Abstract
Objective
Although elevated IL-7 levels were reported in patients with primary Sjӧgren’s syndrome (pSjS), the role of IL-7 in this disease remains unclear. Here we characterized the previously unexplored role of IL-7 in the development and onset of pSjS using C57BL/6.NOD-Aec1Aec2 (B6.NOD-Aec) mouse model that recapitulates human pSjS.
Methods
For gain-of-function studies, recombinant IL-7 or control PBS was injected intraperitoneally (i.p.) into 12-week old B6.NOD-Aec mice for 8 weeks. For loss-of-function studies, neutralizing anti-IL-7 receptor antibody or its isotype control IgG was administered i.p. into 16-week old B6.NOD-Aec mice. Salivary flow measurement, histological and flow cytometric analysis of salivary glands, and serum antinuclear antibody assay were performed to assess various disease parameters.
Results
Administration of exogenous IL-7 accelerated, whereas blockade of IL-7 receptor signaling almost completely abolished the development of pSjS, based on salivary gland inflammation and apoptosis, autoantibody production and secretory dysfunction. IL-7 positively regulated IFN-γ-producing Th1 and CD8 T cells in the salivary glands without affecting IL-17. Moreover, IL-7 enhanced the expression of CXCR3 ligands in a T cell- and IFN-γ-dependent fashion. Accordingly, IFN-γ induced a human salivary gland epithelial cell line to produce CXCR3 ligands. IL-7 also increased the level of TNF-α, another Th1-associated cytokine that can facilitate tissue destruction and inflammation.
Conclusion
IL-7 plays a pivotal pathogenic role in SjS, which is underpinned by an enhanced Th1 response and IFN-γ-CXCR3 ligand-mediated lymphocyte infiltration of target organs. These results suggest that targeting IL-7 pathway may be a potential future strategy to prevent and treat SjS.
Keywords: Primary Sjӧgren’s Syndrome, Autoimmune disease, IFN-γ, CXCR3 ligands
Sjӧgren’s syndrome (SjS) is a chronic autoimmune disease affecting 2–4 million Americans (1). The major immune-mediated pathological events of SjS are lymphocytic infiltration of salivary and lacrimal glands and production of autoantibodies, which together cause destruction and dysfunction of these exocrine glands (1, 2). SjS patients suffer from xerostomia and keratoconjunctivitis, which are often accompanied by systemic inflammation (1, 2). SjS can occur in the form of primary SjS (pSjS) or secondary SjS, which is associated with other connective tissue diseases (1, 3). The etiology of SjS is still unclear, although viral infection and genetic predisposition are implicated as likely triggers (1, 2, 4–7). Both autoreactive T and B cells play essential roles in the autoimmune responses that cause tissue inflammation, autoantibody production and clinical onset of SjS (2, 3, 8–10). T cell-derived cytokines, including IFN-γ, IL-4 and IL-17, exert essential but distinct functions in SjS pathogenesis (11–15). However, signals and factors that can affect the differentiation and functions of the autoreactive effector T cells in SjS remain unclear.
IL-7 is a crucial cytokine that support T cell development and homeostasis (16–18). Recent research has revealed additional roles of IL-7 in promoting differentiation and functions of multiple effector T cell subsets, especially IFN-γ- or IL-17-producing T cells (19–22). Consequently, IL-7 plays a pathogenic role in multiple autoimmune diseases, including rheumatoid arthritis (RA) (22–24), inflammatory bowel disease (IBD) (25–27) and experimental autoimmune encephalomyelitis (EAE) (19). The levels of IL-7 and the frequency of IL-7Rα+ T cells in the salivary glands of pSjS patients are significantly higher than non-SjS sicca patients and they correlate with disease severity (28, 29). Moreover, IL-7 treatment of peripheral blood T cells from pSjS patients increases the production of T helper (Th) 1 and Th17 cytokines (28, 30). These lines of evidence suggest a possible pathogenic role of IL-7 in pSjS.
Many studies have shown that IL-7 enhances the function and expansion of IFN-γ-producing Th1 cells (20–23, 28, 30, 31), which constitutes a chief mechanism by which IL-7 facilitates IBD (25–27), RA (21, 22), type-1 diabetes (18, 32) and EAE (19). IFN-γ promotes SjS development both at the pre-immunological and the immunological phase (11, 13). In addition to IFN-γ, IL-7 enhances secretion of TNF-α from peripheral blood T cells of RA patients (21, 22) and pSjS patients (28). TNF-α is implicated in numerous inflammatory and autoimmune disorders and its levels are elevated in SjS patients (11). Alone or in concert with IFN-γ, TNF-α induces death and secretory dysfunction of salivary gland cells in vitro via multiple mechanisms (33–36).
In the present study, we investigated the in vivo role of IL-7 in the development and onset of pSjS using C57BL/6.NOD-Aec1Aec2 (B6.NOD-Aec) mice, a well-defined model of pSjS (37, 38). By using both gain- and loss-of-function approaches, we demonstrated that IL-7 plays a crucial role in the development and onset of pSjS in B6.NOD-Aec mice by enhancing Th1 response and IFN-γ-dependent CXCR3 ligand expression in the salivary glands. Therefore, we defined a previously unexplored role of IL-7 in the development of pSjS-like autoimmune exocrinopathy and revealed critical underlying mechanisms.
Materials and Methods
Mice
C57BL/6, RAG1−/− and IFN-γ−/− mice were purchased from the Jackson Laboratory and C57BL/6.NOD-Aec1Aec2 mice were from University of Florida, and kept under pathogen-free conditions. All experiments were carried out under the guidelines of the Institutional Animal Care and Use Committee at the Forsyth Institute.
Histology and immunofluorescence staining
Tissue samples were fixed in 4 % paraformaldehyde, embedded in paraffin and sectioned to 5 µm thickness. Sections were then stained with hematoxylin and eosin (H&E) and examined for leukocyte infiltration. Some sections were subjected to deparaffinization, re-hydration and antigen retrieval. They were then incubated with PE-anti-CXCL9 (MIG-2F5.5) or goat anti-mouse CXCL10 (C-19) at 4°C overnight, followed by Alexa Fluor 488-anti-goat-IgG. The stained samples were examined with a Leica laser scanning confocal microscope (Leica Microsystems). Images were average projections of three optical sections and processed with the Leica confocal software.
Antibodies and cytokines
Cells were stained and analyzed on a FACSAria III cell sorter (Becton Dickinson), with dead cells excluded by forward light scatter. The following fluorescence-conjugated Abs were used: CD4 (GK1.5), CD8α (536-7), TCR-β (H57-597), IL-7R (A7R34) and IL-17 (TC11-18H10.1) and anti-mouse CXCL9 (MIG-2F5.5) were from BioLegend; CD19 (ebio1D3) and IFN-γ (XMG1.2) from eBioscience; anti-mouse CXCL10 (C-19) from Santa Cruz Biotechnology; and antihuman CXCL9 (B8-11) and -10 (6D4/D6/G2) from BD Pharmingen. Monoclonal rat-anti-mouse IL-7Rα (A7R34) and its isotype control rat-IgG2a (2A3) were from BioXcell.
Preparation of single cell suspension
Submandibular salivary glands, submandibular lymph nodes or spleen were cut into small fragments, placed in a grinder and processed with a tissue homogenizer. Tissue homogenates were filtered through a 100 µm nylon mesh, washed, and removed of erythrocytes with ACK lysing buffer. The single cells were resuspended in culture medium.
Ex vivo T cell stimulation and intracellular cytokine staining
Singles cells prepared from various organs were stimulated in vitro with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 µM; both from Calbiochem) for 4 hours, with the addition of monensin (eBioscience) in the final 2 hours. Cells were then stained for surface markers and intracellular cytokines with the intracellular cytokine staining kit (eBioscience, Biolegend) following the manufacturers’ instructions.
Real-time RT-PCR
Total RNA was reverse-transcribed into cDNA using Oligo (dT) and M-MLV reverse transcriptase (Promega). The cDNA was subjected to real-time PCR amplification (Qiagen) for 40 cycles with annealing and extension temperature at 60°C, on a LightCycler 480 Real-Time PCR System (Roche). Primer sequences are: mouse IL-7 forward, 5’-GGAACTGATAGTAATTGCCCG-3’; reverse, 5’-TTCAACTTGCGAGCAGCACG-3’, IFN-γ forward, 5’-GGATGCATTCATGAGTATTGC-3’; reverse, 5’-CTTTTCCGCTTCCTGAGG-3’, IL-17 forward, 5’-GCGCAAAAGTGAGCTCCAGA-3’; reverse 5’-ACAGAGGGATATCTATCAGGG-3’, TNF-α forward, 5’-CCTTTCACTCACTGGCCCAA-3’; reverse, 5’-AGTGCCTCTTCTGCCAGTTC-3’, mouse CXCL9 forward, 5’-CCCTCAAAGACCTCAAACAGT-3’; reverse, 5’-AGCCGGATCTAGGCAGGTT-3’, mouse CXCL10 forward, 5’-CCAGTGAGAATGAGGGCCAT-3’; reverse, 5’-CCGGATTCAGACATCTCTGC-3’. Other sequences will be provided upon request.
ELISA
Mouse IL-7 (Biolegend) concentration in serum, and human CXCL9 (Peprotech), CXCL10 (R&D) and IL-7 (Biolegend) concentration in supernatants from in vitro HSG cell cultures were determined using ELISA kits according to the manufacturer’s protocols.
In vivo administration of anti-IL-7Rα antibody and rh IL-7
Female B6.NOD-Aec mice were i.p. injected with 100 µg of control IgG or anti-IL-7Rα 3 times weekly, starting from 16 weeks of age. For in vivo IL-7 administration, female B6.NOD-Aec mice were i.p. injected with 5 µg recombinant human IL-7 (Biological Resources Branch, National Cancer Institute) 3 times weekly for 8 weeks, starting from 12 weeks of age. Mice were then sacrificed and organs harvested for analysis.
In situ apoptosis detection
Paraffin embedded tissue sections were de-paraffinized, hydrated and then subjected to in situ apoptosis assay using Trevigen TACS.XL In Situ Apoptosis Detection kit (purchased from R&D Systems) according to the manufacturer’s instruction. Briefly, re-hydrated tissue sections were partially digested with proteinase-K for 20 min and then endogenous peroxidases were inactivated by incubation in 3% H2O2. DNA fragmentation was then detected following the manufacturer’s protocol.
Detection of serum antinuclear antibodies (ANA)
ANA in mouse sera were detected using HEp-2 (human epithelial cell) substrate slides (INOVA Diagnostics). Briefly, fixed HEp-2 substrate slides were overlaid with 1:40 diluted mouse sera and incubated for 1 hour at room temperature in a humidified chamber. After PBS washes, the substrate slides were incubated with 1:100 diluted Alexa Fluor 568-conjugated goat-anti-mouse IgG (Invitrogen) for 1 hour at room temperature. After PBS washes, the slides were analyzed under a FSX100 fluorescence microscope (Olympus) at 200× magnification. All images were obtained with the FSX-BSW software with constant exposure of 0.1 second (Olympus).
Measurement of salivary flow rate
Non-anesthetized mice were weighed and given an i.p injection of 100 µl PBS-based secretagogue solution containing isoproterenol (0.02 mg/ml) and pilocarpine (0.05 mg/ml). One minute after secretagogue injection, saliva was collected continuously for 5 minutes from the oral cavity of mice with a micropipette. The volume of saliva was measured and normalized to the body weight.
In vitro culture and treatment of HSG cells
0.1 × 106 HSG cells were seeded in each well of a 6-well culture plate and incubated in the presence or absence of rh IFN-γ (50 ng/ml) or rh IL-7 (50 ng/ml) for 1 or 3 days, with monensin added in the last 4 hours of culture. The supernatants were collected for ELISA assay and the cells were analyzed for intracellular chemokines.
Statistical analysis
All statistical significance was determined by Student’s t-test (two-tailed, two-sample equal variance). P values smaller than 0.05 were considered as statistically significant.
Results
Characterization of infiltrating T cells and IL-7 expression in salivary glands of B6.NOD-Aec mice
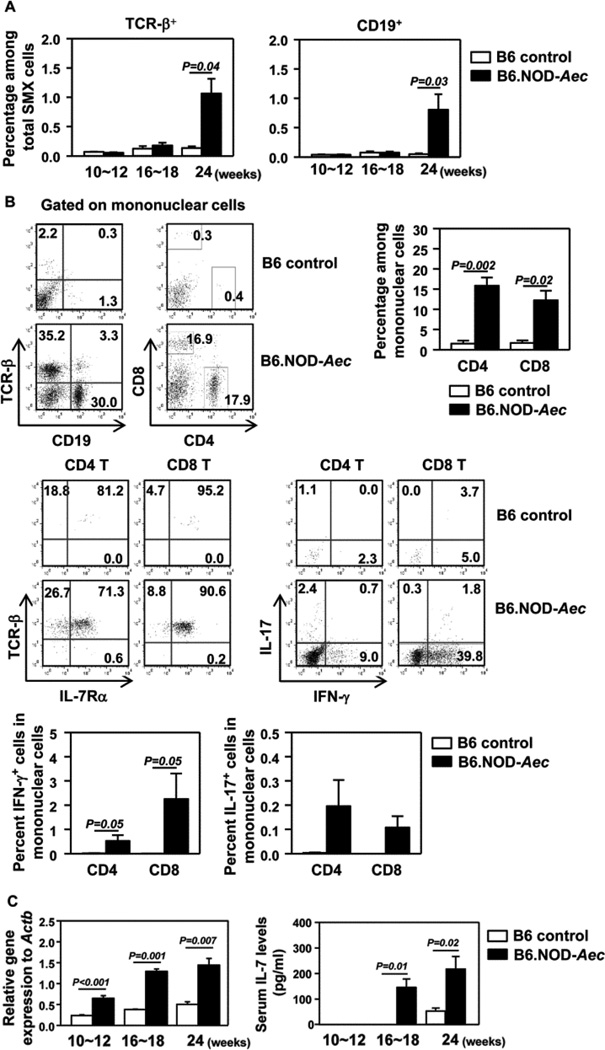
B6.NOD-Aec mice are C57BL/6 mice that carry Idd3 and Idd5 genetic segments derived from NOD mice and develop human pSjS-like exocrinopathy (37, 38). Previous reports showed that B6.NOD-Aec mice develop lymphocytic infiltration in exocrine glands at 12–16 weeks of age and substantial secretory dysfunction at 16–20 weeks of age (13, 37). Since the disease course and severity may vary among different animal facilities, we first examined the time course of disease development in female B6.NOD-Aec or control C57BL/6 (B6) mice belonging to 3 age groups, 10–12, 16–18 and 24 weeks. No inflammation was observed in submandibular salivary glands (SMX) of control B6 mice in any age group (Fig. S1A). No inflammation was detected in the SMX of B6.NOD-Aec mice at 10–12 or 16–18 weeks of age, but clear leukocyte infiltration and foci were detected at age 24 weeks (Fig. S1A). Flow cytometric analysis showed that SMX from B6.NOD-Aec mice contained markedly higher percentages of TCR-β+ T cells and CD19+ B cells than control B6 mice at age 24 weeks (Fig. 1A). Within SMX-infiltrating mononuclear cells, significantly higher proportions of CD4 and CD8 T cells, and B cells were observed in B6.NOD-Aec mice compared to control mice (Fig. 1B), with the majority of T cells being IL-7Rα+ (Fig. 1B). Significant proportions of CD4 and CD8 T cells produced IFN-γ and a small proportion of CD4 T cells produced IL-17 (Fig. 1B). The proportions of T and B cells in submandibular gland draining lymph nodes (dr LN) were comparable between B6.NOD-Aec and control mice, but CD4 and CD8 T cells from the B6.NOD-Aec mice contained markedly higher percentages of IFN-γ+ and IL-17+ cells (Fig. S1B and S1C).
Figure 1. B6.NOD-Aec mice have elevated IL-7 levels.
(A) Percen-tage of TCR-β+ or CD19+ cells in total SMX cells. (B) Flow cytometry of lymphocyte populations in SMX-infiltrating mononuclear cells and intracellular cytokines in CD4 and CD8 T cells, from 24 weeks old control or B6.NOD-Aec mice (upper panels). Percentages of CD4 and CD8 T cells and IFN-γ+ or IL-17+ T cells among mononuclear cells in SMX (lower panels). (C) Real-time PCR analysis of IL-7 mRNA levels in SMX from control or B6.NOD-Aec mice, presented relative to that of β-actin (left). Serum levels of IL-7 measured by ELISA (right). Data are representative or the average of analyses of at least 6 mice for each group (2 mice/experiment, 3 independent experiments).
Since IL-7 can enhance both Th1 and Th17 responses (20–22, 31) and IL-7 levels are elevated in pSjS patients (28), we focused our attention on IL-7. Simulating human pSjS patients, B6.NOD-Aec mice exhibited markedly higher IL-7 mRNA levels in the SMX and higher IL-7 concentrations in the sera than control mice in all age groups examined (Fig. 1C). Moreover, IL-7 levels in B6.NOD-Aec mice increased significantly between 10–12 and 16–18 weeks of age (Fig. 1C). Thus, B6.NOD-Aec mice developed SjS-like lymphocytic infiltration in the salivary glands, which was accompanied by elevated IL-7 levels.
Administration of exogenous IL-7 enhances Th1 response and accelerates the development and onset of pSjS
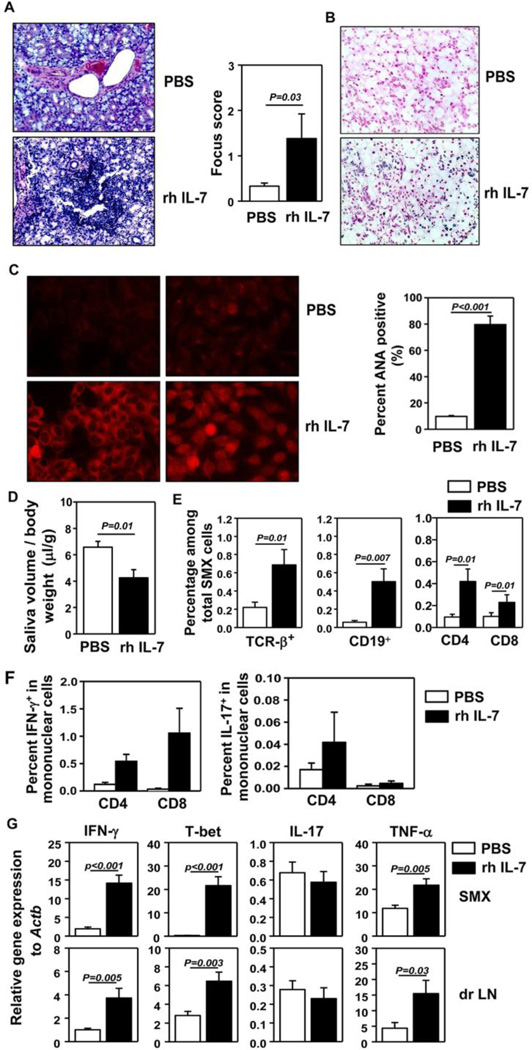
To assessed whether administration of IL-7 could facilitate the development of pSjS, we administered 5 µg recombinant human (rh) IL-7 intraperitoneally (i.p.) into B6.NOD-Aec mice, 3 times weekly starting from age 12 weeks. After 8 weeks of IL-7 treatment, we examined the mice for various disease parameters of SjS. Histological analysis showed leukocyte infiltration in the SMX of IL-7-treated mice, whereas no such infiltration was detected in PBS-treated controls (Fig. 2A). In situ assay for tissue apoptosis showed that IL-7 administration substantially aggravated apoptosis of SMX (Fig. 2B). Analysis of serum antinuclear antibodies (ANA) against a human epithelial cell line, HEp-2, showed that IL-7 treatment markedly increased the percentages of mice that were serum ANA positive, as indicated by the presence of antibodies against HEp-2 substrates in the serum detected by a fluorescence-conjugated anti-mouse IgG antibody (Fig. 2C). Both nuclear and cytoplasmic staining patterns were detected, consistent with a previous report (39). IL-7-treated mice showed significantly decreased saliva flow rate upon pilocarpine-stimulation compared to control mice (Fig. 2D), indicating an exacerbated secretory dysfunction. Hence, IL-7 administration accelerated the development and onset of pSjS.
Figure 2. Administration of exogenous IL-7 accelerates the development and onset of pSjS.
PBS or rh IL-7 were administered to 12 week old B6.NOD-Aec mice 3 times weekly for 8 weeks. (A) H&E staining of SMX sections (left) and focus score of immune cell foci in SMX (right). (B) In situ TUNEL staining of SMX sections for detection of tissue apoptosis. (C) Detection of serum ANA (left) and percentages of mice that are serum ANA positive (right). (D) Stimulated saliva flow rate normalized to body weight. (E) Percentage of lymphocyte populations among total SMX cells. (F) Percentage of IFN-γ+ or IL-17+ T cells among mononuclear cells in SMX. (G) Real-time PCR analysis of gene expression in SMX and dr LN, presented relative to that of β-actin. Data are representative or the average of analyses of 9 mice for each group (3 mice/experiment, 3 independent experiments).
We next assessed the changes in inflammatory T cell subsets and cytokines induced by IL-7 treatment. Significantly more mononuclear leukocytes, determined by forward- and side-scatter profiles, were present in SMX of IL-7-treated mice than in PBS-treated controls (Fig. S2A, left panels). The percentages of CD4, CD8 T cells and B cells among SMX-infiltrating mononuclear cells (Fig. S2A, right panels) and their percentages among total SMX cells (Fig. 2E) were markedly increased by IL-7-treatment. The frequencies of CD4 and CD8 T cells that produced IFN-γ in SMX, spleen and dr LN were elevated by IL-7 treatment (Fig. S2B). The percentages of IFN-γ+ T cells among total mononuclear cells in SMX, spleen and dr LN were all significantly increased by IL-7 treatment (Fig. 2F, left panel; and Fig. S2C). In comparison, IL-7 treatment did not increase the proportion of T cells that produced IL-17 (Fig. S2B, left panels) and the frequency of IL-17+ T cells among the total SMX-infiltrating mononuclear cells in a statistically significant fashion (Fig. 2F, right panels). IL-7-treated mice had higher amounts of IFN-γ, TNF-α and T-bet mRNA in the SMX and dr LN than control mice (Fig. 2G), whereas IL-17 mRNA levels were not affected (Fig. 2G). Hence, exogenous IL-7 administration preferentially increased Th1 responses in the target tissues and accelerated the development and onset of pSjS.
Blockade of IL-7Rα reduces Th1 response and inhibits the development and onset of SjS
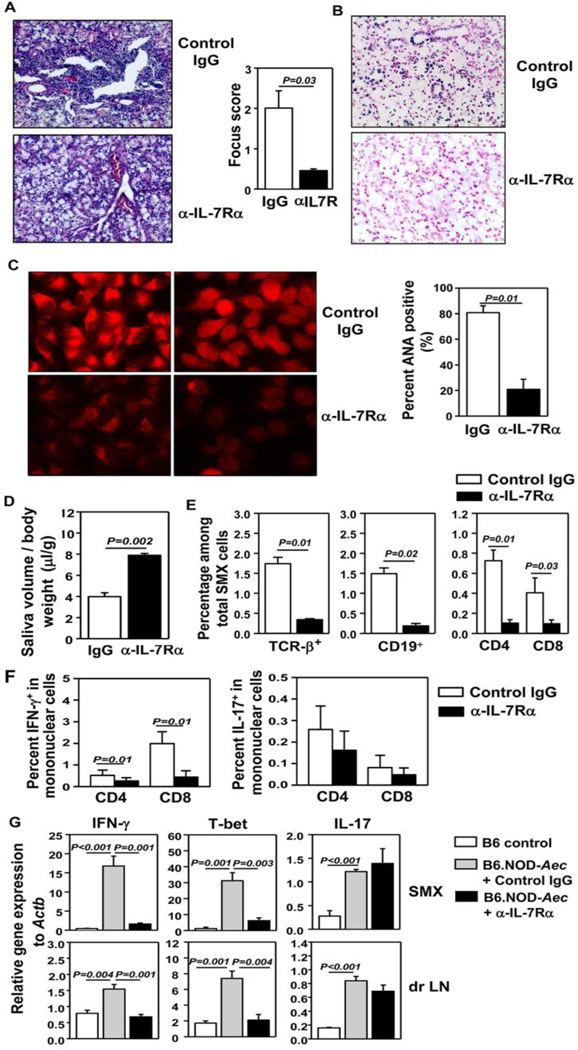
We next assessed whether blockade of endogenous IL-7 activity can inhibit disease development by administration of a non-depleting monoclonal anti-IL-7Rα antibody, previously shown to inhibit IL-7R signaling in vivo (40, 41). We i.p. injected 100 µg anti-IL-7Rα or its isotype control IgG into B6.NOD-Aec mice 3 times weekly, starting from age 16 weeks. After 8 weeks, we measured various disease parameters. Histological analysis showed substantial leukocyte infiltration in the SMX in IgG-treated control mice, which were 24 weeks old at the time of analysis (Fig. 3A). In comparison, leukocyte infiltration was barely detectable in anti-IL-7Rα-treated mice (Fig. 3A). IL-7Rα blockade significantly diminished apoptosis of SMX tissues (Fig. 3B). We particularly examined the tissue areas with no obvious leukocyte infiltrations and the apoptotic cells included both acinar cells and ductal cells of salivary glands (Fig. 3B). IL-7Rα blockade also drastically reduced the percentages of mice that were serum ANA positive (Fig. 3C). Finally, IL-7Rα blockade markedly improved the salivary flow rate, (Fig. 3D). Therefore, blockade of endogenous IL-7R signaling impeded the development and onset of pSjS. Further analysis showed that IL-7Rα blockade significantly reduced the percentages of mononuclear leukocytes in the SMX (Fig. S3A). It also reduced the percentages of CD4, CD8 T cells and B cells in SMX-infiltrating mononuclear cells (Fig. S3A) and their percentages among total SMX cells (Fig. 3E). The proportions of CD4 and CD8 T cells that produced IFN-γ, in both SMX and lymphoid tissues, were reduced by anti-IL-7Rα-treatment (Fig. S3B). The percentages of IFN-γ+ T cells among SMX-infiltrating mononuclear cells were markedly decreased by IL-7Rα blockade (Fig. 3F, left panel). In comparison, IL-7Rα blockade did not significantly affect IL-17+ T cells in SMX (Fig. 3F, right panel) or in lymphoid tissues (Fig. S3C). Consistent with these results, B6.NOD-Aec mice had higher mRNA levels of IFN-γ, TNF-α and T-bet in the SMX than control B6 mice, and that this increase was completely abolished by IL-7Rα blockade (Fig. 3G and S3D). In contrast, IL-17 mRNA level, which was considerably higher in B6.NOD-Aec than control B6 mice, was not affected by IL-7Rα blockade (Fig. 3G). Hence, blockade of endogenous IL-7 activity in B6.NOD-Aec mice preferentially reduced Th1 responses at target sites.
Figure 3. Blockade of IL-7Rα inhibits development and onset of pSjS.
Control IgG or anti-IL-7Rα antibody was injected to 16 week old B6.NOD-Aec mice 3 times weekly for 8 weeks. (A) H&E staining of SMX sections (left) and focus score of immune cell infiltration in SMX (right). (B) In situ TUNEL staining of SMX sections for detection of tissue apoptosis. (C) Detection of serum ANA (left), and percentages of mice that are serum ANA positive (right). (D) Stimulated saliva flow rate normalized to body weight. (E) Percentage of lymphocyte populations in total SMX cells. (F) Percentage of IFN-γ+ or IL-17+ T cells within mononuclear cells in SMX. (G) Real-time PCR analysis of gene expression in SMX and dr LN, presented relative to that of β-actin. Data are representative or the average of analyses of 9 mice for each group (3 mice/experiment, 3 independent experiments).
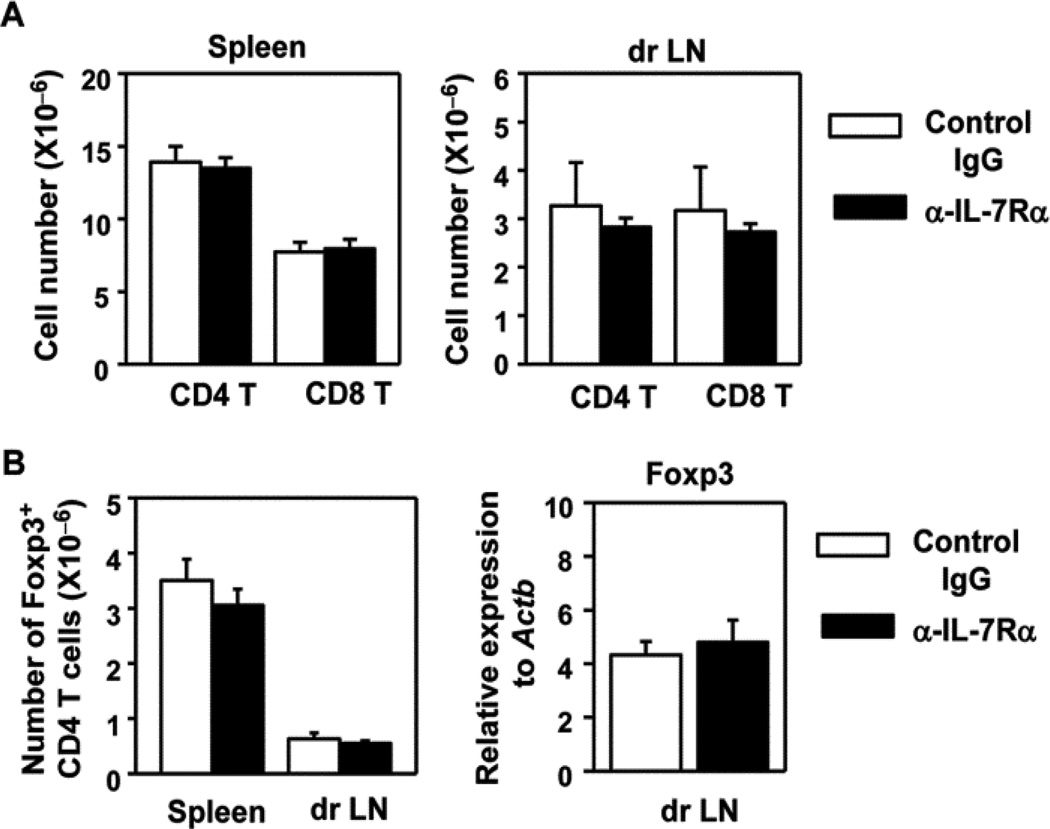
IL-7 is a critical factor for the development and homeostasis of naive T cells. We found that the anti-IL-7Rα treatment regimen we used did not significantly reduce the overall T cells in either spleen or dr LN (Fig. 4A). Hence, this anti-IL-7Rα treatment regimen prevented the development and onset of SjS and reduced Th1 responses at the target sites without affecting overall T cell numbers in lymphoid organs. Importantly, IL-7Rα blockade did not affect the numbers of Foxp3+ CD4 T cells, which are the regulatory T cells (Treg), in either dr LN or spleen (Fig. 4B, left panel). Moreover, Foxp3 mRNA levels in dr LN were not affected by IL-7Rα blockade (Fig. 4B, right panel). Hence, the effects of IL-7 on Th1 responses and SjS development are not caused by altered numbers of Tregs. In conclusion, blockade of endogenous IL-7R signaling inhibits Th1 responses and prevents the development and onset of pSjS.
Figure 4. Anti-IL-7Rα treatment does not affect the numbers of total T cells or Tregs in dr LN and spleen.
Control IgG or anti-IL-7Rα antibody was injected to 16 week old B6.NOD-Aec mice 3 times weekly for 8 weeks. (A) Absolute numbers of CD4 and CD8 T cells in spleen and dr LN (n = 5). (B) Absolute numbers of Foxp3+ Tregs (left) and Foxp3 mRNA levels (right) in dr LN. Data are the average of analyses of 9 mice (3 mice/experiment, 3 independent experiments).
IL-7 upregulates CXCR3 ligand expression in SMX in an IFN-γ- and T cell-dependent fashion
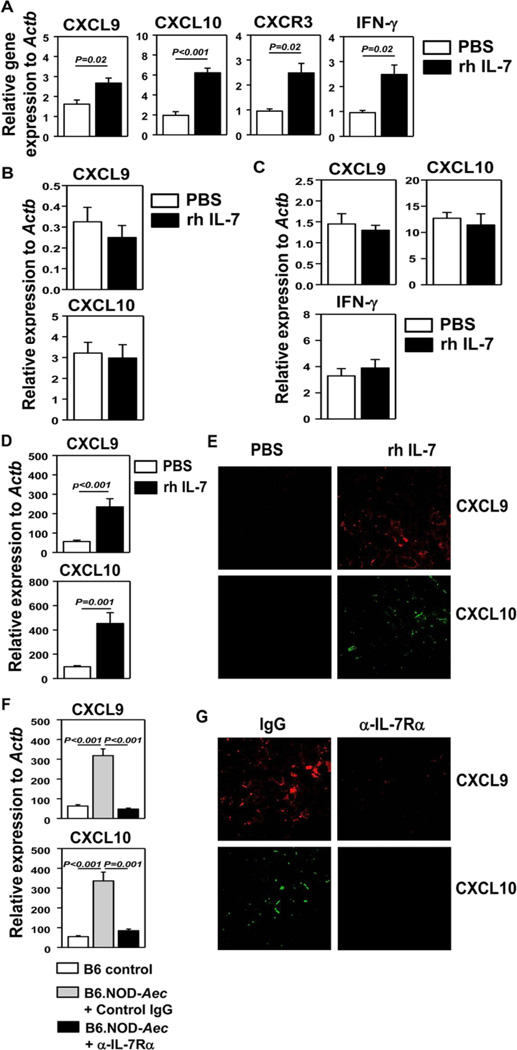
IFN-γ-producing T cells and NK cells express chemokine receptor CXCR3 (42–44). The ligands for CXCR3 include CXCL9, -10 and -11, which are often elevated in the target tissues in autoimmune settings to facilitate recruitment of effector T cells (42–44). Since IFN-γ is a potent inducer of CXCR3 ligands (42–44), we postulated that IL-7-induced upregulation of IFN-γ in SMX will enhance CXCR3 ligand expression. We thereby i.p. administered rh IL-7 into B6 mice and found that IL-7 treatment increased IFN-γ, CXCL9 and -10 gene expression in the SMX 1 day later, while having no effects on CXCL11 (Fig. 5A, and data not shown). CXCR3 mRNA levels were also increased by IL-7, probably as a result of elevated expression of its ligands (Fig. 5A). IL-7 did not upregulate CXCL9 and -10 expression in IFN-γ−/− mice (Fig. 5B), indicating that IL-7 requires IFN-γ to upregulate these CXCR3 ligands in the SMX. Furthermore, IL-7 treatment did not upregulate IFN-γ and CXCR3 ligand expression in RAG1−/− mice (Fig. 5C), indicating that T cells were the major source of IFN-γ in response to IL-7. These results indicated that IL-7 could rapidly upregulate CXCR3 ligands in the SMX in an IFN-γ- and T cell-dependent fashion.
Figure 5. Administration of IL-7 promotes CXCR3 ligand expression in SMX.
Real-time PCR analysis of gene expression in SMX of B6 mice (A), IFN-γ−/− mice (B) or RAG1−/− mice (C) that have received injection of rh IL-7 or PBS 24 hours earlier. (D) CXCR3 ligand mRNA levels in SMX from the same B6.NOD-Aec mice treated with PBS or rh IL-7 described in Figure 2. (E) IF staining of CXCL9 (red) or CXCL10 (green) in SMX from mice described in D. (F) CXCR3 ligand mRNA levels in SMX from B6 control mice and IgG- or anti-IL-7Rα-treated B6.NOD-Aec mice described in Figure 3. (G) IF staining of CXCL9 (red) or CXCL10 (green) in SMX from mice described in F. Data are the average of analyses of 6 – 9 mice for each group (2–3 mice/experi-ment, 3 independent experiments).
We next assessed whether IL-7 administration also induces similar events in B6.NOD-Aec mice in SjS. Indeed, B6.NOD-Aec mice that were treated with rh IL-7 for 8 weeks had markedly higher levels of CXCL9 and CXCL10 mRNA in the SMX than PBS-treated controls (Fig. 5D). Immunofluorescence (IF) staining also showed higher levels of CXCL9 and -10 proteins in the SMX of IL-7-treated B6.NOD-Aec mice (Fig. 5E). Conversely, IL-7Rα blockade in B6.NOD-Aec mice reduced the amount of CXCL9 and -10 mRNA (Fig. 5F) and protein (Fig. 5G) in the SMX to similar levels observed in control B6 mice. Thus, IL-7 enhanced CXCR3 ligand expression in the SMX of both B6.NOD-Aec and B6 mice, in an IFN-γ- and T cell-dependent manner. We reasoned that IL-7 does this by enhancing the levels of T cell-derived IFN-γ.
IFN-γ potently induces CXCR3 ligand expression in human salivary gland epithelial cells
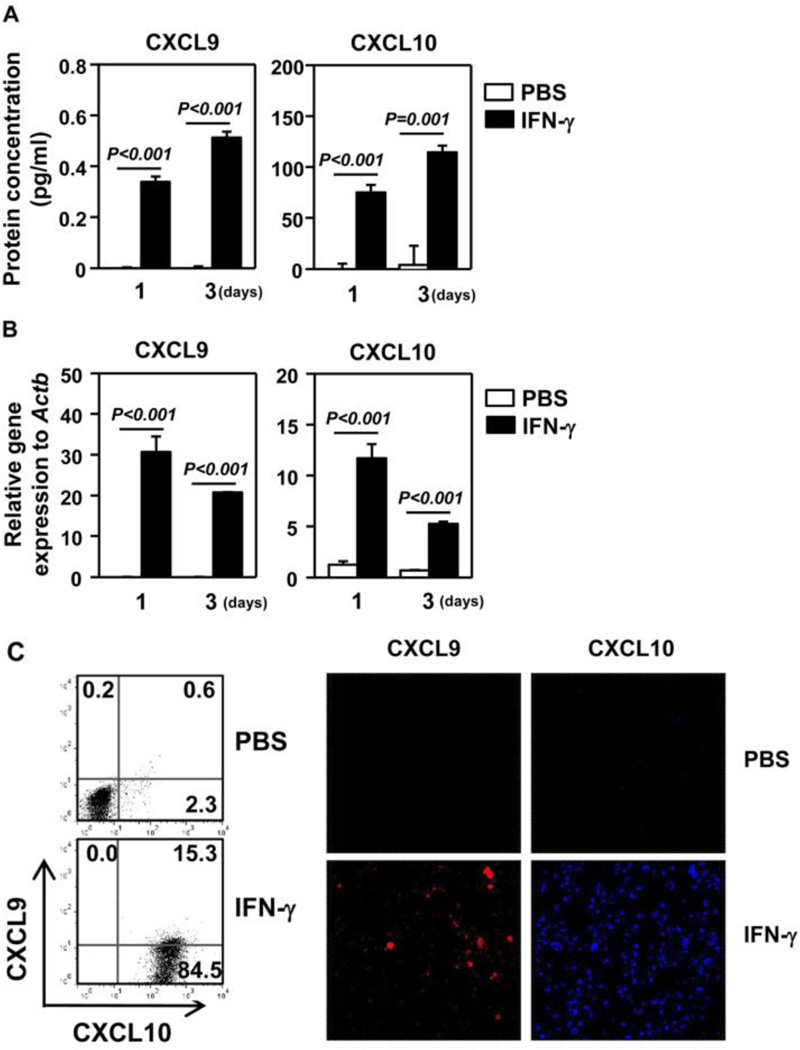
CXCR3 ligands can be produced by a variety of immune cells and non-immune tissue cells. To directly determine the effect of IL-7 and IFN-γ on the production of CXCR3 ligands by salivary gland epithelial cells, we treated HSG cells, a human salivary gland adenocarcinoma cell line, with IL-7 or IFN-γ in vitro and measured expression of CXCR3 ligands. ELISA assay showed that IFN-γ, but not IL-7, greatly increased the concentration of CXCL9 and -10 in HSG culture supernatants after 1 day or 3 days of treatment (Fig. 6A, and data not shown). This is consistent with the findings in mice in that the effect of IL-7 on CXCR3 ligands requires IFN-γ. Furthermore, IFN-γ treatment markedly increased the amount of CXCL9 and -10 mRNA (Fig. 6B) and protein (Fig. 6C) in HSG cells, as determined by RT-PCR, flow cytometric analysis of intracellular protein and IF staining. Hence, IFN-γ, but not IL-7, can induce expression and production of CXCR3 ligands by human salivary gland epithelial cells.
Figure 6. IFN-γ induces expression of CXCR3 ligands in HSG cells.
HSG cells were incubated with PBS or IFN-γ for 1 or 3 days. (A) ELISA assay of CXCL9 and -10 concentrations in culture supernatants. (B) CXCL9 and -10 mRNA levels. (C) Flow cytometry of intracellular CXCL9 and -10 expression (left) and IF staining of CXCL9 and -10 (right) from HSG cells treated with PBS or IFN-γ for 3 days. Data are representative or the average of analyses of 6 independent samples each group (3 samples/experiment, 2 independent experiments).
Discussion
The present study revealed a crucial pathogenic role of IL-7 in the development and onset of pSjS-like autoimmune exocrinopathy using a well-defined mouse model of pSjS. We showed that B6.NOD-Aec mice have increased levels of IL-7 in target organs and sera, which replicates the finding in human patients. We demonstrated that IL-7 plays an indispensable role in the development and clinical onset of this disease. Mechanistically, IL-7 is essential for the aberrantly enhanced Th1 responses and IFN-γ production in the target sites, which leads to subsequent upregulation of CXCR3 ligands to facilitate lymphocytic infiltration of the target tissues.
Previous reports have shown elevated IL-7 levels in the salivary glands and saliva of pSjS patients (28, 30). Here we defined a crucial requirement for IL-7 in the development of pSjS and identified IL-7 as an important new player for this disease. We showed that IL-7 positively regulated the number of IFN-γ-producing CD4 and CD8 T cells in the salivary glands and that T cells were responsible for the production of IFN-γ in response to IL-7. Indeed, many studies have shown the promoting effects of IL-7 on Th1 responses (20–23, 31). Since IFN-γ has been shown to promote both early and late pathogenic events in SjS (11, 13), the positive regulation of IFN-γ is perhaps a chief mechanism by which IL-7 facilitates the development of pSjS. We further demonstrated that IL-7-induced IFN-γ enhanced the expression of CXCR3 ligands in the salivary glands, thereby facilitating the recruitment of more IFN-γ-producing T cells. Hence, IL-7, IFN-γ, CXCR3 ligands and the recruited of CXCR3+ IFN-γ-producing T cells form a positive feed-forward loop to exacerbate salivary gland inflammation and other subsequent pathological changes. Importantly, the anti-IL-7Rα treatment regimen we used did not affect the physiological levels of IL-7R signaling that are required for normal T cell homeostasis, but selectively inhibited the excessive IL-7R signaling associated with SjS conditions. Thus, this regimen provides the foundation for future development of therapeutic strategies that will ablate excessive IL-7R signaling without compromising overall immunity. Furthermore, IL-7Rα blockade did not affect the numbers of Tregs in the lymphoid organs, and therefore, the promoting effects of IL-7 on Th1/Tc1 responses and SjS development are not consequences of altered Treg numbers.
CXCL9 and -10 are expressed in the salivary glands of pSjS patients but not in healthy individuals (45), which was confirmed by our own studies (data not shown). The main cell types producing CXCR3 ligands in the SMX in response to IL-7-induced IFN-γ require further characterization. We demonstrate that IFN-γ can induce the production of CXCL10 and -9 from a human salivary gland epithelial cell line, consistent with a previous report showing that IFN-γ induces these chemokines in human salivary gland explants (45). Thus, salivary gland epithelial cells may be crucial sources of CXCR3 ligands during SjS development. Future studies will address the role of CXCR3 and its ligands in SjS and determine whether the pathogenic effects of IL-7 are dependent on these molecules.
In addition to IFN-γ, we showed that IL-7 increased TNF-α levels, corroborating previous reports that IL-7 enhances TNF-α production from T cells of pSjS patients (28) and RA patients (21, 22). In vitro studies have shown that TNF-α and IFN-γ, alone or cooperatively, can induce apoptosis and secretory dysfunction of salivary gland cells (11, 33–36). Accordingly, we showed that IL-7 markedly enhances apoptosis of salivary gland tissues, which could result from both the direct action of TNF-α and IFN-γ, and indirect action via general enhancement of tissue inflammation. Our future studies will determine the specific functions of IFN-γ or TNF-α in SMX apoptosis in vivo by specifically blocking these cytokines and determine whether the effects of IL-7 on SMX apoptosis are dependent on these cytokines.
Compared to its effects on Th1 responses, we found that the effects of IL-7 on Th17 responses are much less consequential, at least in the B6.NOD-Aec model. The importance of IL-7 for pathogenic IFN-γ production was previously shown in multiple autoimmune diseases (25–27). In the case of RA, IL-7 is required for optimal IL-17 production in addition to IFN-γ (21, 22). Thus, the in vivo role of IL-7 can differ in different disease settings, probably as a result of varying cellular compositions and cytokine milieu.
B6.NOD-Aec mice have elevated levels of IL-7 in target organs and serum, similar to several other autoimmune disease models (21, 46). The regulation of IL-7 expression by external signals is not well understood. Recent studies have shown that tissue production of IL-7 can be regulated by toll-like receptor (TLR) signals (47) and IFN-γ (48). Currently, we are investigating whether IL-7 expression can be induced in the salivary glands by TLR signaling, one possible trigger of SjS.
The present study focused on the functions of IL-7 in the development and onset of SjS. Our ongoing and future studies will determine the effects of both exogenous and endogenous IL-7 on the persistence of SjS after disease onset and to assess whether IL-7Rα blockade can reverse or ameliorate the established disease, which will provide crucial basis for the future development of anti-IL-7Rα therapeutic strategies. We will also characterize the functions of IL-7 in the pathologies of lacrimal glands, the other primary target site of pSjS. Finally, the triggers and pathogenic mechanisms for pSjS are complicated and multifactorial. The various mouse models may each recapitulate part of the pathogenic pathways or one subtype of human pSjS. Therefore, the role of IL-7 needs to be further tested with other mouse models, such as Id3−/− mice (49), and human cells or tissues.
In summary, the present study demonstrates crucial pathogenic functions of IL-7 in pSjS-like autoimmune exocrinopathy in a well-defined model of pSjS. This knowledge will enable us to further investigate the role of IL-7 in other models of this disease and in human samples, in order to comprehensively understand IL-7 in various aspects of SjS and to develop novel therapeutic strategies.
Supplementary Material
Acknowledgments
We thank the Forsyth Institute animal facility for maintaining animals and Biological Resources Branch, DCTD, NCI for providing rh IL-7. This study was supported by grants from the National Institutes of Health (P30DE020751).
Footnotes
The authors have no competing financial interests.
References
- 1.Lee BH, Tudares MA, Nguyen CQ. Sjogren's syndrome: an old tale with a new twist. Arch Immunol Ther Exp (Warsz) 2009;57(1):57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren's syndrome. Nat Rev Rheumatol. 2010;6(9):529–537. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 3.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren's syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32(3):252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R, Deshmukh US, Zheng L, Fu SM, Ju ST. X-linked Foxp3 (Scurfy) mutation dominantly inhibits submandibular gland development and inflammation respectively through adaptive and innate immune mechanisms. Journal of immunology. 2009;183(5):3212–3218. doi: 10.4049/jimmunol.0804355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN. Novel animal models for Sjogren's syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmun. 2006;27(4):289–296. doi: 10.1016/j.jaut.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009;38(1):42–47. doi: 10.1111/j.1600-0714.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandula SR, Scindia YM, Dey P, Bagavant H, Deshmukh US. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjogren's syndrome-like disease. Oral Dis. 2011;17(8):801–807. doi: 10.1111/j.1601-0825.2011.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity. 2004;21(4):551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen CQ, Cha SR, Peck AB. Sjogren's syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front Biosci. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren's syndrome in Id3 knockout mice. Immunology. 2007;122(1):73–79. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome: potential therapeutic targets. Ann Rheum Dis. 2010;69(6):945–948. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brayer JB, Cha S, Nagashima H, Yasunari U, Lindberg A, Diggs S, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjogren's syndrome. Scand J Immunol. 2001;54(1–2):133–140. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 13.Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren's syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60(6):552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis and rheumatism. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen CQ, Sharma A, Lee BH, She JX, McIndoe RA, Peck AB. Differential gene expression in the salivary gland during development and onset of xerostomia in Sjogren's syndrome-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Arthritis Res Ther. 2009;11(2):R56. doi: 10.1186/ar2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nature immunology. 2010;11(3):257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nature immunology. 2011;12(6):478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105(8):2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee LF, Axtell R, Tu GH, Logronio K, Dilley J, Yu J, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci Transl Med. 2011;3(93) doi: 10.1126/scitranslmed.3002400. 93ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144(4):601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Ann Rheum Dis. 2006;65(Suppl 3):iii69–iii74. doi: 10.1136/ard.2006.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, et al. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis and rheumatism. 2010;62(9):2716–2725. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 23.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-Cell immunity in health and disease. Critical reviews in immunology. 2008;28(4):325–339. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 24.Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology (Oxford) 2008;47(6):753–759. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara T, Nemoto Y, Kanai T, Kameyama K, Okamoto R, Tsuchiya K, et al. Upregulated IL-7 receptor alpha expression on colitogenic memory CD4+ T cells may participate in the development and persistence of chronic colitis. Journal of immunology. 2011;186(4):2623–2632. doi: 10.4049/jimmunol.1000057. [DOI] [PubMed] [Google Scholar]
- 26.Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, et al. IL-7 Is essential for the development and the persistence of chronic colitis. Journal of immunology. 2007;178(8):4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki M, Yajima T, Tanabe M, Fukui K, Okada E, Okamoto R, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. Journal of immunology. 2003;171(3):1556–1563. doi: 10.4049/jimmunol.171.3.1556. [DOI] [PubMed] [Google Scholar]
- 28.Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, Bijlsma JW, et al. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjogren's syndrome correlates with increased inflammation. Arthritis and rheumatism. 2010;62(4):969–977. doi: 10.1002/art.27318. [DOI] [PubMed] [Google Scholar]
- 29.Bikker A, Kruize AA, Wenting M, Versnel MA, Bijlsma JW, Lafeber FP, et al. Increased interleukin (IL)-7Ralpha expression in salivary glands of patients with primary Sjogren's syndrome is restricted to T cells and correlates with IL-7 expression, lymphocyte numbers and activity. Ann Rheum Dis. 2012;71(6):1027–1033. doi: 10.1136/annrheumdis-2011-200744. [DOI] [PubMed] [Google Scholar]
- 30.Bikker A, Moret FM, Kruize AA, Bijlsma JW, Lafeber FP, van Roon JA. IL-7 drives Th1 and Th17 cytokine production in patients with primary SS despite an increase in CD4 T cells lacking the IL-7Ralpha. Rheumatology (Oxford) 2012;51(6):996–1005. doi: 10.1093/rheumatology/ker448. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15(5):528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 32.Lee LF, Logronio K, Tu GH, Zhai W, Ni I, Mei L, et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc Natl Acad Sci U S A. 2012;109(31):12674–12679. doi: 10.1073/pnas.1203795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295(5):C1191–C1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol. 2012;302(9):C1331–C1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauley KM, Gauna AE, Grichtchenko II, Chan EK, Cha S. A secretagogue-small interfering RNA conjugate confers resistance to cytotoxicity in a cell model of Sjogren's syndrome. Arthritis and rheumatism. 2011;63(10):3116–3125. doi: 10.1002/art.30450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamachi M, Kawakami A, Yamasaki S, Hida A, Nakashima T, Nakamura H, et al. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. J Lab Clin Med. 2002;139(1):13–19. doi: 10.1067/mlc.2002.120648. [DOI] [PubMed] [Google Scholar]
- 37.Cha S, Nagashima H, Brown VB, Peck AB, Humphreys-Beher MG. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjogren's syndrome) on a healthy murine background. Arthritis and rheumatism. 2002;46(5):1390–1398. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 38.Cha S, Nagashima H, Peck AB, Humphreys-Beher MG. IDD3 and IDD5 alleles from nod mice mediate Sjogren's syndrome-like autoimmunity. Adv Exp Med Biol. 2002;506(Pt B):1035–1039. doi: 10.1007/978-1-4615-0717-8_146. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen CQ, Kim H, Cornelius JG, Peck AB. Development of Sjogren's syndrome in nonobese diabetic-derived autoimmune-prone C57BL/6.NOD-Aec1Aec2 mice is dependent on complement component-3. Journal of immunology. 2007;179(4):2318–2329. doi: 10.4049/jimmunol.179.4.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 41.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 43.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89(2):207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis and rheumatism. 2002;46(10):2730–2741. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Quintial R, Lawson BR, Scatizzi JC, Craft J, Kono DH, Baccala R, et al. Systemic autoimmunity and lymphoproliferation are associated with excess IL-7 and inhibited by IL-7Ralpha blockade. PloS one. 2011;6(11):e27528. doi: 10.1371/journal.pone.0027528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30(3):447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, et al. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. Eur J Immunol. 2010;40(9):2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- 49.Guo Z, Li H, Han M, Xu T, Wu X, Zhuang Y. Modeling Sjogren's syndrome with Id3 conditional knockout mice. Immunol Lett. 2011;135(1–2):34–42. doi: 10.1016/j.imlet.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.