulated Developmental Process. Plant Cell. 1991 Feb;3(2):191–201. doi: 10.1105/tpc.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
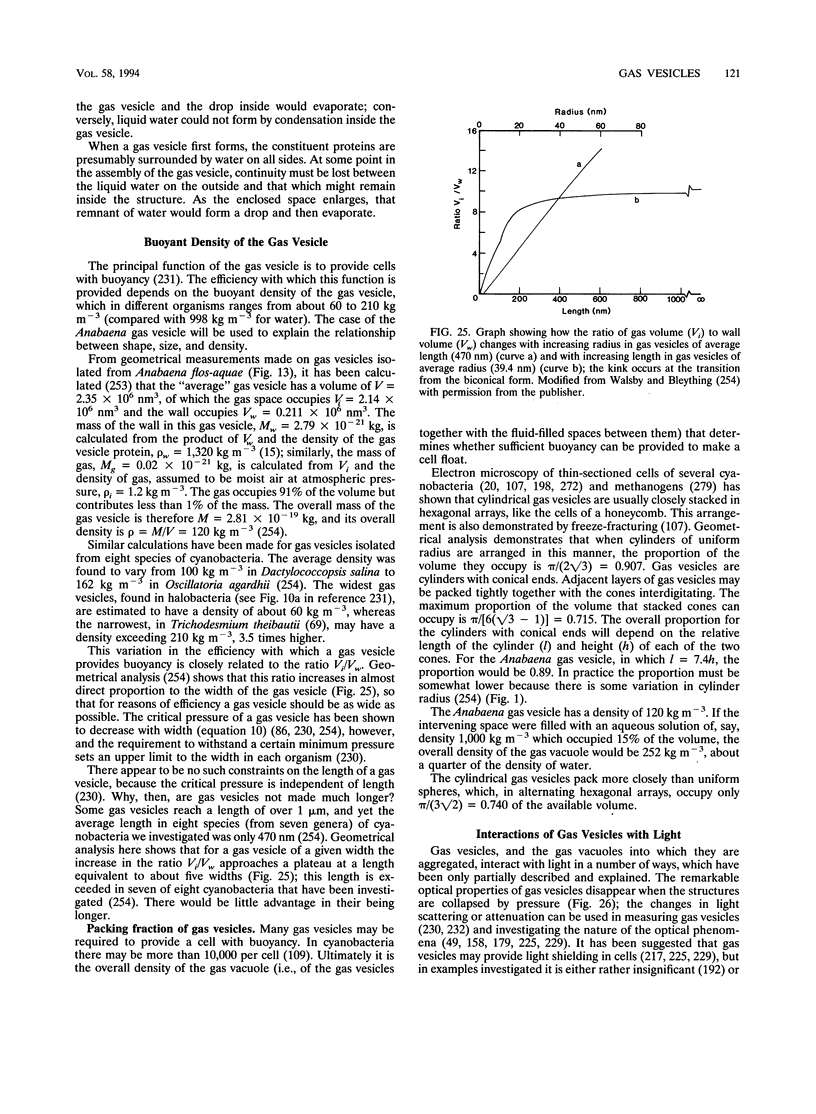
- Damerval T., Houmard J., Guglielmi G., Csiszar K., Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch R. N., Sjoblad R. D. Flagellar structure and function in eubacteria. Annu Rev Microbiol. 1980;34:69–108. doi: 10.1146/annurev.mi.34.100180.000441. [DOI] [PubMed] [Google Scholar]
- Dubelaar G. B., Visser J. W., Donze M. Anomalous behaviour of forward and perpendicular light scattering of a cyanobacterium owing to intracellular gas vacuoles. Cytometry. 1987 Jul;8(4):405–412. doi: 10.1002/cyto.990080410. [DOI] [PubMed] [Google Scholar]
- Easterbrook K. B., Willison J. H., Coombs R. W. Arrangement of morphological subunits in bacterial spinae. Can J Microbiol. 1976 May;22(5):619–629. doi: 10.1139/m76-092. [DOI] [PubMed] [Google Scholar]
- Englert C., Horne M., Pfeifer F. Expression of the major gas vesicle protein gene in the halophilic archaebacterium Haloferax mediterranei is modulated by salt. Mol Gen Genet. 1990 Jul;222(2-3):225–232. doi: 10.1007/BF00633822. [DOI] [PubMed] [Google Scholar]
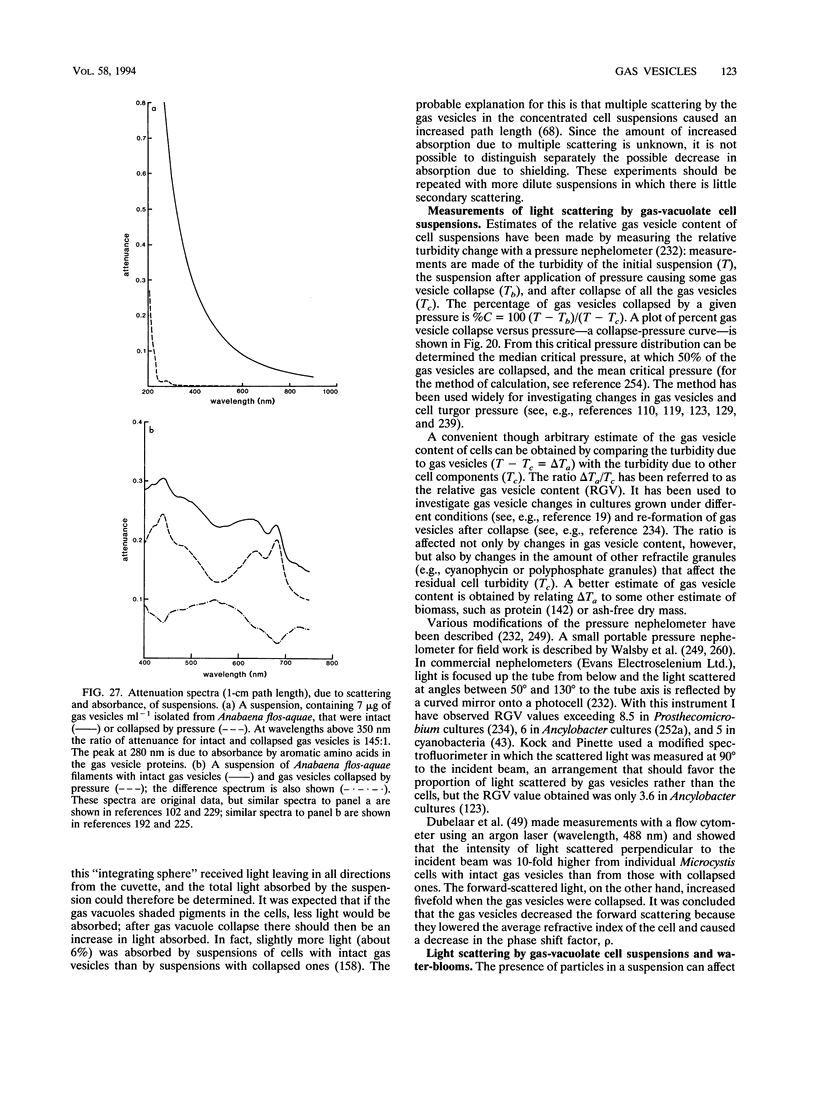
- Englert C., Krüger K., Offner S., Pfeifer F. Three different but related gene clusters encoding gas vesicles in halophilic archaea. J Mol Biol. 1992 Sep 20;227(2):586–592. doi: 10.1016/0022-2836(92)90914-6. [DOI] [PubMed] [Google Scholar]
- Englert C., Pfeifer F. Analysis of gas vesicle gene expression in Haloferax mediterranei reveals that GvpA and GvpC are both gas vesicle structural proteins. J Biol Chem. 1993 May 5;268(13):9329–9336. [PubMed] [Google Scholar]
- Englert C., Wanner G., Pfeifer F. Functional analysis of the gas vesicle gene cluster of the halophilic archaeon Haloferax mediterranei defines the vac-region boundary and suggests a regulatory role for the gvpD gene or its product. Mol Microbiol. 1992 Dec;6(23):3543–3550. doi: 10.1111/j.1365-2958.1992.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Falkenberg P., Buckland B., Walsby A. E. Chemical composition of gas vesicles isolated from Anabaena flos-aquae. Arch Mikrobiol. 1972;85(4):304–309. doi: 10.1007/BF00549268. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985 May;54(2):374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S. J., Turner S., Olsen G. J., Barns S., Lane D. J., Pace N. R. Evolutionary relationships among cyanobacteria and green chloroplasts. J Bacteriol. 1988 Aug;170(8):3584–3592. doi: 10.1128/jb.170.8.3584-3592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Gorlenko V. M., Lebedeva E. V. Novye zelenye serobakterii s vyrostami. Mikrobiologiia. 1971 Nov-Dec;40(6):1035–1039. [PubMed] [Google Scholar]
- Gorlenko V. M., Lokk S. I. Vertikal'noe raspredelenie i osobennosti vidovogo sostava mikroorganizmov nekotorykh stratifitsirovannykh ozer Estonii. Mikrobiologiia. 1979 Mar-Apr;48(2):351–359. [PubMed] [Google Scholar]
- Griffiths A. E., Walsby A. E., Hayes P. K. The homologies of gas vesicle proteins. J Gen Microbiol. 1992 Jun;138(6):1243–1250. doi: 10.1099/00221287-138-6-1243. [DOI] [PubMed] [Google Scholar]
- Halladay J. T., Jones J. G., Lin F., MacDonald A. B., DasSarma S. The rightward gas vesicle operon in Halobacterium plasmid pNRC100: identification of the gvpA and gvpC gene products by use of antibody probes and genetic analysis of the region downstream of gvpC. J Bacteriol. 1993 Feb;175(3):684–692. doi: 10.1128/jb.175.3.684-692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P. K., Buchholz B., Walsby A. E. Gas vesicles are strengthened by the outer-surface protein, GvpC. Arch Microbiol. 1992;157(3):229–234. doi: 10.1007/BF00245155. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Lazarus C. M., Bees A., Walker J. E., Walsby A. E. The protein encoded by gvpC is a minor component of gas vesicles isolated from the cyanobacteria Anabaena flos-aquae and Microcystis sp. Mol Microbiol. 1988 Sep;2(5):545–552. doi: 10.1111/j.1365-2958.1988.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Walsby A. E., Walker J. E. Complete amino acid sequence of cyanobacterial gas-vesicle protein indicates a 70-residue molecule that corresponds in size to the crystallographic unit cell. Biochem J. 1986 May 15;236(1):31–36. doi: 10.1042/bj2360031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B. B., Hemmingsen E. A. Rupture of the cell envelope by induced intracellular gas phase expansion in gas vacuolate bacteria. J Bacteriol. 1980 Aug;143(2):841–846. doi: 10.1128/jb.143.2.841-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P., Pankratz S. H. Study of bacterial populations in natural environments by use of submerged electron microscope grids. Z Allg Mikrobiol. 1970;10(8):589–605. [PubMed] [Google Scholar]
- Horne M., Englert C., Pfeifer F. Two genes encoding gas vacuole proteins in Halobacterium halobium. Mol Gen Genet. 1988 Aug;213(2-3):459–464. doi: 10.1007/BF00339616. [DOI] [PubMed] [Google Scholar]
- Horne M., Englert C., Wimmer C., Pfeifer F. A DNA region of 9 kbp contains all genes necessary for gas vesicle synthesis in halophilic archaebacteria. Mol Microbiol. 1991 May;5(5):1159–1174. doi: 10.1111/j.1365-2958.1991.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Horne M., Pfeifer F. Expression of two gas vacuole protein genes in Halobacterium halobium and other related species. Mol Gen Genet. 1989 Sep;218(3):437–444. doi: 10.1007/BF00332407. [DOI] [PubMed] [Google Scholar]
- Häder D. P. Photosensory behavior in procaryotes. Microbiol Rev. 1987 Mar;51(1):1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. D., Haug A., Jost M., Graber D. R. Ultrastructural and conformational changes in gas vacuole membranes isolated from Microcystis aeruginosa. Arch Biochem Biophys. 1969 Dec;135(1):296–303. doi: 10.1016/0003-9861(69)90543-8. [DOI] [PubMed] [Google Scholar]
- Jones D. D., Jost M. Isolation and chemical characterization of gas-vacuole membranes from Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1970;70(1):43–64. doi: 10.1007/BF00691059. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Hackett N. R., Halladay J. T., Scothorn D. J., Yang C. F., Ng W. L., DasSarma S. Analysis of insertion mutants reveals two new genes in the pNRC100 gas vesicle gene cluster of Halobacterium halobium. Nucleic Acids Res. 1989 Oct 11;17(19):7785–7793. doi: 10.1093/nar/17.19.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Jones D. D. Morphological parameters and macromolecular organization of gas vacuole membranes of Microcystis aeruginosa Kuetz. emend. Elenkin. Can J Microbiol. 1970 Mar;16(3):159–164. doi: 10.1139/m70-028. [DOI] [PubMed] [Google Scholar]
- Jost M., Jones D. D., Weathers P. J. Counting of gas vacuoles by electron microscopy in lysates and purified fractions of Microcystis aeruginosa. Protoplasma. 1971;73(3):329–335. doi: 10.1007/BF01273937. [DOI] [PubMed] [Google Scholar]
- Klemer A. R., Feuillade J., Feuillade M. Cyanobacterial blooms: carbon and nitrogen limitation have opposite effects on the buoyancy of oscillatoria. Science. 1982 Mar 26;215(4540):1629–1631. doi: 10.1126/science.215.4540.1629. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Pinette M. F. Nephelometric determination of turgor pressure in growing gram-negative bacteria. J Bacteriol. 1987 Aug;169(8):3654–3663. doi: 10.1128/jb.169.8.3654-3663.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The protein burden of lac operon products. J Mol Evol. 1983;19(6):455–462. doi: 10.1007/BF02102321. [DOI] [PubMed] [Google Scholar]
- Konopka A. E. Inhibition of gas vesicle production in Microcyclus aquaticus by L-lysine. Can J Microbiol. 1977 Apr;23(4):363–368. doi: 10.1139/m77-054. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Lara J. C., Staley J. T. Isolation and characterization of gas vesicles from Microcyclus aquaticus. Arch Microbiol. 1977 Mar 1;112(2):133–140. doi: 10.1007/BF00429325. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Staley J. T., Lara J. C. Gas vesicle assembly in Microcyclus aquaticus. J Bacteriol. 1975 Jun;122(3):1301–1309. doi: 10.1128/jb.122.3.1301-1309.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz M. J., Ballou C. E. Analysis of Halobacterium halobium gas vesicles. J Bacteriol. 1973 Jun;114(3):1058–1067. doi: 10.1128/jb.114.3.1058-1067.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasil'nikov N. A., Duda V. I. Ul'trastruktura kolpachkov na sporakh anaéorbnykh bakterii. Dokl Akad Nauk SSSR. 1968 Apr 1;179(4):970–973. [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H., Omang S., Steensland H. On the gas vacuoles of the halobacteria. Arch Mikrobiol. 1967;59(1):197–203. doi: 10.1007/BF00406332. [DOI] [PubMed] [Google Scholar]
- Marr A. G. Growth rate of Escherichia coli. Microbiol Rev. 1991 Jun;55(2):316–333. doi: 10.1128/mr.55.2.316-333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Lowe G., Berg H. C. The proton flux through the bacterial flagellar motor. Cell. 1987 Jun 5;49(5):643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Oliver C., Stadtman E. R. Inactivation of glutamine synthetase by a purified rabbit liver microsomal cytochrome P-450 system. Arch Biochem Biophys. 1985 Jul;240(1):319–329. doi: 10.1016/0003-9861(85)90037-2. [DOI] [PubMed] [Google Scholar]
- Paerl H. W. Partitioning of CO(2) Fixation in the Colonial Cyanobacterium Microcystis aeruginosa: Mechanism Promoting Formation of Surface Scums. Appl Environ Microbiol. 1983 Jul;46(1):252–259. doi: 10.1128/aem.46.1.252-259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Blaseio U., Horne M. Genome structure of Halobacterium halobium: plasmid dynamics in gas vacuole deficient mutants. Can J Microbiol. 1989 Jan;35(1):96–100. doi: 10.1139/m89-015. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Englert C. Function and biosynthesis of gas vesicles in halophilic Archaea. J Bioenerg Biomembr. 1992 Dec;24(6):577–585. doi: 10.1007/BF00762350. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Characterization of plasmids in halobacteria. J Bacteriol. 1981 Jan;145(1):369–374. doi: 10.1128/jb.145.1.369-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., cohen-Bazire G. Some properties of the green bacterium Pelodictyon clathratiforme. Arch Mikrobiol. 1967;59(1):226–236. doi: 10.1007/BF00406336. [DOI] [PubMed] [Google Scholar]
- Pinette M. F., Koch A. L. Turgor pressure responses of a gram-negative bacterium to antibiotic treatment, measured by collapse of gas vesicles. J Bacteriol. 1988 Mar;170(3):1129–1136. doi: 10.1128/jb.170.3.1129-1136.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J., Jost M. Physiological effects of the presence and absence of gas vacuoles in the blue-green alga, Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Microbiol. 1976 Nov 2;110(23):225–231. doi: 10.1007/BF00690231. [DOI] [PubMed] [Google Scholar]
- Powell R. S., Walsby A. E., Hayes P. K., Porter R. Antibodies to the N-terminal sequence of GVPa bind to the ends of gas vesicles. J Gen Microbiol. 1991 Oct;137(10):2395–2400. doi: 10.1099/00221287-137-10-2395. [DOI] [PubMed] [Google Scholar]
- Simon R. D. Halobacterium strain 5 contains a plasmid which is correlated with the presence of gas vacuoles. Nature. 1978 May 25;273(5660):314–317. doi: 10.1038/273314a0. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Peat A., Bailey C. J. The isolation and characterisation of gas-cylinder membranes and alpha-granules from Anabaena flos-aquae D 124. Arch Mikrobiol. 1969;65(2):87–97. doi: 10.1007/BF00693312. [DOI] [PubMed] [Google Scholar]
- Staley J. T., Irgens R. L., Herwig R. P. Gas vacuolate bacteria from the sea ice of antarctica. Appl Environ Microbiol. 1989 Apr;55(4):1033–1036. doi: 10.1128/aem.55.4.1033-1036.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968 May;95(5):1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W. Walsby's square bacterium: fine structure of an orthogonal procaryote. J Bacteriol. 1981 Oct;148(1):352–360. doi: 10.1128/jb.148.1.352-360.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surek B., Pillay B., Rdest U., Beyreuther K., Goebel W. Evidence for two different gas vesicle proteins and genes in Halobacterium halobium. J Bacteriol. 1988 Apr;170(4):1746–1751. doi: 10.1128/jb.170.4.1746-1751.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Mazel D., Bryant D. A., Houmard J. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7223–7236. doi: 10.1093/nar/13.20.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasselli A. G., Mast E., Janes W., Schiltz E. The complete amino acid sequence of adenylate kinase from baker's yeast. Eur J Biochem. 1986 Feb 17;155(1):111–119. doi: 10.1111/j.1432-1033.1986.tb09465.x. [DOI] [PubMed] [Google Scholar]
- Van Baalen C., Brown R. M., Jr The ultrastructure of the marine blue green alga, Trichodesmium erythraeum, with special reference to the cell wall, gas vacuoles, and cylindrical bodies. Arch Mikrobiol. 1969;69(1):79–91. doi: 10.1007/BF00408566. [DOI] [PubMed] [Google Scholar]
- Van Ert M., Staley J. T. A new gas vacuolated heterotrophic rod from freshwaters. Arch Mikrobiol. 1971;80(1):70–77. doi: 10.1007/BF00410581. [DOI] [PubMed] [Google Scholar]
- Van Ert M., Staley J. T. Gas-vacuolated strains of Microcyclus aquaticus. J Bacteriol. 1971 Oct;108(1):236–240. doi: 10.1128/jb.108.1.236-240.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaland J. R., Branton D. Gas vacuole development in a blue-green alga. Science. 1969 Mar 21;163(3873):1339–1341. doi: 10.1126/science.163.3873.1339. [DOI] [PubMed] [Google Scholar]
- Waaland J. R., Waaland S. D., Branton D. Gas vacuoles. Light shielding in blue-green algae. J Cell Biol. 1971 Jan;48(1):212–215. doi: 10.1083/jcb.48.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Walsby A. E. Molecular weight of gas-vesicle protein from the planktonic cyanobacterium Anabaena flos-aquae and implications for structure of the vesicle. Biochem J. 1983 Mar 1;209(3):809–815. doi: 10.1042/bj2090809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E., Armstrong R. E. Average thickness of the gas vesicle wall in Anabaena flos-aquae. J Mol Biol. 1979 Apr 5;129(2):279–285. doi: 10.1016/0022-2836(79)90281-x. [DOI] [PubMed] [Google Scholar]
- Walsby A. E., Hayes P. K. Gas vesicle proteins. Biochem J. 1989 Dec 1;264(2):313–322. doi: 10.1042/bj2640313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterbury J. B., Willey J. M., Franks D. G., Valois F. W., Watson S. W. A cyanobacterium capable of swimming motility. Science. 1985 Oct 4;230(4721):74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]
- Weathers P. J., Jost M., Lamport D. T. The gas vacuole membrane of Microcystis aeruginosa. A partial amino acid sequence. Arch Biochem Biophys. 1977 Jan 15;178(1):226–244. doi: 10.1016/0003-9861(77)90188-6. [DOI] [PubMed] [Google Scholar]
- Weidinger G., Klotz G., Goebel W. A large plasmid from Halobacterium halobium carrying genetic information for gas vacuole formation. Plasmid. 1979 Jul;2(3):377–386. doi: 10.1016/0147-619x(79)90021-0. [DOI] [PubMed] [Google Scholar]
- Whitton B. A., Peat A. On Oscillatoria redkei Van Goor. Arch Mikrobiol. 1969;68(4):362–376. doi: 10.1007/BF00408860. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Sporulation and further nutritional characteristics of Desulfotomaculum acetoxidans. Arch Microbiol. 1981 Jul;129(5):401–402. doi: 10.1007/BF00406471. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhilina T. N. Tonkoe stroenie metanosartsiny. Mikrobiologiia. 1971 Jul-Aug;40(4):674–680. [PubMed] [Google Scholar]









