Abstract
Oplopanax horridus is a plant native to North America. Previous reports have demonstrated that this herb has antiproliferative effects on cancer cells but study mostly focused on its extract or fractions. Because there has been limited phytochemical study on this herb, its bioactive compounds are largely unknown. We recently isolated and identified 13 compounds, including six polyynes, three sesquiterpenes, two triterpenoids, and two phenolic acids, of which five are novel compounds. In this study, we systemically evaluated the anticancer effects of compounds isolated from O. horridus. Their antiproliferative effects on a panel of human colorectal and breast cancer cells were determined using the MTS assay. Cell cycle distribution and apoptotic effects were analyzed by flow cytometry. The in vivo antitumor effect was examined using a xenograft tumor model. Among the 13 compounds, strong antiproliferative effects were observed from falcarindiol and a novel compound oplopantriol A. Falcarindiol showed the most potent antiproliferative effects, significantly inducing pro-apoptosis and cell cycle arrest in the S and G2/M phases. The anticancer potential of falcarindiol was further verified in vivo, significantly inhibiting HCT-116 tumor growth in an athymic nude mouse model at 15 mg/kg. We also analyzed the relationship between polyyne structures and their pharmacological activities. We observed that both the terminal hydroxyl group and double bond obviously affected their anticancer potential. Results from this study supplied valuable information for future semi-synthesis of polyyne derivatives to develop novel cancer chemopreventive agents.
Keywords: Oplopanax horridus, Phytochemistry, Polyynes, Oplopantriol A, Falcarindiol, Anticancer, Cell cycle, Apoptosis, Structure-activity relationship
Introduction
Cancer is a major public health problem in the United States and other Western countries (Siegel et al., 2012). Current treatment of cancer generally employs surgical resection combined with chemotherapy using cytotoxic drugs and radiation therapy (Buchholz, 2009; Paoletti et al., 2010). Because these therapies are only moderately successful, novel approaches for the treatment of cancer are required.
Complementary and alternative medicine (CAM) is an approach that is gaining more attention for cancer management (Davis et al., 2012; Wang et al., 2012a). The emergence of CAM represents a natural experiment of huge dimensions, as millions of Americans have begun self-medicating with natural products (Bell, 2010; Lin and Chen, 2012). Natural products have been valuable sources of new therapeutic candidate compounds (Hait and Hambley, 2009; Xu et al., 2011); an analysis of the number of chemotherapeutic agents and their sources indicates that nearly 80% of approved drugs are derived from natural compounds (Cragg et al., 2009). With the advent of new isolation and screening technologies, more active compounds with enhanced anticancer properties could be found (Kharwar et al., 2011; Randhawa and Alghamdi, 2011).
Oplopanax horridus (Sm.) Miq. (Araliaceae), or Devil's club, is a shrub distributed around the Pacific Northwest of North America, from Alaska and the southwestern Yukon Territory down to Oregon, Idaho, and Montana (Calway et al., 2012). As an herbal medicine, O. horridus has a rich history of use by the Pacific indigenous peoples from over 38 linguistic groups for the treatment of upwards of 34 categories of medical conditions. These medical conditions range from arthritis to cancer (Lantz et al., 2004).
Although recent pharmacological studies have observed that O. horridus possesses antidiabetic, antiviral, antibacterial, and cancer chemopreventive potential, chemical studies of this plant are very limited, so the active compounds were not previously identified (McCutcheon et al., 1995; Tai et al., 2006). Recently, we conducted a phytochemical isolation of O. horridus root bark and obtained a series of compounds. Their structures were elucidated by a combination of spectroscopic analyses. Five of them, specifically the two polyynes oplopantriols A and B (Huang et al., 2010) and the three phenolic glycosides, oplopanphesides A, B, and C, are novel compounds (Huang et al., 2011).
Regarding the anticancer potential evaluation of O. horridus, previous studies focused on its extract or fractions (Tai et al., 2006; Wang et al., 2010) so the effect of individual compounds from O. horridus is largely unknown. In this study, we systemically evaluated the antiproliferative activities of 13 isolated compounds using a panel of human colorectal cancer cell lines and a breast cancer cell line. We observed that two polyynes, one of which was a novel compound, possessed significant anticancer activity. Falcarindiol had the most potent effects while still being safe to normal small intestine epithelial cells. The mechanisms behind the actions of falcarindiol were explored, and its antitumor potential was verified using an in vivo xenograft animal model.
Materials and methods
Chemicals and reagents
All solvents were of high-performance liquid chromatography grade. Cell culture plasticware was obtained from Falcon Labware (Franklin Lakes, NJ) and Techno Plastic Products (Trasadingen, Switzerland). Glutamine, insulin trypsin, McCoy's 5A, Leibovitz's L-15, RPMI-1640 and DMEM media, and phosphate buffered saline were obtained from Mediatech, Inc. (Herndon, VA, USA). Penicillin and streptomycin were obtained from Sigma-Aldrich (St. Louis, MO). The CellTiter 96 Aqueous Solution Cell Proliferation Assay, an MTS assay kit, was obtained from Promega (Madison, WI). PI/RNase staining buffer was obtained from BD Biosciences Pharmingen (San Diego, CA). An annexin V-FITC apoptosis detection kit was obtained from BD Biosciences (Rockville, MD).
Plant materials
Root bark of Oplopanax horridus (Sm.) Miq. from Oregon, USA was obtained from Pacific Botanicals, LLC and was authenticated by a botanist. The voucher specimens were deposited in the Tang Center for Herbal Medical Research at the University of Chicago.
Extraction, compound isolation and structural identification
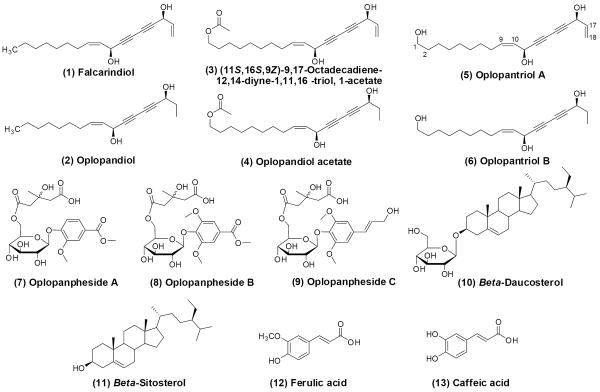
Air-dried, powdered root bark of O. horridus was extracted with 80% ethanol under reflux, suspended in water, then extracted with petroleum ether (60–90 °C), ethyl acetate, and n-butanol successively to give three fractions. The ethyl acetate fraction was separated by silica gel, RP-C18 silica gel, and preparative HPLC to afford compounds 1–6 and 11. The n-butanol fraction was separated by D-101 macroporous resin, normal phase silica gel, RP-C18 silica gel, and preparative HPLC to afford compounds 7–10, 12, and 13. Structures of isolated compounds were determined by a combination of spectroscopic analyses, including IR, 1H and 13C NMR, hydrogen-hydrogen correlation spectroscopy (H-H COSY), heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple bond coherence (HMBC), mass spectroscopic data, and chemical methods. The compounds are identified as (1) falcarindiol, (2) oplopandiol, (3) (11S,16S,9Z)-9,17-octadecadiene-12,14-diyne-1,11,16-triol,1-acetate, (4) oplopandiol acetate, (5) oplopantriol A, (6) oplopantriol B (Huang et al., 2010); (7) oplopanpheside A, (8) oplopanpheside B, (9) oplopanpheside C (Huang et al., 2011); (10) β-daucosterol, (11) β-sitosterol, (12) ferulic acid, and (13) caffeic acid (Huang, 2012) (Fig. 1).
Fig. 1.
Chemical structures of compounds isolated from the root bark of Oplopanax horridus. These compounds can be separated into four groups: Compounds (1)–(6), polyynes; Compounds (7)–(9), sesquiterpenes; Compounds (10) and (11), triterpenoids; and Compounds (12) and (13), phenolic acids.
Cell lines and cell culture
The human colorectal cancer cell lines HCT-116 (McCoy's 5A) and SW-480 (Leibovitz's L-15), human breast cancer cell line MCF-7 (RPMI-1640), and rat small intestine epithelial cell line IEC-6 (DMEM) were purchased from American Type Culture Collection (Manassas, VA, USA) and grown in the indicated media supplemented with 10% FBS and 50 IU penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37 °C.
Cell proliferation analysis
Compounds were dissolved in DMSO. Cancer cells or rat small intestine epithelial cells were seeded in 96-well plates. After 1 day, various concentrations of compounds were added to the wells. The final concentration of DMSO was 0.5%. Controls were exposed to a culture medium containing 0.5% DMSO without drugs. Cell proliferation was evaluated using an MTS assay according to the manufacturer's instructions. Briefly, after 48 h treatment, the medium was replaced with 100 μl of fresh medium and 20 μl of MTS reagent (CellTiter 96 Aqueous Solution) in each well, and then the plate was returned to the incubator for 1–2 h. A 60-μl aliquot of medium from each well was transferred to an ELISA 96-well plate and its absorbance at 490 nm was recorded. Results are expressed as percent of control (0.5% DMSO control set at 100%).
Cell cycle analysis
The HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed and the cells were treated with different concentrations of falcarindiol. The cells were incubated for 48 h before harvesting. Next, the cells were gently fixed with 80% ethanol in a freezer for 2 h and then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 300 μl of PBS containing 40 μg/ml PI and 0.1 mg/ml RNase, incubated in a dark room for 20 min at room temperature and analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR). For each measurement, at least 10,000 cells were counted.
Apoptosis assay
The HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed and the falcarindiol was added. After treatment for 48 h, the cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then the culture medium containing FBS (and floating cells) was added to inactivate the trypsin. After being pipetted gently, the cells were centrifuged for 5 min at 1500g. The supernatant was removed and the cells were stained with annexin V-FITC and PI according to the manufacturer's instructions. Cells were analyzed immediately after staining using a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
In vivo xenograft tumor model and xenogen bioluminescence imaging
Female athymic nude mice (4–6 weeks of age, Harlan, Indianapolis, IN) were used. The use and care of animals was carried out under the guidelines approved by the Institutional Animal Care and Use Committee. Subconfluent HCT-116-Luc cells were harvested and resuspended in PBS. Before inoculation, cell viability was tested by 0.4% trypan blue exclusion assay (viable cells > 90%). For subcutaneous injection, approximately 1×106 HCT-116-Luc cells in 50 μl PBS were injected into both flanks of each mouse. Starting the same day, falcarindiol was intraperitoneally (IP) administered at doses of 15 mg/kg (body weight) every day. Control mice were injected with the vehicle. Animal whole body optical imaging was carried out as described previously (He et al., 2011). Briefly, animals were subjected to Xenogen IVIS 200 imaging system (Caliper Life Sciences, Hopkinton, MA) for imaging weekly after tumor cell inoculation. D-Luciferin sodium salt (Gold Biotechnology, St. Louis, MO) at 100 mg/kg body weight in 0.1 ml sterile PBS was administered IP as a substrate before imaging. The acquired pseudo images were collected by superimposing the emitted light over the grayscale photographs of the animal. Quantitative image analysis was performed with Xenogen's Living Image V2.6.1 software.
Statistical analysis
Data are presented as mean ± standard error (SE). A one-way ANOVA was employed to determine statistical significance of the results. In some cases, Student's t-test was used for comparing two groups. The level of statistical significance was set at p < 0.05.
Results
Effects of 13 compounds on colorectal and breast cancer cell proliferation
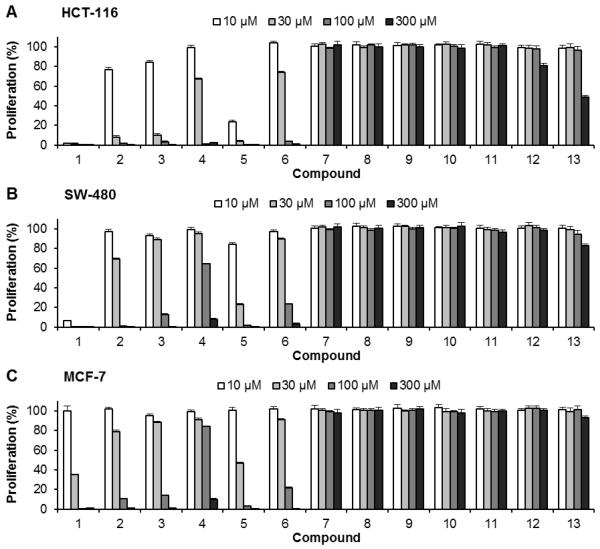
(Huang, 2012) We evaluated the antiproliferative effects of 13 compounds using two human colorectal cancer cell lines HCT-116 and SW-480 and one human breast cancer cell line MCF-7. As shown in Fig. 2, the 13 compounds exhibited different antiproliferative effects on the three cancer cell lines. At the tested concentrations (10–300 μM), compounds 7–11 did not inhibit cancer cell growth in either of the three cell lines. Compounds 12 and 13 showed some antiproliferative effects on two colorectal cancer cells at 300 μM, but such effects were not observed in MCF-7 cells. However, compounds 1–6 showed different potential for cell growth inhibition in the three cancer cell lines.
Fig. 2.
Effects of 13 compounds isolated from Oplopanax horridus on proliferation of cancer cells. Human colorectal cancer cell lines (A) HCT-116 and (B) SW-480, and (C) human breast cancer cell line MCF-7 were employed to evaluate the antiproliferative effects of isolated compounds. Cells were treated with 10–300 μM of compounds for 48 h, and cell proliferation was assayed by the MTS method. Results were normalized to each control in percentage and expressed as average ± standard error of the three experiments (solvent vehicle set at 100%). Compound number is the same as Fig. 1.
In HCT-116 cells, compounds 4 and 6 showed moderate antiproliferative effects and at 30 and 100 μM, HCT-116 cell growth was inhibited by 32.7% and 98.8% for compound 4, and 26.0% and 96.5% for compound 6, respectively (all p < 0.01 vs. control). Compounds 2 and 3 showed stronger effects; when treated with 30 μM, cancer cell growth was inhibited by 91.7% and 89.8%, respectively (both p < 0.01). Compounds 1 and 5 showed the most potent antiproliferative effects. At 10 μM, compound 5 (oplopantriol A) inhibited cell growth by 76.4% (p < 0.01), while compound 1 (falcarindiol) inhibited cell growth by 98.1% (p < 0.01). Falcarindiol at 10 μM almost completely inhibited HCT-116 cell growth (Fig. 2A).
Similar effects were also observed in SW-480 colorectal cancer cells, but the inhibition potential of compounds 1–6 on this cell line was lower than that of HCT-116 cells (Fig. 2B). Antiproliferative effects of compounds 1–6 on MCF-7 cells were weaker than on SW-480 cells, but all six compounds showed dose-dependent effects on MCF-7 cells. Moreover, falcarindiol and oplopantriol A showed the most potent antiproliferative effects (Fig. 2C).
Antiproliferative effects of two potent compounds on HCT-116 and SW-480 cells
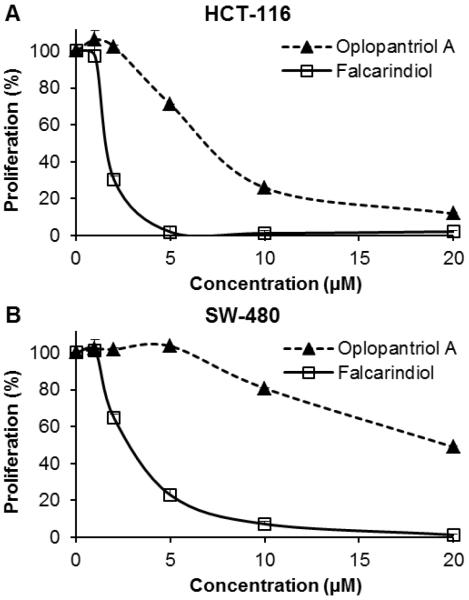
Since two human colorectal cancer cell lines were more sensitive to polyynes, and cell growth was almost absolutely inhibited by the two most potent compounds falcarindiol and oplopantriol A at low concentrations, we tested the antiproliferative effects of the two compounds in even lower concentrations. As shown in Fig. 3, the dose dependent effects of the two compounds were observed by treatment with concentrations of 1–20 μM.
Fig. 3.
Antiproliferative effect of falcarindiol (compound 1) and oplopantriol A (compound 5) on human colorectal cancer cells. (A) HCT-116 and (B) SW-480 cells were treated with 1–20 μM of falcarindiol or oplopantriol A for 48 h, and cell proliferation was assayed by the MTS method. Results were normalized to each control in percentage and expressed as average ± standard error of the three experiments.
For oplopantriol A, the newly identified compound, treatment concentrations of 5, 10, and 20 μM, inhibited HCT-116 cell proliferation by 69.0%, 74.1%, and 88.2%, respectively (Fig. 3A). In SW-480 cells, treatment with 10 and 20 μM inhibited cell proliferation by 19.2% and 51.1%, respectively (Fig. 3B). Compared to oplopantriol A, falcarindiol showed more potent antiproliferative effects. When treated with 2 and 5 μM of falcarindiol, HCT-116 cell proliferation was inhibited by 69.7% and 98.1%, respectively (Fig. 3A). Although at the same concentrations, falcarindiol showed less antiproliferative effects on SW-480 cells, its potential is significantly higher than that of oplopantriol A (Fig. 3B). Thus, falcarindiol is most potent antiproliferative compound identified from O. horridus.
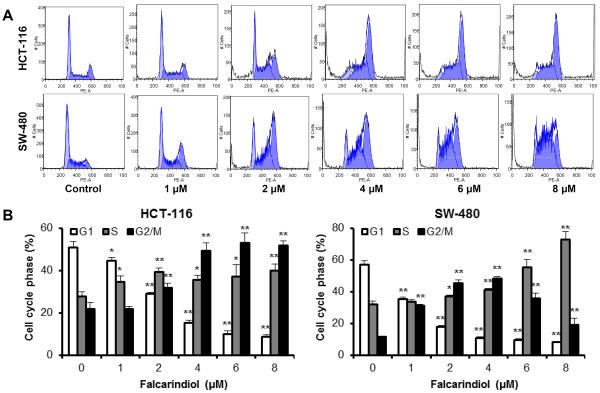
Effects of falcarindiol on colorectal cancer cell cycle distribution
Antiproliferative evaluation suggested that among the 13 isolated compounds, falcarindiol is the most active. To examine whether falcarindiol's cell growth inhibition was because of cell cycle arrest at a specific phase, cell cycle profiles were determined using flow cytometry after staining with PI. As shown in Fig. 4, the effects of falcarindiol on the cell cycle profile were observed at concentrations as low as 1 μM. Treatment of HCT-116 cells with 2 μM of falcarindiol for 48 h increased the percentages of cells in both the S and G2/M phases to 39.2% and 31.8%, respectively, compared to 27.7% and 21.6% in the vehicle treated cells (all p < 0.01). When treatment concentration was increased, most cells were in the G2/M phase.
Fig. 4.
Cell cycle analysis of HCT-116 and SW-480 cells treated with falcarindiol. HCT-116 and SW-480 cells were treated with 1–8 μM of falcarindiol for 48 h, then fixed in ethanol and stained with propidium iodide. DNA content was determined by flow cytometry. (A) Representative histograms of the DNA content in each experimental group. (B) Percentage of each cell cycle phase with various treatments or with control. Data are presented as the mean ± standard error of triplicate experiments. *p < 0.05, ** p < 0.01 vs. control.
Treatment of SW-480 cells also changed their cell cycle profile but differently than that of the HCT-116 cells (Fig. 4A). 2 μM of falcarindiol increased both the S and G2/M phases, but most cells were in the G2/M phase (45.4% compared to 11.3% in the control, p < 0.01). With an increase in treatment concentration, more cells were in the S phase. After treatment with 8 μM of falcarindiol, 72.9% cells were in the S phase (Fig. 4B). Thus, in both cancer cell lines, falcarindiol significantly increased the number of cancer cells in the S and G2/M phases.
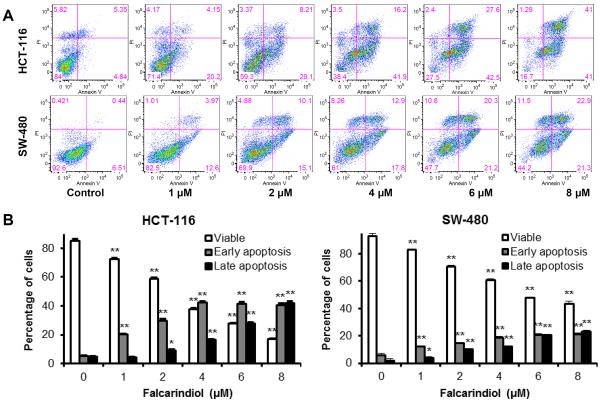
Apoptotic induction effects of falcarindiol on colorectal cancer cells
To explore the potential mechanism through which falcarindiol inhibits cell growth, cell apoptosis was assayed by flow cytometry after double staining with annexin V and PI in both human colorectal cancer cell lines. Annexin V can be detected in both the early and late stages of apoptosis. PI enters the cell in late apoptosis or necrosis. Viable cells were negative for both annexin V and PI (lower left quadrant); early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic or necrotic cells displayed both positive annexin V and PI (upper right quadrant).
As shown in Fig. 5, apoptotic cells increased significantly following treatment with 1–8 μM of falcarindiol for 48 h. After treatment with 6 μM of falcarindiol, the percentage of early and late apoptotic cells increased to 41.6% and 27.8%, respectively, for HCT-116 cells (control: 5.1% and 4.9%); and 20.8% and 20.7% for SW-480 cells (control: 5.8% and 1.8%). The results demonstrate that falcarindiol significantly induces cell apoptosis in both human colorectal cancer cell lines. In addition, similar to its antiproliferative effects (Fig. 3), falcarindiol's apoptotic induction activity is more potent in HCT-116 than in SW-480 cells (Fig. 5).
Fig. 5.
Apoptotic analysis of HCT-116 and SW-480 cells treated with falcarindiol. HCT-116 and SW-480 cells were treated with 1–8 μM of falcarindiol for 48 h, then stained with annexin V/propidium iodide (PI) before the extent of apoptosis was determined by flow cytometry. (A). Representative scatter plots of PI (y-axis) versus annexin V (x-axis). (B). Percentage of viable early apoptotic and late apoptotic cells. Data are presented as the mean ± standard error of triplicate experiments. *p < 0.05, ** p < 0.01 vs. control.
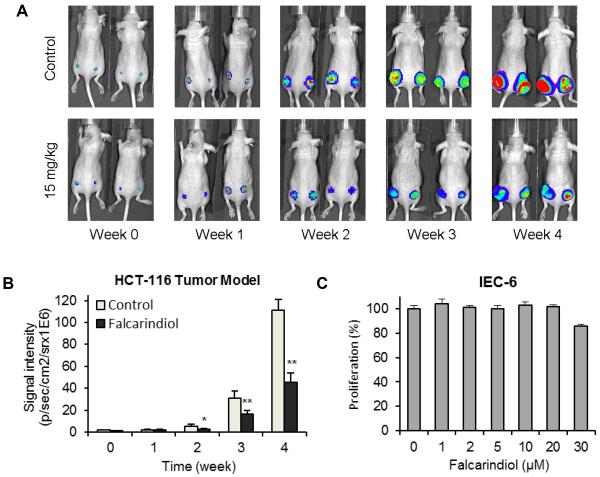
Antitumor effects in xenograft tumor model and safety evaluation of falcarindiol
To evaluate the in vivo antitumor potential of falcarindiol, firefly luciferase-tagged HCT-116 cells were inoculated into the flanks of athymic nude mice. Beginning on day 1, animals were also administered with falcarindiol at 15 mg/kg or vehicle intraperitoneally every day. Tumor growth was measured by xenogeny bioluminescence imaging on a weekly basis. Representative xenogen imaging results at weeks 0–4 are shown in Fig. 6A. Tumor size at indicated time points as assessed by imaging signal intensities is summarized in Fig. 6B. The data showed that the falcarindiol treatment group exhibited significantly decreased xenogeny imaging signal intensities compared with the control group. Quantitative analysis revealed that falcarindiol significantly inhibited xenograft tumor growth in the 2nd week after falcarindiol administration (p < 0.05). Weeks 3 and 4 exhibited more significant antitumor effects than week 2 (both p < 0.01).
Fig. 6.
In vivo antitumor observation using a xenograft model and safety evaluation of falcarindiol. (A) Firefly luciferase-tagged HCT-116 cells were injected into both flanks of athymic mice subcutaneously (n = 10/group), and the tumor sizes after treatment with solvent control or 15 mg/kg/day of falcarindiol were measured on a weekly basis by xenogen bioluminescence imaging. Representative xenogen imaging results are shown. (B) Quantitative analysis of xenogen bioluminescence imaging. Average tumor sizes at the indicated time points are represented with imaging signal intensities (in photons/second/cm2/steradian) as mean ± standard error. *p < 0.05, **p < 0.01 vs. control. (C) Safety evaluation of falcarindiol on normal intestinal cells. The IEC-6 rat small intestine epithelial cells were treated with 1–30 μM of falcarindiol for 48 h, and cell proliferation was determined.
A rat small intestine epithelial cell line, IEC-6, was used to evaluate the safety of falcarindiol. Fig. 6C shows the activities of falcarindiol on the proliferation of IEC-6 cells. At concentrations of 1–20 μM, falcarindiol did not inhibit cell growth. Compared with the control (100%), the cell viabilities of falcarindiol on IEC-6 cells are 101.5% at 20 μM, and 85.5% at 30 μM. Cell growth was almost absolutely inhibited in both of the two colorectal cancer cell lines when treated with 10 μM of falcarindiol (Fig. 3). Thus, these results suggest that falcarindiol showed significant antitumor activity in the HCT-116 xenograft tumor model and was relatively safe to normal intestinal cells.
Discussion
Oplopanax is a small genus, consisting of three species: O. elatus, O. japonicus, and O. horridus. Because O. elatus and O. japonicus are found in the East Asian countries of China, Korea, and Japan, countries with a long history of herbal medicine use, the breadth and depth of research on the phytochemistry and pharmacology of the two species is higher than that of O. horridus (Calway et al., 2012). Relatively more literature was found to report progress on the former two species; however, anticancer related reports were still limited even for the two Asian species.
Compared to the sporadic distribution of the two Asian species, O. horridus has a more widespread distribution from Alaska to Oregon. Wild resources of O. horridus are plentiful, but research on O. horridus is just at the beginning stage. Based on the literature search, several polyynes and volatile compounds were isolated from O. horridus (Kobaisy et al., 1997; Calway et al., 2012). Pharmacological studies on O. horridus were only focused on its antibacterial, antidiabetic, and antimalignancy properties, and the components used in previous studies were mostly herbal extracts or fractions (Wang et al., 2010; Calway et al., 2012). The biological activities of single compounds isolated from O. horridus are largely unknown.
Because the commonly used part of O. horridus is the root bark (Lantz et al., 2004), we recently performed phytochemical studies on the root bark of this plant (Huang et al., 2010; Huang et al., 2011). Thirteen compounds were isolated and identified. Based on their chemical structures, they can be divided into four groups: polyynes (compounds 1–6), sesquiterpenes (compounds 7–9), triterpenoids (compounds 10 and 11), and phenolic acids (compounds 12 and 13). Among these compounds, the two polyynes (5) oplopantriol A, and (6) oplopantriol B (Huang et al., 2010), and the three sesquiterpenes (7) oplopanpheside A, (8) oplopanpheside B, and (9) oplopanpheside C (Huang et al., 2011) are novel compounds.
In this study, we systemically evaluated the 13 compounds' anticancer activities. An MTS assay showed that the sesquiterpenes and triterpenoids did not show any antiproliferative effects at the tested concentrations. Phenolic acids showed weak antiproliferative effects. On the other hand, all six polyynes showed dose-dependent antiproliferative effects of varying strengths. These polyyne compounds were isolated from the hydrophobic fraction of the root bark extract (Huang et al., 2010). This was consistent with our previous observations that the hydrophobic fraction showed the most potent antiproliferative activity (Sun et al., 2010a; Sun et al., 2010b). Two polyynes, including the novel compound oplopantriol A, showed strong antiproliferative activity (Fig. 2).
Polyyne natural products are interesting for their wide variety of ecological functions, and surprising mode of biosynthesis. Research into the metabolism of polyynes has revealed that they can be biosynthesized by plants and fungi (Minto and Blacklock, 2008). However, it is uncertain whether the polyynes from O. horridus were produced by the plants or phytofungi. This will be observed in future studies.
The two human colorectal cancer cell lines HCT-116 and SW-480 were more sensitive to the potent polyynes (Fig. 3). Thus, they were selected for further observation. The two cell lines differ in p53 expression. HCT-116 has p53 wild-type, while SW-480 has mutated p53. Tumor suppressor gene p53 is thought to be important in the cellular response to chemotherapeutic agent-induced cell death. Mutations in p53 have been shown to correlate with increased resistance to chemotherapeutic agents in cancer cells (Din et al., 2004). Our results showed that the two polyynes falcarindiol and oplopantriol A significantly inhibited cell growth in these two cell lines in a concentration-dependent manner and that HCT-116 was more sensitive than SW-480.
Since falcarindiol showed the most potent antiproliferative effects among all of the isolated compounds, evaluation of its antiproliferative mechanism was carried out. Cell cycle assay showed that the response to falcarindiol in the two cell lines on is different. Falcarindiol arrested HCT-116 cells in the S phase at a lower concentration but in the G2/M phase at a higher concentration. Interestingly, falcarindiol arrested SW-480 cells in the G2/M phase at a lower concentration, but in S phase at a higher concentration. Apoptotic assay showed that HCT-116 was more sensitive than SW-480 cells, suggesting that falcarindiol's anticancer activity is in part due to the induction of cell cycle arrest and apoptosis and that p53 may participate in the mechanism (Palozza et al., 2009; Wang et al., 2012b).
In vivo antitumor evaluation supported the in vitro observation. After mice received 15 mg/kg of falcarindiol, HCT-116 tumor growth was significantly inhibited from weeks 2–4 (Fig. 6). Furthermore, a rat small intestine epithelial cell line was used to determine the safety of falcarindiol on the normal intestinal cell line. Falcarindiol did not show significant antiproliferative effects at 10 μM, the concentration at which HCT-116 and SW-480 cancer cell growth was absolutely inhibited, suggesting that falcarindiol showed cancer cell selective inhibition activity and was thus safe for normal cells.
Based on their chemical structures and observed biological activities, we explored the structure-activity relationship of compounds from O. horridus on cancer chemoprevention. Within the four chemical groups, cell growth inhibition effects were not observed by the compounds in the sesquiterpene and triterpenoid groups. Phenolic acids showed lower antiproliferative effects compared with the polyynes, while the effects of caffeic acid are more potent. The polyyne group showed significant antiproliferative effects on all three cancer cell lines. This result suggested that the core structure of polyynes is important for antiproliferative activity. Previous studies show that polyyne compounds including falcarindiol possess antitumor and antibiotic activities (Wagner et al., 2013). In this study, we observed anti-colorectal cancer activity of isolated compounds and identified two polyynes, including a novel compound, that are potent colorectal cancer chemopreventive compounds. For future animal and clinical studies, as an alternative approach, large quantities of falcarindiol and other polyynes could be obtained through fermentation on an industrial scale (Radic and Strukelj, 2012).
With regard to this polyyne group, there are three pairs of compounds: compounds 1 and 2; 3 and 4; and 5 and 6 grouped by similarity of structure. Regarding their biological effects, compounds 1, 3, and 5 showed more potent activity, suggesting that the terminal double bond increases anticancer activity. Among the three compounds, oplopantriol A can be considered a basic structure. If the hydroxyl group at C-1 was esterified with an acetate group, its antiproliferative effect was decreased, but if the hydroxyl group at C-1 was eliminated, its antiproliferative effect was increased significantly. However, compared to the derivation of the hydroxyl group at C-1, the terminal double bond is more important for maintaining anticancer potential. This structure-activity assay supplied important information for semi-synthesis of this group of compounds to find novel anticancer agents.
Conclusion
To identify active anticancer compounds in Oplopanax horridus, the anticancer potentials of 13 compounds isolated from this plant, including six polyynes, three sesquiterpenes, two triterpenoids and two phenolic acids, were evaluated. Sesquiterpenes and triterpenoids did not show any antiproliferative effects at tested concentrations. Phenolic acids showed weak antiproliferative effects, while the six polyynes showed dose dependent antiproliferative effects in human colorectal and breast cancer cells. Two polyynes, falcarindiol and oplopantriol A, significantly inhibited cell growth, and falcarindiol had the most potent effects. An in vivo xenograft tumor model supported the in vitro observation that 15 mg/kg of falcarindiol significantly inhibited HCT-116 tumor growth. A structure-activity relationship assay showed that elimination of the hydroxyl group at C-1 increased anticancer activity, and that the terminal double bond is the key structure maintaining the anticancer potential of polyyne compounds. Mechanism observation suggested that falcarindiol induced cancer cell death in part by cell cycle arrest and induction of pro-apoptosis, and that the tumor suppressor protein p53 may participate in its cancer chemoprevention.
Acknowledgements
This work was supported in part by the NIH/NCCAM AT004418 and AT005362, NIH GM074197, NIH/NCI CA149275, DOD W81XWH-10-1-0077, and the University of Macau grant (UL015/09-Y1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell RM. A review of complementary and alternative medicine practices among cancer survivors. Clinical Journal of Oncology Nursing. 2010;14:365–370. doi: 10.1188/10.CJON.365-370. [DOI] [PubMed] [Google Scholar]
- Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. The New England Journal of Medicine. 2009;360:63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- Calway T, Du GJ, Wang CZ, Huang WH, Zhao J, Li SP, Yuan CS. Chemical and pharmacological studies of Oplopanax horridus, a North American botanical. Journal of Natural Medicines. 2012;66:249–256. doi: 10.1007/s11418-011-0602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chemical Reviews. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- Davis EL, Oh B, Butow PN, Mullan BA, Clarke S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. The Oncologist. 2012;17:1475–1481. doi: 10.1634/theoncologist.2012-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. British Journal of Cancer. 2004;91:381–388. doi: 10.1038/sj.bjc.6601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait WN, Hambley TW. Targeted cancer therapeutics. Cancer Research. 2009;69:1263–1267. doi: 10.1158/0008-5472.CAN-08-3836. discussion 1267. [DOI] [PubMed] [Google Scholar]
- He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH, Huang E, Gao Y, Yang K, Wagner ER, Wang L, Tang N, Luo J, Liu X, Li M, Bi Y, Shen J, Luther G, Hu N, Zhou Q, Luu HH, Haydon RC, Zhao Y, He TC. Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses tumor growth of human colorectal cancer. Molecular Pharmacology. 2011;79:211–219. doi: 10.1124/mol.110.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WH. Ph.D. Thesis. University of Macau; Macau: 2012. Chemical Investigation on Root Barks of Oplopanax horridus; p. 268. [Google Scholar]
- Huang WH, Zhang QW, Meng LZ, Yuan CS, Wang CZ, Li SP. Oplopanphesides A-C, three new phenolic glycosides from the root barks of Oplopanax horridus. Chemical and Pharmaceutical Bulletin. 2011;59:676–679. doi: 10.1248/cpb.59.676. [DOI] [PubMed] [Google Scholar]
- Huang WH, Zhang QW, Wang CZ, Yuan CS, Li SP. Isolation and identification of two new polyynes from a North American ethnic medicinal plant--Oplopanax horridus (Smith) Miq. Molecules. 2010;15:1089–1096. doi: 10.3390/molecules15021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Natural Product Reports. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- Kobaisy M, Abramowski Z, Lermer L, Saxena G, Hancock RE, Towers GH, Doxsee D, Stokes RW. Antimycobacterial polyynes of Devil's Club (Oplopanax horridus), a North American native medicinal plant. Journal of Natural Products. 1997;60:1210–1213. doi: 10.1021/np970182j. [DOI] [PubMed] [Google Scholar]
- Lantz TC, Swerhun K, Turner NJ. Devil's club (Oplopanax horridus): An ethnobotanical review. Herbal Gram. 2004;62:33–48. [Google Scholar]
- Lin JG, Chen YH. The role of acupuncture in cancer supportive care. The American Journal of Chinese Medicine. 2012;40:219–229. doi: 10.1142/S0192415X12500176. [DOI] [PubMed] [Google Scholar]
- McCutcheon AR, Roberts TE, Gibbons E, Ellis SM, Babiuk LA, Hancock RE, Towers GH. Antiviral screening of British Columbian medicinal plants. Journal of Ethnopharmacology. 1995;49:101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minto RE, Blacklock BJ. Biosynthesis and function of polyacetylenes and allied natural products. Progress in Lipid Research. 2008;47:233–306. doi: 10.1016/j.plipres.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P, Torelli C, Boninsegna A, Simone R, Catalano A, Mele MC, Picci N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Letters. 2009;283:108–117. doi: 10.1016/j.canlet.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- Radic N, Strukelj B. Endophytic fungi: the treasure chest of antibacterial substances. Phytomedicine. 2012;19:1270–1284. doi: 10.1016/j.phymed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed) - a review. The American Journal of Chinese Medicine. 2011;39:1075–1091. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS. Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.) Journal of Ethnopharmacology. 2010a;132:280–285. doi: 10.1016/j.jep.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Li XL, Wang CZ, Williams S, Yuan CS. Improving anticancer activities of Oplopanax horridus root bark extract by removing water-soluble components. Phytotherapy Research. 2010b;24:1166–1174. doi: 10.1002/ptr.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai J, Cheung S, Cheah S, Chan E, Hasman D. In vitro anti-proliferative and antioxidant studies on Devil's Club Oplopanax horridus. Journal of Ethnopharmacology. 2006;108:228–235. doi: 10.1016/j.jep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Wagner H, Ulrich-Merzenich G, editors. Evidence and Rational Based Research on Chinese Drugs. chapter 1. Springer; New York: 2013. pp. 525pp. 1–25. [Google Scholar]
- Wang CZ, Aung HH, Mehendale SR, Shoyama Y, Yuan CS. High performance liquid chromatographic analysis and anticancer potential of Oplopanax horridus: comparison of stem and berry extracts. Fitoterapia. 2010;81:132–139. doi: 10.1016/j.fitote.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Calway T, Yuan CS. Herbal medicines as adjuvants for cancer therapeutics. The American Journal of Chinese Medicine. 2012a;40:657–669. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, Musch MW, Bissonnette M, Chang EB, Yuan CS. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. International Journal of Oncology. 2012b;40:1970–1976. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. The American Journal of Chinese Medicine. 2011;39:15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]