Abstract
For patients with end-stage lung diseases, lung transplantation is the only available therapeutic option. However, the number of suitable donor lungs is insufficient and lung transplants are complicated by significant graft failure and complications of immunosuppressive regimens. An alternative to classic organ replacement is desperately needed. Engineering of bioartificial organs using either natural or synthetic scaffolds is an exciting new potential option for generation of functional pulmonary tissue for human clinical application. Natural organ scaffolds can be generated by decellularization of native tissues; these acellular scaffolds retain the native organ ultrastructure and can be seeded with autologous cells toward the goal of regenerating functional tissues. Several decellularization strategies have been employed for lung, however, there is no consensus on the optimal approach. A variety of cell types have been investigated as potential candidates for effective recellularization of acellular lung scaffolds. Candidate cells that might be best utilized are those which can be easily and reproducibly isolated, expanded in vitro, seeded onto decellularized matrices, induced to differentiate into pulmonary lineage cells, and which survive to functional maturity. Whole lung cell suspensions, endogenous progenitor cells, embryonic and adult stem cells, and induced pluripotent stem (iPS) cells have been investigated for their applicability to repopulate acellular lung matrices. Ideally, patient-derived autologous cells would be used for lung recellularization as they have the potential to reduce the need for post-transplant immunosuppression. Several studies have performed transplantation of rudimentary bioengineered lung scaffolds in animal models with limited, short-term functionality but much further study is needed.
Keywords: lung, tissue engineering, decellularization, recellularization, stem cells
Introduction
Many devastating lung diseases including chronic obstructive pulmonary diseases (COPD), idiopathic pulmonary fibrosis (IPF), and cystic fibrosis, among others, have no cure and cause significant morbidity and mortality. Further, unlike other major diseases, lung diseases, notably COPD are increasing in prevalence and COPD is predicted to be the third leading cause of death worldwide by the year 20201,2. Currently, patients with end stage lung diseases are limited to lung transplantation as their only treatment option. Unfortunately, there are few available lungs for transplant, 5 year survival after lung transplantation is only approximately 50%, and transplantation recipients require lifelong immunosuppression1,2. New options are desperately needed.
A promising and rapidly growing area of investigation is that of ex vivo bioengineering of functional lung tissue that could then be implanted into patients with diseases such as COPD or IPF. This could be accomplished by utilizing either biologically-derived or fabricated 3 dimensional (3D) matrices or other artificial scaffolding seeded with autologous stem, progenitor, or other cells obtained from the eventual transplant recipient. The use of autologous cells would eradicate the need for lifelong immunosuppressive drugs. These approaches have been successfully utilized in regeneration of other tissues including skin, vasculature, cartilage, bone, and trachea and more recently more complex organs including heart and liver3–15. Synthetic constructs offer one option and a number of different synthetic scaffold materials and manufacturing technologies have been evaluated for use to produce matrices for ex vivo lung parenchymal development and for the study of growth factors and mechanical forces on lung remodeling16–21. These studies have included implantation of various scaffolds impregnated with stem or other cells in order to produce functioning lung tissue22–25. Comparable approaches have been utilized to study creation of pulmonary vascular networks from synthetic scaffolds and to investigate effects of vascular endothelial cells on development of airway and alveolar epithelial tissues26,27. However, current state-of-the-art manufacturing technologies are unable to recapitulate the complex 3- dimensional architecture of the lung and, further, robust schemes for successful implantation and clinical use of synthetic lung scaffolds remain unknown.
An alternative approach is to utilize whole lungs in which all cells and cellular materials are removed leaving an intact 3-dimensional scaffold comprised of innate extracellular matrix (ECM) proteins in a bio-mimetically similar 3-dimensional architecture. This approach, termed decellularization, preserves native airway and vascular structure and provides an acellular matrix for cell seeding and functional recellularization3,28–30. This approach also provides a novel culture system to study cell-matrix interactions and environmental factors such as mechanical stretch on lung cell growth and development. This technique was originally described many years ago, one classic example is by Lwebuga- Mukasa and colleagues in 1986 in which a decellularized rat lung was utilized to study the effect of the basement membrane on growth of type II alveolar epithelial (AEII) cells30. The technique was re-invigorated in 2010 and a number of laboratories are currently exploring this approach (Table 1) 31–44.
Table 1.
Compiled Studies of Ex Vivo Lung Bioengineering Using Decellularized Whole Lung Scaffolds
| Reference | Scaffold | Objective | Method Of Decellularization |
Timing Of Decellularizat ion Process |
Endpoints |
|---|---|---|---|---|---|
|
Kuttan Lung 1981 (29) |
Alveolar Basement Membrane (Calf, Dog, Rabbit, Adult/Newb orn Rat) |
Study Basement Membrane |
Filtered Distal Lung Homogenate, Saline, 4% Triton- X100 With Protease Inhibitors, Nahco3 Rinse, Distilled H2o Rinse. |
26–52 Hours Depending On Homogenate Volume |
Histology, Immunofluorescen ce, Electron Microscopy, Amino Acid Analysis, Carbohydrate Analysis |
|
Lwega Mukasa et al. Exp Lung Res 1986 (30) |
Acellular Alveolar Versus Amniotic Basement Membranes |
Differentiatio n Pattern On Different Basement Membranes |
Distilled H2O, 0.1% Triton X100, 2% SDC, NaCl, Pancreatic DNAase Type 1S |
> 2 Days | Cell Attachment And Morphology |
|
Price et al Tissue Engineeri ng Part A 2010 (31) |
Mouse (Female C57/BL6) Acellular Lungs |
Effect Of Matrix On Geospacial Engraftment Of E17 Fetal Lung Homogenate |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, SDC, NaCl, Porcine Pancreatic DNAase |
3 Days (Approximatel y 63 Hrs) |
Histology, Quantification Of ECM Proteins, Immunofluorescen ce, SEM, Function With Flexivent, Bioreactor With Fetal Type II Cells |
|
Petersen et al Science 2010 (32) |
Rat Acellular Lungs (Male Fischer 344) |
Development Of Bioartificial Lung For Orthotopic Transplantati on |
Vascular Perfusion Only (1–5ml/Min With Less Than 20 Mmhg Arterial Pressure) CHAPS, NaCl, EDTA, PBS |
4 Hours | Histology, Immunofluorescen ce, DNA Quantification Assay, Collagen Assay, GAG Assay, Western Blots, SEM, TEM, Micro- CT Imaging |
|
Cortiella et al Tissue Eng Part A 2010 (34) |
Rat Acellular Lung (Sprague Dawley) |
Comparison Of Matrices Including Decellularized Rat Lung In Ability To Support mESCs |
Fast Freeze/Thaw Cycles, 1%SDS, Dnaase, Rnaase, PBS, Penicillin/Streptom ycin, Amphotericin, DMEM |
>6weeks | Quantification Of DNA, Immunohistochemi stry, Confocal Microscopy, Flow Cytometry, 2 Photon Microscopy, Presence Of Spa |
|
Ott et al Nature Med 2010 (33) |
Rat Acellular Lung (Sprague Dawley) |
Development Of Bioartificial Lung For Orthotopic Transplantati on |
Vascular Perfusion Only: Pulmonary Artery Pressure Kept Constant At 80cmh2o, Heparinized PBS With 0.1% SDS, Deionized Water, Triton X100, And PBS With Penicillin, Streptomycin, Amphotericin B |
3 Days (Approximatel y 75 Hrs) Including Incubation With Antibiotics |
Histology, Morphology, Mechanical Function, Fluoroscopy, Gas Exchange, Transplantation, Protein Analysis |
|
Song, Ott et al. Ann Thorac Surg 2011 (37) |
Rat Acellular Lung (Sprague Dawley) |
Orthotopic Transplantati on |
Vascular Perfusion Only: Pulmonary Artery Pressure Kept Constant At 80cmh2o, Heparinized PBS With 0.1% SDS, Deionized Water, Triton X100, And PBS With Penicillin, Streptomycin, Amphotericin B |
3 Days (Approximatel y 75 Hrs) Including Incubation With Antibiotics |
Histology, Immunohistochemi stry, Morphology, Fluoroscopy, Functional Analysis, Transplantation Seeded Lungs With Fetal Pulmonary Cells And Pulmonary Artery And Vein With Endothelial Cells |
|
Shamis et al. Tiss Eng Part C 2011 (36) |
Rat Acellular Liver And Lung (Lewis) |
Cellular Differentiatio n On 3D In Vitro Scaffold |
Lung Lobes Cut Into 300 Micron Thick, 0.5% Tritonx100, 10mm Ammonia, Mechanical Disruption, PBS, Distilled Water |
N/A | Histology, TEM, Environmental Scanning, PCR, Immunohistochemi stry, Liquid Chromatography With Tandem Mass Spectrometry |
|
Daly et al Tissue Eng Part A 2011 (39) |
Mouse Acellular Lung (C57BL/6; BALB/C) |
Initial Binding And Recellularizati on Of Mscs In Acellular Scaffold; Directed Seeding With Integrin Blocking |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, 2% SDC, NaCl, Pancreatic DNAase Type 1S |
3 Days (Approximatel y 72 Hrs) |
Histology, Immunofluorescen ce, EM, Perfusion To Assess Vascular Continuity, Mass Spectrometry, Western Blot, Lung Mechanics With Flexivent, Innoculation Of Bone Marrow Derived MSCs |
|
Wallis et al. Tissue Engineeri ng Part C 2011 (41) |
Mouse Acellular Lung And Lung Slices (BALB/C) |
Comparison Of Detergent- Based Decellularizati on Protocols |
Airway And Vascular Perfusion. 3 Different Protocols Tested: 1) H2O, 0.1% Triton X-100, 2% SDC, NaCl, Porcine Pancreatic DNAase; 2) PBS, 0.1%SDS, 0.1%Triton X-100; 3) PBS, CHAPS, NaCl, EDTA, DNAase, FBS |
3 Days (Approximatel y 72 Hrs) |
Immunohistochemi stry, Mass Spectrometry, Western, Mechanical Analysis, Gelatinase, Dnaase, Rnaase, Comparative Recellularization With MSCs And C10 Epithelial Cells |
|
Bonvillain et al Tissue Eng Part A 2012 (38) |
Normal Rhesus Macaque Acellular Lung |
Initial Binding And Recellularizati on Of MSCs In Acellular Scaffold |
Airway And Vascular Perfusion: PBS, EDTA, Penicillin/Streptom ycin At Initial Harvest: Pulmonary Artery: PBS+Heparin+ Sodium Nitroprusside With Pressures 25- 30mmhg; Then Trachea And Vasculature: Deionized H2O, 0.1%Tritonx100, 2%SDC, NaCl, Bovine Pancreatic DNAase |
2–3 Days (Approximatel y 48–72 Hrs) |
Histology, Morphology, Immunohistochemi stry, Western Blot, Genomic DNA, Proteomics, Seeding With Bone Marrow And Adipose Derived Rhesus MSCs |
|
Longmire et al. Cell Stem Cell 2012 (61) |
Mouse Acellular Lung And Lung Slices (C57/BL6) |
Seeding With And Differentiation Of mESCs- Derived Endodermal Lung Precursors |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, 2% SDC, NaCl, Pancreatic DNAase Type 1S |
3 Days (Approximatel y 72 Hrs) |
Evaluation Of Ability To Differentiate mESCs Into Lung Precursor Cells |
|
Jensen et al. Tissue Eng Part C 2012 (40) |
Mouse Acellular Lung (C57BL/6) |
Comparison Of Timing Of Decellularizati on, Coating Of Decellularized Matrices, And Support Of mESCs Differentiated Into Alveolar Epithelial Cells |
Airway And Vascular Perfusion: 0.1% Triton X100, 2%SDC, NaCl, Porcine Pancreatic DNAase, PBS |
1 Vs 3 Days (Approximatel y 24 Hours Vs 50 Hours) |
Histology, Morphology, EM, Western Blot, Gelatinase Assay, Immunofluorescen ce, Mechanical Properties With Flexivent, Support Of Differentiated mESCs Within Scaffold, Subcutaneous Implantation Of Scaffold |
|
Petersen et al. Cells Tissues Organs 2012 (44) |
Rat Acellular Lung (Fisher 344) |
Comparison Of Different Detergent- Based Decellularizati on Protocols |
2 Approaches: 1- Vascular Perfusion CHAPS, NaCl, EDTA, PBS; 2- NaCl, EDTA, SDS |
4 Hours | Histology, Collagen Assay, Elastin Assay, GAG Assay, DNA Assay, Mechanical Testing With Linear Strips |
|
Booth et al. Am J Resp Crit Care 2012 (56) |
Human Normal And Fibrotic Acellular Lungs |
Development Of An In Vitro System For Normal And Fibrotic Matrices |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, PBS, 2%SDC, NaCl, DNAase, Mgso4, Cacl2; 0.18% Paracetic Acid/4.8% Ethanol |
3 Days (Approximatel y 72 Hrs) |
Histology, Western Blot, PCR, AFM, Mass Spectrometry, EM; Fibroblasts Were Seeded In To Normal And Fibrotic Lungs And Assayed For Gene And Protein Expression Changes |
|
Bonenfant et al Biomateri als 2013 (42) |
Mouse Acellular Lung And Lung Slices (C57BL/6) |
Effect Of Time To Necropsy, Length Of Storage, And 2 Different Methods Of Sterilization Of Construct |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, 2% SDC, NaCl, Pancreatic Deoxyribonuclease Type 1S, Mgso4, Cacl2, Penicillin, Streptomycin |
3 Days (Approximatel y 72 Hrs) |
Histology, Immunohistochemi stry, Morphology, Mass Spectrometry, Seeded Lungs With MSCs And C10 Epithelial Cell Line |
|
Sokocevic et al Biomateri als 2013 (43) |
Mouse Acellular Lung And Lung Slices (C57BL/6) |
Effect Of Recipient Age And Elastase, Or Bleomycin Injury On Decellularizati on And Recellularizati on |
Airway And Vascular Perfusion: Distilled H2O, 0.1% Triton X100, 2% SDC, NaCl, Pancreatic Deoxyribonuclease Type 1S, MgSo4, CaCl2, Penicillin, Streptomycin |
3 Days (Approximatel y 72 Hrs) |
Histology, Immunohistochemi stry, Mass Spectrometry, Inoculation With MSCs And C10 Cells |
Abbreviations Used:
AFM- Atomic Force Microscopy
CaCl2-Calcium Chloride
CHAPS-3- [(3-Cholamidopropyl)Dimethylammonio]-1-Propanesulfonate Hydrate
cmh2o: Centimeters of Water (Pressure)
CT: Computed Tomography
DMEM: Dulbecco’s Modified Eagle’s Medium
DNAase: Deoxyribonuclease
DNA- Deoxyribonucleic Acid
E17- Embryonic Day 17
ECM- Extracellular Matrix
EDTA- Ethylenediaminetetraacetic Acid
FBS: Fetal Bovine Serum
GAG- Glycosaminoglycan
H2O- Water
mESCs: Embryonic Stem Cells
MgSO4- Magnessium Sulfate
mM: Millimolar
mmhg: Millimeters Of Mercury
MSC: Mesenchymal Stem Cells
NaCl- Sodium Chloride
NaHCO3- Sodium Biocarbonate
PBS- Phosphate Buffered Saline
PCR: Polymerase Chain Reaction
RNAase: Ribonuclease
SDC- Sodium Deoxycholate
SEM- Scanning Electron Microscopy
SDS: Sodium Dodecyl Sulfate
SpA: Surfactant Protein A
TEM- Transmission Electron Microscopy
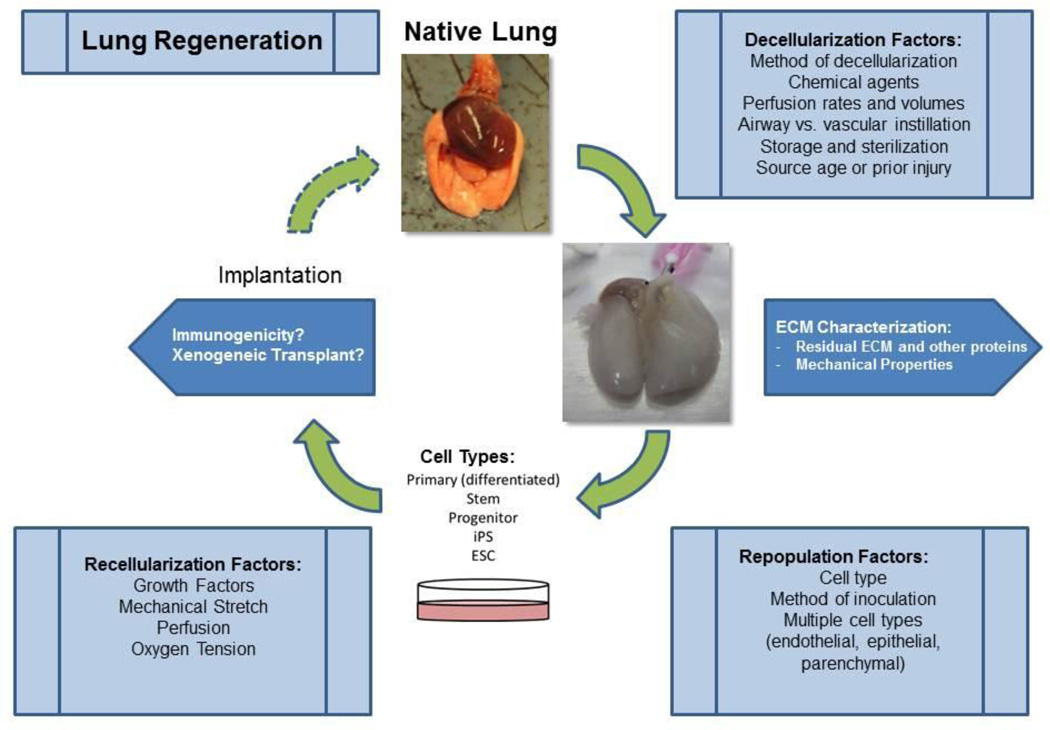
In this review, we will address some of the critical factors involved in the theoretical and practical considerations for use of decellularized whole lungs (alternatively referred to as acellular) for ex vivo lung regeneration. These include decellularization and recellularization procedures as well as consideration of the potential immunogenicity of the scaffolds (schematic in Figure 1). We will also speculate as to the logistics involved in implementation of this approach for lung diseases. Finally, we discuss the feasibility of employing acellular scaffolds for ‘repopulation assays’ of stem-progenitor cells.
Figure 1. Schematic for optimal decellularization, recellularization, and implantation.
Review
Decellularization
Methods of Decellularization
Creation of organ scaffolds requires removal of the native cell population while minimizing alterations to the dimensions and mechanical characteristics of the organ, the structural support for the airway, vascular and lymphatic networks, and to the composition of the native matrix including important cell binding ligands3. Common methods for decellularization of lung tissue pieces include sonication, sieving, and extraction of thin pieces of lung tissue and digestion with acetic acid followed by sonication. While useful techniques for developing in vitro systems to study lung biology, these methods did not preserve the 3 dimensional architecture of the lung. Recently, several techniques have emerged for decellularizing whole lungs which retain the 3 dimensional architecture as well as key extracellular matrix proteins (Table 2, Figure 2). These techniques vary significantly with use of different combinations of physical, ionic, chemical and enzymatic methods and procedure times that vary between 2 hours to 7 weeks. Detergent-based decellularization is a frequently utilized approach and commonly used detergents include Triton X100, sodium deoxycholate (SDC), sodium dodecyl sulfate (SDS), and CHAPS in addition to hypertonic lysis of cells with NaCl as well as a DNAase and/or RNAase. Furthermore, some investigators employed both vascular and airway perfusion with these agents while others have only perfused through the vasculature. As such, significant differences in histologic appearance of the decellularized lungs and in content of both ECM and other retained proteins occur in the various published works evaluating the quality of decellularized lungs. How these differences might affect recellularization or potential immunogenicity of the implanted scaffold are still poorly understood. Some proposed criteria for optimal decellularization include complete absence of visible cellular or nuclear material on histological examination, less than 50ng of dsDNA per 1 mg of dry weight of the extracellular matrix scaffold and remnant DNA molecules shorter than 200 bp3,40. However, further study is needed to understand and define optimal endpoints for decellularization. Other criteria such as retention of specific ECM components and maintenance of macro and micro-mechanical properties are likely critical parameters in defining optimal decellularized scaffolds.
Table 2.
Agents Commonly Used During De-Cellularization
| Agent | Properties |
|---|---|
| Triton X-100 | Nonionic detergent used to solubilize proteins; mild non-denaturing detergent |
| Sodium Deoxycholate (SDC) | Water soluble ionic detergent used for disrupting and dissociated protein interaction |
| Sodium Dodecyl Sulfate (SDS) | Anionic surfactant used for lysing cells and unraveling proteins |
| 3-[(3-cholamidopropyl)dimethylammonio]-1- propanesulfonate (CHAPS) |
Non-denaturing zwitterionic detergent used to solubilize proteins |
| Ethylenediaminetetracetic acid (EDTA) | Chelating agent that binds to calcium and prevents joining of cadherins between cells, preventing clumping of cells grown in liquid suspension, and detaching adherent cells. Can also be used to inhibit metalloproteinases. |
| Antibiotics | Typically Penicillin, Streptomycin, and an anti mycotic Amphotericin |
| Other | DNAase, RNAase, and heparin |
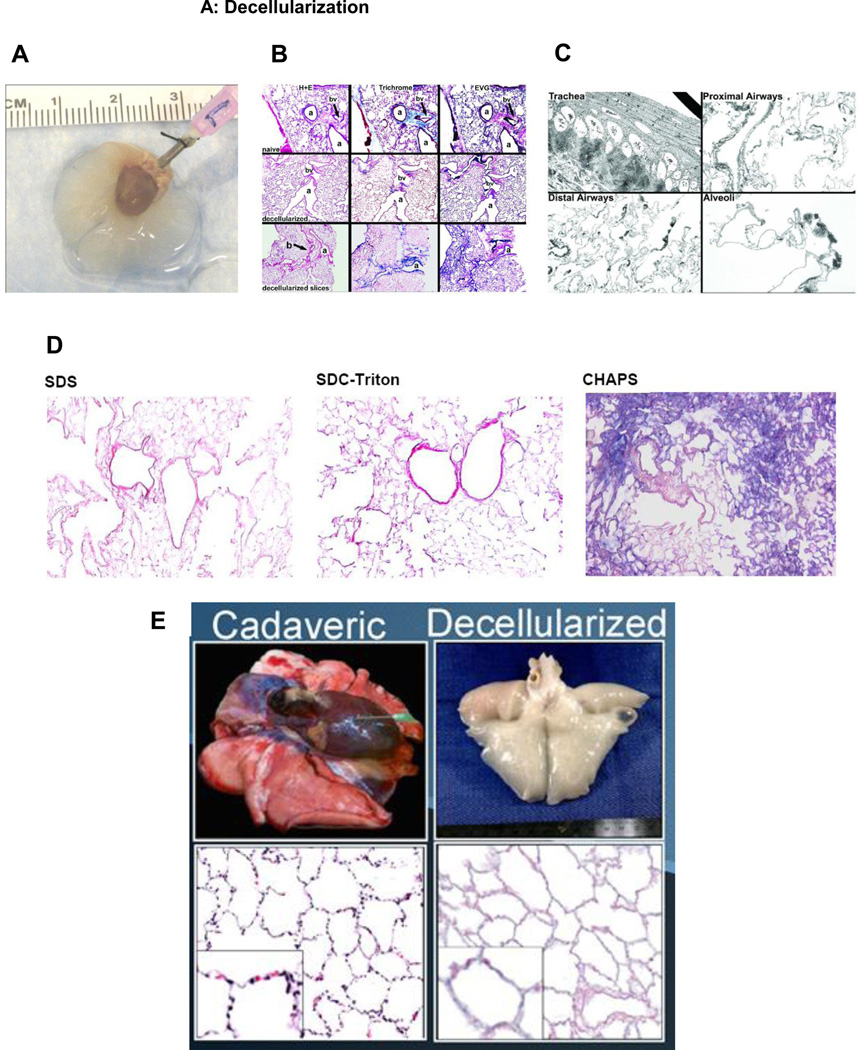
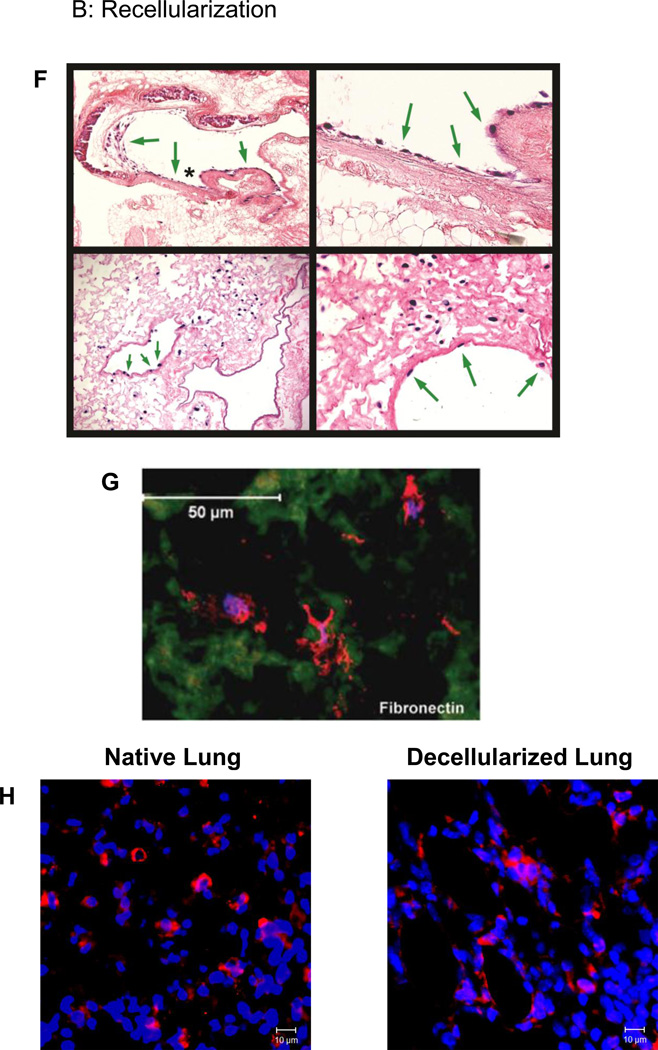
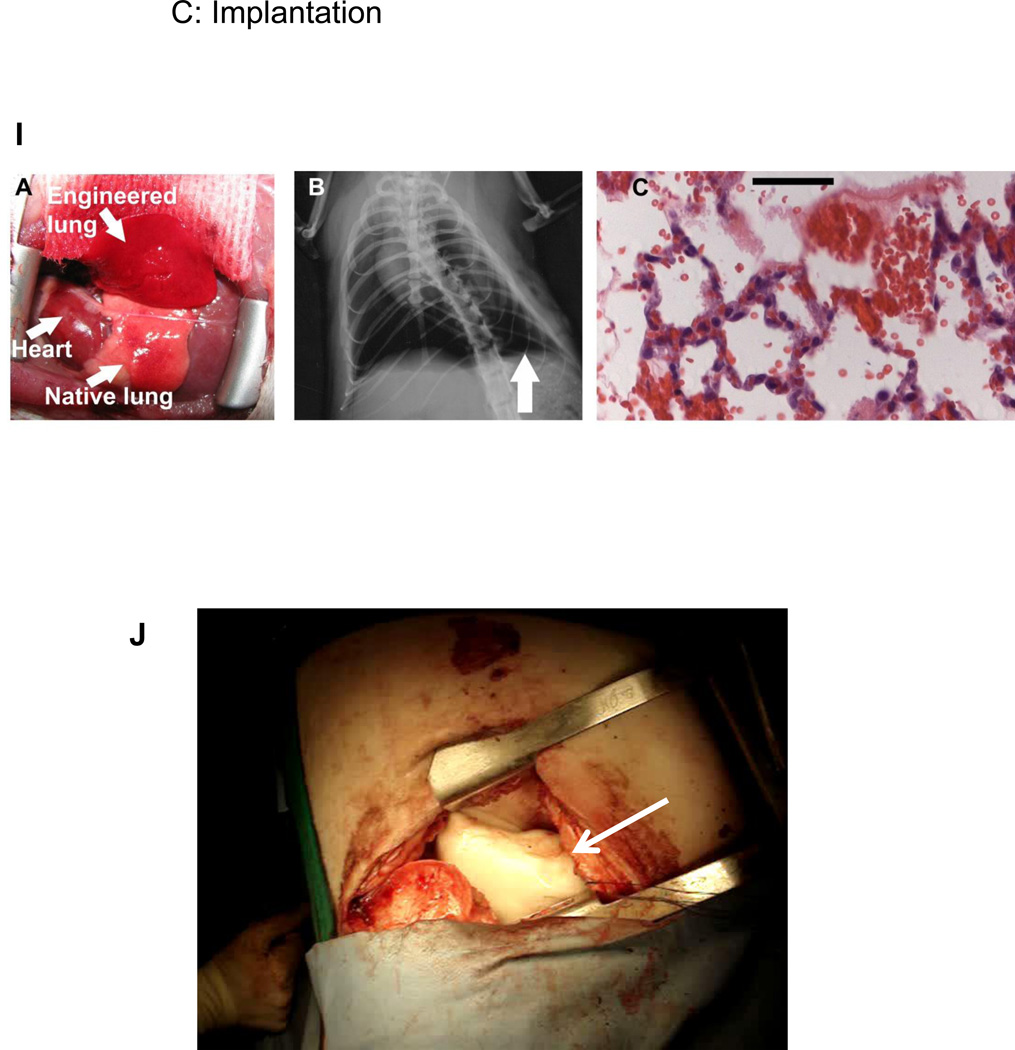
Figure 2. Representative images depicting decellularization, recellularization, and implantation of decellularized lung scaffolds.
A) Whole de-cellularized mouse heart-lung bloc. The trachea is cannulated with a 18 gauge blunted needle. Reprinted with permission from Daly et al., Initial Binding and Re-Cellularization of De-Cellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells Tissue Engineering Part A, Vol. 18, No. 1–2: 1–16, 2012 (39).
B) H and E, Masson’s Trichrome collagen, and Verhoeff’s Van Gieson staining of native mouse lungs, de-cellularized whole mouse lungs, and approximately 1 mm thick slices of mouse de-cellularized lungs. Original magnifications: 100 X. a = airway, bv = blood vessel. Reprinted with permission from Daly et al., Initial Binding and Re-Cellularization of De-Cellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells Tissue Engineering Part A, Vol. 18, No. 1–2: 1–16, 2012 (39).
C) Transmission electron micrograph images of different regions of a representative de-cellularized whole mouse lung are shown. Original magnifications A) 600X, B) 1,000X, C) 1,000 X, D) 3,000X. Reprinted with permission from Daly et al., Initial Binding and Re-Cellularization of De-Cellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells Tissue Engineering Part A, Vol. 18, No. 1–2: 1–16, 2012 (39).
D) Histologic assessment of H and E stained whole mouse lungs de-cellularized using different detergent-based protocols demonstrates significant differences in resulting histologic architecture notably loss loss of detail and parenchymal structure when using CHAPS. a = airway, bv = blood vessel, Original magnifications 100X. Reprinted with permission from Wallis et al., Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 2012; 18: 420–432 (41).
E) Representative gross and histologic images of native and decellularized macaque lungs. Reprinted with permission from Bonvillain et al., A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A 2012; 18: 2437–52 (38)→.
F) Intratracheally inoculated MSCs cultured up to one month in both basal MSC media and in SAGM grow in parenchymal and airway regions of de-cellularized whole mouse lungs. Representative photomicrographs depict MSCs in large airways (upper row) and in parenchymal lung regions (lower row). Green arrows highlight cells growing in large airways, and the asterisk in the upper left-hand image show the region magnified in the upper right-hand image. Original mags are 100X, 400X, 400X and 200X. Reprinted with permission from Daly et al., Initial Binding and Re-Cellularization of De-Cellularized Mouse Lung Scaffolds with Bone Marrow-Derived Mesenchymal Stromal Cells Tissue Engineering Part A, Vol. 18, No. 1–2: 1–16, 2012 (39).
G) Lung-derived MSCs (L-MSCs) inoculated into decellularized sheep lung scaffolds express and grown for 2 weeks express a variety of ECM proteins (fibronectin is depicted) that may help to remodel the scaffold. Reprinted with permission from Ingenito et al,. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Trans 2012; 21: 175–189 (75).
H) Mouse embryonic stem cells (ESCs) pre-differentiated in routine tissue culture to express pro-surfactant protein C (pro-SPC, red stain) maintain pro-SPC expression after culture in decellularized mouse lung scaffolds. Depcited are images of native mouse lung and decllularized mouse lung 1 week after inoculation with SPC-expressing murine ESCs. Original magnification 200X. Reprinted with permission from Jensen et al., A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods 2012; 18: 632–46 (40).
I) Tissue-engineered left lung was implanted into adult Fischer 344 rat recipient and photographed ∼30 minutes later. (B). X-ray image of rat showing the implanted engineered left lung (white arrow) and the right native lung. (C) H and E stain of explanted lung. Red blood cells perfusing septa are evident, and some red blood cells are present in airspaces. Scale bar 50 µm. Reprinted with permission from Petersen et al., Tissue-Engineered Lungs for in Vivo Implantation. Science, 2010 329:538–541 (32).
J) Decellularized sheep lung lobe (white arrow) implanted into an adult sheep establishing both airway and vascular anastomoses with appropriate inflation and vascular perfusion. Hoffman, Finck, Weiss unpublished data.
Residual Extracellular Matrix and Other Proteins
The lung is composed of a variety of cells and associated extracellular matrix (ECM). The ECM has an essential role in prenatal development, postnatal maintenance of normal function, and is known to be an inductive scaffold in directing the remodeling response after injury45–49. The ability of cells to receive organotypic signals from native ECM makes decellularized scaffolds a seemingly better choice than synthetic constructs for tissue engineering. Therefore, retention of key ECM components is essential in the decellularization process. Which combination of ECM proteins must be retained to maintain critical cues for cell functions remains unknown. Further, the detergents utilized during the decellularization process can activate matrix metalloproteinases and thus potentially exacerbate degradation of critical binding epitopes on the remaining ECM proteins41. The major structural and functional molecules in the ECM include glycosaminoglycans (GAGS) and the collagens, elastin, fibronectin, laminin, and vitronectin. Collagens are important structural components of the lung and are responsible for overall mechanical strength. Elastin is an important ECM protein for providing the reversible distension and intrinsic recoil properties of lung tissue. GAGs help control macromolecular and cellular movement across the basal lamina and may play a role in the mechanical integrity of the lung. These matrix molecules are generally highly conserved proteins in eukaryotic organisms and theoretically may explain the absence of an adverse immune response after xenotransplantation as seen with some other decellularized organ transplants such as the tracheal patch14,15. However, some ECM proteins, for example collagen V, are postulated to play significant immunogenic roles in rejection of lung transplantation50. Laminin, fibronectin, elastin, and collagens I and IV have also been found to play a role in trans-membrane cell signaling, cellular differentiation, respiratory mechanics and other pulmonary-specific functions46–49.
The ECM components remaining in decellularized lung scaffolds can be evaluated using a combination of histologic, immunohistochemical, and Western blotting techniques (Table 1). In most instances, the decellularization process largely preserves collagen but results in a moderate loss of elastin31–33,38–43. In one study, comparison of different detergents in the decellularization process revealed that SDS was associated with a greater loss of type 1 collagen and elastin when compared with CHAPS44. Another study demonstrated that different detergent based de-cellularization protocols result in significant differences in histologic appearance, gelatinase activation, distribution of ECM components, and lung mechanics41. However, despite these differences in composition of the lungs, inoculated cells appeared to attach and recellularize the lung regardless of the decellularization protocol utilized41. Furthermore, recent investigations of relevant clinical parameters including effect of donor age, time to necropsy, prior lung injury, length of scaffold storage, and sterilization method demonstrate that each of these parameters can affect the outcome and histology of the decellularized scaffold42–43. Therefore, the choice of the lung utilized, method of decellularization, and clinical storage conditions must, at a minimum, preserve appropriate ligands to allow adherence, spreading, polarization (if appropriate), and in some cases, proliferation of cells.
Mass spectrometry is increasingly utilized to identify a broader range of residual ECM proteins, including isoforms not readily distinguished by the other analytical techniques38,39,41–43. This approach also demonstrates that a wide range of other non-ECM proteins are retained in decellularized scaffolds with the current techniques including intracellular, cytoskeletal, and cell membrane-associated proteins. This suggests that cellular proteins are not all removed by decellularization protocols, presumably due to tight anchoring of transmembrane proteins to ECM ligands. Importantly, the spectrum of retained cellular (non-ECM) proteins differs depending on the decellularization protocol utilized41. The effects of these cellular proteins on recellularization and potential immunogenicity of the scaffold is unknown at present but several retained proteins (e.g. histones) are known to be immunogenic51. Moreover, the range of different GAGs that are retained which may be important for recellularization and contribute to potential immunogenicity has not been well explored52. These are important areas for future study.
Species differences in decellularized lungs
Published data to date includes decellularization of mouse, rat, sheep, macaque, and human lungs. Our collaborative group has also accumulated extensive data on decellularization of pig and cadaveric human lungs (manuscripts in preparation). While there are no obvious substantive differences in the final decellularized lungs from each species, differences in lung and pleural anatomy may significantly affect the decellularization process. Further, decellularizing larger lungs requires practical modifications of detergent-based techniques including close attention to rate and volume of solutions used to perfuse and wash the more cumbersome larger lungs. Special consideration needs to be taken for decellularizing human lungs under appropriate containment conditions including use of appropriate antimicrobial agents and an appropriate post-decellularization sterilization protocol.
Functionality of the Decellularized Scaffold
In vitro evaluation of the “function” of the decellularized scaffolds can be challenging. It is not clear what type of functional assessments best reflect the ability of decellularized lung scaffolds to support recellularization and ultimately development of functional lung tissue. Investigators have explored mechanical function including assessment of lung mechanics39–41 as well as force tension relationships in linear strips of decellularized lungs44. These methods give an indication of the elastance, compliance, resistance, and diffusion properties of the scaffolds. However, in the absence of cells and surfactants, the decellularized lungs are stiff, a factor that must be taken into account for recellularization schemes using bioreactor technology. One potential bioassay to follow over time as the lungs are recellularized is the decrease in elastance (increase in compliance) due to growth and maintenance of a functional population of surfactant-producing cells39. Total lung water, normally controlled by endothelial, lymphatic, and type I alveolar epithelial cells might also be important to monitor but no guidelines have been established for these endpoints.
Recellularization of acellular scaffolds for bioengineering and assays of organotypic repopulation
The lung is comprised of many (>40) cell types that are replenished by resident stem or progenitor cells following injury. However, there is much debate concerning the identification and nature of different types of endogenous lung stem/progenitor cells that can function in repair53–55. Notably, this area of research has been hampered by the lack of robust assays of endogenous stem/progenitor cell function including an accurate repopulation assay. Repopulation assays are critical for verifying the capacity for multipotency, self-renewal (i.e. stemness), and for establishing the heterogeneity of stem, progenitor, and progeny cells, and characterization of cell niches, as evidenced by their long-standing application to studies of hematopoiesis. Repopulation studies using single cells, clonally-derived populations, or mixed cell populations further provide an understanding of their spatial (i.e., homing, niche), functional (secretory, paracrine, matrix synthesis, self-renewal, differentiation), and population (kinetics, heterogeneity) characteristics. As such, one potential novel and important use of decellularized lung scaffolds is as a matrix for repopulation assessments. This further allows unique opportunities to study abnormal matrices obtained from diseased lungs43,56.
Seeding decellularized lung scaffolds with whole lung fetal or post-natal cell suspensions has the potential advantage of providing a model of spontaneous self-assembly and facilitating natural cell-cell interactions that could potentially improve organotypic growth. When fetal rat (E17) whole lung suspensions were delivered intratracheally to decellularized rat lungs and cultured in a bioreactor, cells adherent in the alveolar compartment expressed markers of ATII (CK18 and pro-surfactant C (proSPC)); markers of type I alveolar epithelial (ATI), endothelial, mesenchymal, or club cells (formerly known as Clara cells) were absent31. E17 lung cells are enriched for ATII cells so it is possible that this observation relates to the difficulty to induce ATII cells to convert to ATI cells under these conditions. In contrast, seeding with rat fetal lung cells (E17–20) resulted in multilineage engraftment again, mainly in the alveolar compartment, with expression of markers of ATII cells such as pro-surfactant A (proSPA), proSPC, thyroid transcription factor (TTF-1/Nkx2.1), ATI (T1α), and fibroblasts (vimentin); concurrent inoculation with human umbilical vein endothelial cells (HUVEC) by way of the pulmonary artery showed retention along the entire vasculature and close apposition of endothelial and alveolar epithelial cells suggestive of perfusion of distal lung33. Larger airways were only sparsely engrafted in this model. Neonatal (P7) whole lung cell suspensions exhibited similar multilineage engraftment including evidence of distal airway repopulation with club cells (club cell secretory protein (CCSP+,CC10)) and basal cells (CK14+), as well as alveolar engraftment of ATII cells (proSPC+) and ATI cells (Aqp5+); endothelium engrafted the pulmonary vasculature and formed tight junctions32. While it is difficult to ascertain the precise geospatial distribution of cells, it is clear that fetal or post-natal lung homogenates can repopulate scaffolds with cells exhibiting a wide range of phenotypes with preferential distribution to the alveoli and distal airways.
However, fetal lung cells and transformed cell lines are not practical for clinical use in recellularizing lung scaffolds; therefore, the question arises as to which cells are appropriate for lung bioengineering applications. While there is still only limited experience, the ability of other cell types to home or engraft in specific regions of the lung is emerging (Table 3). Cell types that might be best utilized are those which can be easily and reproducibly isolated, expanded in vitro, seeded to decellularized matrices, induced to differentiate into pulmonary lineage cells, and survive to functional maturity. Each of these criteria requires careful optimization in order to recapitulate the natural tissue environment. In particular, the human lung is comprised of a complex mix of airway and glandular epithelial cells, mucoid cells, neuroendocrine cells, and endogenous progenitor cells; all of which exist in a specific gradient of niches polarized from the proximal to distal airway53. As such, it is logical that recellularization of decellularized lung scaffolds will require multiple cell types to be seeded at various stages of differentiation and at specific locations throughout the airway scaffold for successful tissue regeneration.
Table 3.
Distribution and phenotype of cells seeded onto acellular scaffolds.
| Reference | Cells used for seeding |
Scaffold | Route | Durati on |
Distribution | Final phenotype |
|---|---|---|---|---|---|---|
|
Lwega- Mukasa et al. Exp Lung Res 1986 (30) |
AECII | Acellular alveolar vs. amniotic basement membranes |
Direct seeding |
8 days | N/A | Alveolar matrices: AECI; amnionic membranes AECII |
|
Cortiella et al. Tissue Eng Part A 2010 (34) |
mESC | Rat (Sprague Dawley) acellular lung |
Trachea | 21 days |
Proximal-distal regiospecific CC10, proSP-C expression) |
tracheobronchial: CC10, CK18; distal lung: proSP-C, CD31, PDGFRα |
|
Ott et al. Nature Med 2010 (33) |
HUVEC (DsRed) |
Rat acellular lung |
Pulmonary artery |
9 days | All vessels | endothelial cells |
| A549 | Rat acellular lung |
Trachea | 9 days | Airways/alveoli | airway / alveolar epithelium |
|
| HUVEC (DsRed) |
Rat acellular lung |
Pulmonary artery |
9 days | Entire vasculature |
endothelial cells | |
| Rat fetal lung cells (GD19–20) |
Rat acellular lung |
Trachea | 9 days | Airways/alveoli | proSP-A, proSP-C, Ttf-1/Nkx2.1 (AECII); T1α (AECI); Vimentin (fibroblast) |
|
|
Petersen et al. Science 2010 (32) |
Neonatal (7d) lung epithelial cells (rat) |
Rat acellular lungs (Fischer 344) |
Trachea | 8 days | Alveolar, small airways |
CCSP (Clara cell), proSP-C (AECII), Aqp5 (AECI), CK14(basal cell) |
| Lung vascular endotheliu m (rat) |
Rat acellular lungs (Fischer 344) |
Pulmonary artery |
7 days | Microvascular | CD31 | |
|
Price et al. Tissue Engineering Part A 2010 (31) |
Fetal lung (E17) |
Ms accellular lungs |
Tracheal | 7 days | Alveolar | CK18+/proSP-C+ (AECII); no CD11b, aquaporin-5, CCSP, CD31, or vimentin |
|
Daly et al. Tissue Eng Part A 2011 (39) |
mBM-MSCs | Ms acellular lung |
Trachea | 28 days |
Parenchymal>ai rway (squamous) |
MSCs: No evidence for transdifferentiation |
| C10 -hAECII (non- tumorigenic ) |
Ms acellular lung |
Trachea | 28 days |
Parenchymal | N/A | |
|
Ott et al Ann Thorac Surg 2011 (37) |
Rat fetal (GD17–20) pneumocyte s |
Rat acellular lung |
Trachea | 14 days |
Alveolar/distal bronchioles> trachea/bronchi |
CCSP (airways); TTF- 1, proSP-C (alveolar) |
| HUVEC | Athymic nude rat |
Pulmonary artery |
14 days |
Proximal to distal vasculature |
CD31pos | |
|
Shamis et al Tiss Eng Part C 2011 (36) |
Ms AECII (primary or P2) |
Acellular lung microscaffold |
Direct seeding |
22 days |
Alveolar | proSP-C/SP-C (AECII- like, from primary or cultured AECII); Aqp5, Pdpn (AECI); CCSP (primary) |
|
Wallis et al Tissue Engineering Part C 2011 (41) |
mBM-MSCs | Ms acellular lung |
Trachea | 14 days |
Alveolar | MSCs |
| C10 - hAECII (non- tumorigenic ) |
Ms acellular lung |
Trachea | 14 days |
Large and Small Airways |
squamous morphology |
|
|
Bonvillain et al, Tissue Eng Part A 2012 (38) |
Rhesus BM- MSCs |
Rhesus macaque |
Secondary bronchus |
7 days | Alveolar septae, terminal bronchioles, respiratory bronchioles |
MSCs phenotype |
| Rhesus AD- MSCs |
Rhesus macaque |
Secondary bronchus |
7 days | Alveolar septae, terminal bronchioles, respiratory bronchioles |
MSCs phenotype | |
|
Jensen et al Tissue Eng Part C 2012 (40) |
mESCs diff. to Ttf1pos/proSP-Cpos |
Immersion | 14 day | Alveolar | Ttf1/Nkx2.1, proSP- C (alveolar); PDGFRα (mesenchymal) |
|
|
Longmire et al. Cell Stem Cell 2012 (61) |
mESCs | Ms acellular lung | Trachea | 10 days |
Hypercellular sheets (alveolar) |
ciliated cells (airways); T1αneg (alveoli) |
| Nkx2.1GFP | Ms acellular lung | Trachea | 10 days |
Alveolar | Nkx2.1/T1α (alveoli) | |
|
Bonenfant et al Biomaterials 2013 (42) |
Mouse BM-MSCs |
Ms acellular lung | Trachea | 28 days |
Alveolar | N/A |
| C10 -AECII (non- tumorigen ic) |
Ms acellular lung | Trachea | 28 days |
Alveolar | N/A | |
|
Sokoce vic et al Biomaterials 2013 (43) |
mBM- MSCs |
Ms acellular lung | Trachea | 28 days |
Alveolar | N/A |
| C10 - hAECII (non- tumorigen ic) |
Ms acellular lung | Trachea | 28 days |
Alveolar | N/A | |
Ideally, patient-derived autologous cells would be used for lung recellularization as they have the potential to reduce the need for post-transplant immunosuppression. Terminally differentiated cells derived from adult lungs are highly specialized and lose their proliferative ability by the time they reach this state; therefore, primary airway or vascular cells, while offering the most functionally diverse options for lung tissue engineering, may not be capable of long-term success without a source of progenitor cells for maintenance and repair. A more desirable strategy is one in which stem or progenitor cells are differentiated along pulmonary lineages following programs that mimic fetal lung development. The ideal candidate cells must be easily isolated, expanded in culture, and sustained stably while undergoing tissue-specific differentiation.
Embryonic stem cells (ESCs) derived from the inner mass of in vitro-fertilized embryonic blastocysts have the potential to differentiate into mature cells of all three germ layers. Directed differentiation of ESCs results in the production of lineage-specific progenitor cells that may potentially be used in therapeutic or regenerative applications57. In the laboratory, lineage specification of ESC has been accomplished by recapitulating the developmental environment in vitro. Murine ESCs have been induced to express markers of lung epithelial phenotype including alveolar (TTF-1, SPA and SPC) and airway (CCSP) airway epithelium after specification of definitive endoderm using Activin A to mimic the Nodal signaling pathway followed by adherent cell culture in small airways growth medium or use of more selective differentiation reagants45,58–61. Similar findings using human ESCs have also been reported62,63. Induced pluripotent stem (iPS) cells, an alternative cell type similar to ESCs with less ethical controversy, are derived by re-programming somatic cells to a stem-like state by inducing simultaneous expression of combinations of the transcription factors Oct4, Klf4, Sox2, and cMyc64,65. iPS cells re-establish pluripotency and, like ESCs, with appropriate stimulation, can differentiate into lineages of all three germ layers including those from which the host cell was not derived. A limitation of iPS cells is that they are not free from age-, environment-, and tissue-associated epigenetic modifications; therefore, there is some question as to whether iPS cells will respond developmentally and functionally as do ESCs. Further, iPS cells, like ESCs carry risk of teratoma formation. Nonetheless, iPS cells have been shown to be responsive to developmental stimuli for the specification of anterior foregut endoderm and further differentiation into early lung progenitor populations60,66. An attractive feature of iPS cell generation is that they may be derived autologously from a patient, thereby eliminating the need for allogeneic cells and avoiding much of the controversy associated with ESCs. However, it is unknown as to whether the genetic manipulation required for creating iPS cells or the epigenetic modifications inherent in the initial host cells will have any bearing on the ability of iPS cells to create fully functional tissues that can be used as transplantable substitutes for diseased tissues67.
ESCs or iPS cells or their endoderm derivatives might be expected to reconstitute acellular lung scaffolds efficiently with progeny upon exposure to instructive cues embedded in the matrix. Seeding acellular rat scaffolds with mouse ESCs resulted in both greater survival compared to cells seeded onto non-lung matrices (Matrigel, gelfoam, or collagen) and also apparent differentiation toward multiple lineages that exhibited region-specific distribution, including club cells (CC10+), ATII cells (CK18+, proSPC+), endothelial cells (CD31+), and mesenchymal cells (PDGFRα+)34. Thus it is theoretically possible to observe differentiation of the most primitive of stem cells along the lines of development simply by seeding them on acellular scaffolds. However, the efficiency of this system was not explicitly evaluated. Seeding of decellularized mouse lungs with definitive endoderm derived from ESCs and subsequent culture of lung slices resulted in spontaneous differentiation to elongated type I alveolar epithelium expressing T1α (podoplanin) that distributed along the alveolar septae; in contrast, inoculation with parent ESCs produced hypercellular sheets of disorganized cells lining both the alveoli and some ciliated cells along airways61. Similarly, mouse ESCs differentiated to Nkx2.1+, proSPC+ ATII-like cells directly seeded onto mouse acellular scaffolds that were implanted subcutaneously distributed to airways (FoxJ1pos) and alveolar regions (proSPC+ or PDGFRα+ cells) and maintained phenotype expression for 14 days40. In parallel, host-derived endothelial cells infiltrated the scaffolds suggesting that functional vascularization might occur. These studies also demonstrated that Matrigel as a vehicle for seeding increased the frequency of proSPC, TTF-1, PDGFRα, and FoxJ1 positive cells after 14 days, implying that biomimetic basement membranes may preserve heterogeneity in populations of lung endoderm derived from ESC or iPS cells, particularly in the airway epithelial fractions. However, no data is as yet available examining the behavior of iPS cells at any stage of differentiation in decellularized lung scaffolds.
The use of more committed cells should better define the fidelity of repopulation including region-specific distribution. To date, only a limited number of cell types have been employed, and almost all of these cell types normally inhabit the alveolar compartment. In one study, ATII cells converted to ATI-like cells (flattening, loss of lamellar bodies, formation of tight junctions, and synthesis of pinocytotic vesicles) when cultured on acellular lung but not amniotic matrices, again supporting the specific role of lung matrices to instruct differentiation of progenitor cells30. In a more recent study, primary or passaged ATII cells seeded directly onto acellular lung for 22 days maintained proSP-C and mature SP-C expression with some cells developing expression of Aqp5 and T1α36. Moreover, fresh ATII cells seeded on lung scaffolds showed CC10 expression although the specific distribution of these cells was not defined. In other studies, seeding decellularized mouse lungs with immortalized non-neoplastic mouse ATII cells (C10 cells) resulted in widespread distribution including predominantly small but also large airways as well as alveolar regions41–43. This suggests that transformed, and likely also neoplastic, cell lines will not home specifically to the region inhabited by their cell of origin (i.e. ATII cells) and may exhibit atypical morphologies. While these data do not completely resolve the specificity of homing and engraftment of ATII cells, they suggest that these events are promoted by the acellular scaffolds acknowledging that the composition and quality of the scaffold is a critical factors in retention, survival, phenotype, and function during recellularization. Data concerning regio-specific repopulation of acellular lung scaffolds with specific populations of basal cells, club cells, neuroendocrine cells, submucosal glandular cells, bronchioalveolar stem cells (BASC), ciliated or non-ciliated airway epithelial cells, lung fibroblasts, and microvascular endothelium are lacking.
While the existence of adult endogenous lung epithelial progenitor cells is a topic of great interest to pulmonary biology, it is unclear how acellular scaffolds will influence the phenotype and function of these cells. Importantly, our understanding of the specific features of cellular niches in the lung is limited, and therefore it is unknown how acellular scaffolds succeed or fail to recapitulate those microenvironemnts. In some areas of lung (e.g. basal cell layer) the features of a niche are better understood than in others (e.g. alveolus). Until the features of a niche are known, it may be impossible to distinguish or direct homing after recellularization to a specific niche (i.e., true ‘repopulation’) from stochastic events. Exploiting endogenous stem-progenitor cell populations for bioengineering purposes will be further constrained due to the relatively small number of stem-progenitor cells that inhabit the lung, and the challenges of isolation, culture, and expansion of those cells while preserving their native characteristics. Furthermore it remains unknown as to whether endogenous epithelial progenitor cells obtained from diseased lungs, when placed into an epigenetically new environment (i.e. acellular scaffold) will adapt to these changes by restoring function. Thus while many opportunities exist to study fundamental mechanisms of stem-progenitor cell-scaffold interactions in decellularization-recellularization models, it will be critical to develop acellular scaffolds and culture conditions that replicate normal ‘homeostatic’ conditions before they are used for bona fide ‘repopulation’ assays akin to those assays employed for study of hematopoiesis.
The lung also harbors non-endodermal (mesenchymal) sources of progenitor cells. Tissue stroma in particular appears to be robust sources of mesenchymal stem-like cells that may participate in tissue maintenance, repair, and immune regulation68. Lung-resident mesenchymal stem/stromal cells (L-MSCs) have been identified in mice and humans by fluorescence-activated cells sorting (FACS) for vital dye efflux as well as by adherent cell culture from bronchioalveolar lavage and lung tissue explants69–71. Recent reports demonstrate that L-MSCs contributed to lung repair after bleomycin-induced lung injury and in elastase-mediated injury in murine and ovine models of experimental emphysema70–72. Co-culture of L-MSCs with ATII cells induced the expression of CK18, CK19, occludin, and SPC in L-MSCs suggesting that these lung-resident stromal cells have the ability to differentiate into alveolar-like cells in vitro; however, little is known about whether they accomplish this in vivo73. Like bone marrow-derived MSC, L-MSCs also exhibit the ability to differentiate into endothelial-like cells that take up acetylated-LDL when cultured in endothelial growth medium on appropriate substrates70. L-MSCs may thus be useful in lung bioengineering strategies as they exhibit several desirable characteristics including tissue support, regulation, repair, and potentially regeneration70,71,74,75. For example, ovine L-MSCs were shown to promote epithelial growth in co-culture, engrafted and synthesized provisional matrices (laminin, fibronectin, collagen IV) on acellular sheep lung scaffolds, and promoted tissue healing in a sheep model of emphysema74,75 and murine L-MSCs reversed elastase-induced injury70.
Just as resident mesenchymal stem/stromal cells from the lung may be exploited for bioengineering, more accessible MSCs isolated from bone marrow (BM-MSCs) and other sources including adipose and placental tissues may also be potentially utilized in used in this context. While it has been demonstrated that BM-MSCs and cord blood-derived MSCs can differentiate into pulmonary-like cells in vitro when cultured in specialized media or co-cultured with airway epithelial cells76,77, in vivo engraftment is currently felt to be a rare phenomenon of no likely physiologic or therapeutic significance54,77–79. However, MSCs may hold more potential for recellularizing organ scaffolds and have been utilized in bioengineering schemes utilizing both synthetic and decellularized tracheas14,15. Our collaborative group has investigated the initial interactions of BM-MSCs with decellularized lung parenchyma in both murine and non-human primate models to test their applicability toward regeneration of functional pulmonary tissue38,39,41–43. As with L-MSCs, MSCs from other sources will most likely have a role to provide a stroma and participate in formation of niches for epithelial and endothelial cells 80,81.
To best develop repopulation assays using recellularization of acellular scaffolds, it will be crucial to characterize stem-progenitor cell ‘niches’ in the lung, including the role for supportive stromal cells and the identity of extracellular matrix ligands that presumably control the fate and function of cells within and upon release from the niche. In some areas of lung (e.g. basal cell layer) the features of a niche are better understood than in others (e.g. alveolus). Until the features of a niche are known, it may be impossible to distinguish or direct homing to a niche (‘repopulation’) from stochastic phenomena.
Implantation of Recellularized Scaffolds
Several investigators have performed xenotransplantation of decellularized scaffolds into rodents as well as larger animals. Using decellularized rat lung scaffolds re-epithelialized with a mixture of rat fetal lung homogenate and A549 lung carcinoma cells, and re-endothelialized with human umbilical vein endothelial cells, two groups were able to transplant the repopulated scaffold into rats that had undergone previous pneumonectomy32,33. Prior to implantation, the repopulated scaffolds were able to maintain adequate oxygenation, carbon dioxide exchange, and appropriate pressure/volume relationships. However, once implanted, the grafts developed significant pulmonary edema and/or hemorrhage resulting in respiratory failure after several hours. In a follow-up study, survival for fourteen days was achieved after implantation but graft function progressively declined and the histologic appearance of the graft at necropsy demonstrated significant fibrosis37. Though these studies are technically innovative and provide proof of concept that acellular matrices can be repopulated, transplanted, and maintain a degree of function, they are not yet clinically translatable. In order to have a clinically translatable model, adequate gas exchange, re-creation of intact alveolar and vascular compartments, unidirectional mucociliary clearance, immune surveillance, clearance of infection, and maintenance of physiologic airway pressures and volumes. Thus far, there has been a compartmentalized approach to the respiratory system, separating the vasculature, proximal airways, and distal lung. A successful translatable implantation animal model has not yet been created, but will need to balance all of these requirements. It will likely be a number of years before this is achieved.
Immunogenicity of Implanted Scaffolds
A critical assumption for clinical use of decellularized lung scaffolds is that they will be relatively non-immunogenic and minimize any detrimental host response following implantation. However, ECM and other proteins remaining in decellularized scaffolds can provoke immune responses82–85. Interestingly, some of these may be beneficial as growing literature suggests that decellularized scaffolds can polarize macrophages to anti-inflammatory M2 phenotype with subsequent permissive effects on implanted scaffolds86–88. With respect to lung, proteomic assessments utilizing mass spectrometry and/or western blotting demonstrate that a wide range of residual proteins, including intracellular, nuclear, cytoskeletal, and others can remain in the lungs, despite apparent effective de-cellularization38,39,41–43. Whether these residual proteins provoke immune responses is currently the focus of intense inquiry. Theoretically, despite conservation of ECM proteins, any denuded basement membrane may provoke an immune response, in part to mobilize the necessary cells to cover the “damaged” membrane50. Whether cells inoculated into decellularized scaffolds will secrete ECM and other proteins and remodel the scaffold accordingly is also the subject of intense current investigation39.
Mechanical Factors in Ex Vivo Lung Regeneration
In addition to utilizing the proper cell type(s) to inoculate into the scaffolds and using appropriate growth factors, environmental cues such as mechanical stimuli may also play a critical role. There is a large and growing body of literature that delineates the importance of various mechanical stimuli on regulating development as well as normal and diseased tissue homeostasis in vivo. For example, mechanical stretch is known to induce overexpression of SPC mRNA and protein expression in ATII cells, while shear stress on endothelial cells is critical for VEGF expression. Several studies have examined the biological consequences of mechanotransduction on fetal or adult lung cells in vitro85–88 but there is no available information on effects of stretch on development of lung epithelial tissue from embryonic or adult stem cells or from endogenous lung progenitor cells. It is likely that in addition to derivation of an optimal decellularized scaffold, precise control of the mechanical environment with bioreactor technologies (ie. stimuli mimicking stretch from breathing and shear stress induced by blood flow) will be necessary for a successful regeneration scheme. Other environmental factors such as oxygen tension will likely also play critical roles in recellularization schemes.
Summary
The challenges in developing complex 3-dimensional functional lung tissues ex vivo will be in recapitulating the normal dynamic integrated network of component cells, orientation and function of the fiber network, perfusion ventilation relationships, and immune surveillance, all of which are vital for proper function (92). Whether decellularized lung constructs or synthetic 3-dimensional lung scaffolds achieve these goals is an area of intense excitement and study.
Biographies
Ryan W. Bonvillain PhD (BS Biology/Chemistry, Ph.D. Human Molecular Genetics) is a postdoctoral fellow in the laboratory of Dr. Bruce A. Bunnell at Tulane University in New Orleans. His research interests include lung bioengineering, inflammatory lung diseases, and endogenous tissue stem cells.
Darcy E. Wagner PhD (BS Mechanical Engineering, PhD Bioengineering) is a postdoctoral fellow in the laboratory of Daniel J. Weiss at the University of Vermont. Her research interests include ex vivo lung bioengineering.
Todd J. Jensen, MS (BS Biology/Chemistry, MS Biomedical Science) is a research associate in the laboratory of Christine Finck at the University of Connecticut Health Center. His research interests are lung tissue engineering and utilzing stem cells for therapy.
Eric D. Girard MD (BS Biology) is a research fellow and surgical resident in the laboratory of Christine Finck at the University of Connecticut Health Center. His research interests are lung tissue decellularization and implantation utilizing physiologic bioreactor systems.
Bruce A. Bunnell PhD is Director of the Center for Stem Cell Research and Regenerative and Aron Professor of Gene Therapy at the Tulane University School of Medicine; his research interests are focused on the applications of adult stem cells for the treatment of neurologic and pulmonary diseases and tissue engineering of the lung.
Christine M. Finck MD (BS Biology/Business) is the chair of surgery at Connecticut Children’s Medical Center and associate professor of pediatrics at the University of Connecticut Health Center. Her research interests focus on cell therapy and bioengineering of lung tissue for the treatment of pediatric lung diseases.
Andrew M. Hoffman, DVM, DVSc; Director of the Regenerative Medicine Laboratory, Tufts University Cummings School of Medicine, North Grafton, MA. Research interests include fundamental mechanisms of lung regeneration, stem-progenitor cell biology of lung, and cell therapeutics.
Daniel J. Weiss MD PhD is Professor of Medicine (Pulmonary and Critical Care) with research interests in regenerative medicine approaches for lung diseases.
Contributor Information
Darcy E. Wagner, Department of Medicine, University of Vermont College of Medicine, Burlington, VT 05405.
Ryan W. Bonvillain, Center for Stem Cell Research and Regenerative Medicine, Tulane University School of Medicine, New Orleans, LA 70112.
Todd J. Jensen, Center for Vascular Biology, University of Connecticut Health Center, Farmington, CT 06030.
Eric D. Girard, Department of Surgery, Connecticut Children’s Medical Center, Hartford, CT 06106.
Bruce A. Bunnell, Center for Stem Cell Research and Regenerative Medicine, Department of Pharmacology, Tulane University School of Medicine, New Orleans, LA 70112.
Christine M. Finck, Department of Surgery, Connecticut Children’s Medical Center, Hartford, CT 06106.
Andrew M. Hoffman, Department of Clinical Sciences, Tufts University Cummings School of Veterinary Medicine, Regenerative Medicine Laboratory, North Grafton, MA 01536.
Daniel J. Weiss, Department of Medicine, University of Vermont College of Medicine, Burlington, VT 05405.
References
- 1.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Eisner MD, Anthonisen N, Coultas D, et al. An Official American Thoracic Society Public Policy Statement: Novel Risk Factors and the Global Burden of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF, Weiss DJ, Caplan A, et al. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 4.Orlando G, Baptista P, Birchall M, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Trans Int. 2011;24:223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wertheim JA, Baptista PM, Soto-Gutierrez A. Cellular therapy and bioartificial approaches to liver replacement. Curr Op in Org Trans. 2012;17:235–240. doi: 10.1097/MOT.0b013e3283534ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott H, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 7.Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials. 2012;33:3388–3400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Totonelli G, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401–3410. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman JM, De Coppi P, Elliott MJ, et al. Airway tissue engineering. Exp Op on Biol Therapy. 2011;11:1623–1635. doi: 10.1517/14712598.2011.623696. [DOI] [PubMed] [Google Scholar]
- 10.Baiguera S, Del Gaudio C, Jaus MO, et al. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials. 2012;33:3662–3672. doi: 10.1016/j.biomaterials.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Haag J, Baiguera S, Jungebluth P, et al. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials. 2012;33:780–9. doi: 10.1016/j.biomaterials.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Haykal S, Soleas JP, Salna M, et al. Evaluation of the structural integrity and extracellular matrix components of tracheal allografts following cyclical decellularization techniques: comparison of three protocols. Tissue Engineering -Part C: Methods. 2012;18:614–623. doi: 10.1089/ten.TEC.2011.0579. [DOI] [PubMed] [Google Scholar]
- 13.Hinderer S, Schesny M, Bayrak A, et al. Engineering of fibrillar decorin matrices for a tissue-engineered trachea. Biomaterials. 2012;33:5259–5266. doi: 10.1016/j.biomaterials.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 14.Jungebluth P, Bader A, Baiguera S, et al. The concept of in vivo airway tissue engineering. Biomaterials. 2012;33:4319–4326. doi: 10.1016/j.biomaterials.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Jungebluth P, Moll G, Baiguera S, et al. Tissue-engineered airway: a regenerative solution. Clinical Pharmacology & Therapeutics. 2012;91:81–93. doi: 10.1038/clpt.2011.270. [DOI] [PubMed] [Google Scholar]
- 16.Mondrinos MJ, Koutzaki S, Jiwanmall E, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 17.Lin YM, Boccaccini AR, Polak JM, et al. Biocompatibility of poly-DL-lactic acid (PDLLA) for lung tissue engineering. J Biomater Appl. 2006;21:109–118. doi: 10.1177/0885328206057952. [DOI] [PubMed] [Google Scholar]
- 18.Cortiella J, Nichols JE, Kojima K, et al. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006;12:1213–1225. doi: 10.1089/ten.2006.12.1213. [DOI] [PubMed] [Google Scholar]
- 19.Mondrinos MJ, Koutzaki S, Lelkes PI, et al. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol. 2007;293:L639–L650. doi: 10.1152/ajplung.00403.2006. [DOI] [PubMed] [Google Scholar]
- 20.Nichols JE, Cortiella JC. Engineering of a complex organ progress toward development of a tissue-engineered lung. Proc Am Thorac Soc. 2008;5:723–730. doi: 10.1513/pats.200802-022AW. [DOI] [PubMed] [Google Scholar]
- 21.Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. European Cells & Materials. 2010;19:284–299. doi: 10.22203/ecm.v019a27. [DOI] [PubMed] [Google Scholar]
- 22.Andrade CF, Wong AP, Waddell TK, et al. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 23.Ingenito EP, Sen E, Tsai LW, et al. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J of Tiss Eng & Regen Med. 2010;4:259–272. doi: 10.1002/term.237. [DOI] [PubMed] [Google Scholar]
- 24.Miller C, George S, Niklason L. Developing a tissue-engineered model of the human bronchiole. J of Tiss Eng & Regen Med. 2010;4:619–627. doi: 10.1002/term.277. [DOI] [PubMed] [Google Scholar]
- 25.Tsunooka N, Hirayama S, Medin JA, et al. A novel tissue-engineered approach to problems of the postpneumonectomy space. Annals of Thoracic Surgery. 2011;91:880–886. doi: 10.1016/j.athoracsur.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Hoganson DM, Pryor H, III, Vacanti JP. Tissue Engineering and Organ Structure: A Vascularized Approach to Liver and Lung. Pediatric Research. 2008;63:520–526. doi: 10.1203/01.pdr.0000305879.38476.0c. [DOI] [PubMed] [Google Scholar]
- 27.Mondrinos MJ, Koutzaki SH, Honesto M, et al. In Vivo Pulmonary Tissue Engineering: Contribution of Donor-Derived Endothelial Cells to Construct Vascularization. Tissue Engineering Part A. 2008:361–368. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- 28.Finck CM, Weiss DJ. Embryonic stem cells and repair of lung injury. Molecular Therapy. 2010;18:460–461. doi: 10.1038/mt.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuttan R, Spall RD, Duhamel RC, et al. Preparation and composition of alveolar extracellular matrix and incorporated basement membrane. Lung. 1981;159:333–345. doi: 10.1007/BF02713933. [DOI] [PubMed] [Google Scholar]
- 30.Lwebuga-Mukasa JS, Ingbar DH, et al. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 31.Price AP, England KA, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 34.Cortiella J, Niles J, Cantu A, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 35.Petersen TH, Calle EA, Colehour MB, et al. Bioreactor for the long-term culture of lung tissue. Cell Transp. 2011;20:1117–1126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamis Y, Hasson E, et al. Organ-specific scaffolds for in vitro expansion, differentiation, and organization of primary lung cells. Tissue Eng Part C Methods. 2011;17:861–870. doi: 10.1089/ten.tec.2010.0717. [DOI] [PubMed] [Google Scholar]
- 37.Song JJ, Kim SS, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Bonvillain RW, Danchuk S, Sullivan DE, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–2452. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daly AB, Wallis JM, Borg ZD, et al. Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Engineering Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen T, Roszell B, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallis JM, Borg ZD, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonenfant NR, Sokocevic D, et al. The effects of storage and sterilization on decellularized and re-cellularized whole lung. Biomaterials. 2013;34:3231–3245. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokocevic D, Bonenfant NR, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34:3256–3269. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen TH, Calle EA, Colehour MB, et al. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rippon HJ, Polak JM, Qin M, et al. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- 46.Lin YM, Zhang A, Rippon HJ, et al. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A. 2010;16:1515–1526. doi: 10.1089/ten.TEA.2009.0232. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes H, Mentink A, Bank R, et al. Endogenous collagen influences differentiation of human multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:1693–1702. doi: 10.1089/ten.TEA.2009.0341. [DOI] [PubMed] [Google Scholar]
- 48.Mariani TJ, Sandefur S, Pierce RA. Elastin in lung development. Exp Lung Res. 1997;23:131–145. doi: 10.3109/01902149709074026. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev Biol. 2006;294:271–279. doi: 10.1016/j.ydbio.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Weber DJ, Wilkes DS. The role of autoimmunity in obliterative bronchiolitis after lung transplantation. Am J Physiol - Lung Cell & Mol Physiol. 2013;304:L307–L311. doi: 10.1152/ajplung.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 52.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 53.McQualter JL, Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 54.Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau AN, Goodwin M, Kim CF, et al. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther: J Am Soc Gene Ther. 2012;20:1116–1130. doi: 10.1038/mt.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Booth AJ, Hadley R, Cornett AM, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J of Resp & Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Roszell B, Mondrinos MJ, Seaton A, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green MD, Chen A, Nostro MC, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mou H, Zhao R, Sherwood R, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longmire TA, Ikonomou L, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samadikuchaksaraei A, Cohen S, Isaac K, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Haviland DL, Burns AR, et al. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Sommer AG, Rozelle SS, Sullivan S, et al. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp. 2012;68:4327. doi: 10.3791/4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotech. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong SG, Dunbar CE, Winkler T. Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol Ther. 2013;21:272–281. doi: 10.1038/mt.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 69.Lama VN, Smith L, Badri L, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffman AM, Paxson JA, Mazan MR, et al. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev. 2011;10:1719–1792. doi: 10.1089/scd.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ingenito EP, Tsai L, Murthy S, et al. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Trans. 2012;21:175–189. doi: 10.3727/096368910X550233. [DOI] [PubMed] [Google Scholar]
- 72.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011;29:725–735. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong X, Sun Z, Cui D, et al. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol Int. 2012 doi: 10.1002/cbin.10240. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Ingenito EP, Sen E, et al. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J Tissue Eng Regen Med. 2010;4:259–272. doi: 10.1002/term.237. [DOI] [PubMed] [Google Scholar]
- 75.Ingenito EP, Tsai L, et al. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Trans. 2012;21:175–189. doi: 10.3727/096368910X550233. [DOI] [PubMed] [Google Scholar]
- 76.Wang G, Bunnell BA, Painter RG, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of Lung Epithelium from Human Cord Blood-Derived Mesenchymal Stem Cells. Am J Resp Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loi R, Beckett T, Goncz KK, et al. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding BS, Nolan DJ, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volckaert T, Dill E, et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Franz S, Rammelt S, Scharnweber D, et al. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 83.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Seminars in Immunology. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw AS, Filbert EL. Scaffold proteins immune-cell signalling. Nature Reviews Immunology. 2009;9:47–56. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- 85.Daly KA, Liu S, Agrawal V, et al. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials. 2012;33:91–101. doi: 10.1016/j.biomaterials.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 86.Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown BN, Ratner BD, Goodman SB, et al. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown BN, Londono R, Tottey S, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomaterialia. 2012;8:978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez-Esteban J, Tsai SW, Sang J, et al. Effects of mechanical forces on lung-specific gene expression. Am J Med Sci. 1998;316:200. [PubMed] [Google Scholar]
- 90.Boudreault F, Tschumperlin DJ. Stretch-induced mitogen-activated protein kinase activation in lung fibroblasts is independent of receptor tyrosine kinases. Am J Resp Cell & Mol Biol. 2010;43:64–73. doi: 10.1165/rcmb.2009-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Z, Wang Y, Nayak PS, et al. Stretch-induced Fetal Type II Cell Differentiation Is Mediated via ErbB1-ErbB4 Interactions. J Biol Chem. 2012;287:18091–18102. doi: 10.1074/jbc.M111.313163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weibel ER. It takes more than cells to make a good lung. Am J Respir Crit Care Med. 2013;187(4):342–346. doi: 10.1164/rccm.201212-2260OE. [DOI] [PubMed] [Google Scholar]