Abstract
The inhibitor of apoptosis protein survivin is expressed in most cancers. Using the conditional PTEN deletion mouse model, we previously reported that survivin levels increase with prostate tumor growth. Here we evaluated the functional role of survivin in prostate tumor growth. First, we demonstrated that mice lacking the survivin gene in prostate epithelium were fertile and had normal prostate growth and development. We then serially, from about 10–56 weeks of age, evaluated histopathologic changes in the prostate of mice with PTEN deletion combined with survivin mono- or bi-allelic gene deletion. While within this time period most of the animals with wild-type or monoallelic survivin deletion developed adenocarcinomas, the most severe lesions in the biallelic survivin deleted mice were high-grade prostatic intra-epithelial neoplasia with distinct histopathology. Many atypical cells contained large hypertrophic cytoplasm and desmoplastic reaction in the prostatic intra-epithelial neoplasia lesions of this group was minimal until the late ages. A reduced proliferation index as well as apoptotic and senescent cells were detected in the lesions of mice with compound PTEN/survivin deficiency throughout the time points examined. Survivin deletion was also associated with reduced tumor expression of another inhibitor of apoptosis member, the X-linked inhibitor of apoptosis. Our findings suggest that survivin participates in the progression of prostatic intraepithelial neoplasia to adenocarcinoma, and that survivin interference at the prostatic intraepithelial neoplasia stages may be a potential therapeutic strategy to halt or delay further progression.
Introduction
Survivin is a 142-amino acid residue protein that belongs to the family of inhibitor of apoptosis proteins (IAP). Due to its high expression in most human cancers and its role in promoting cell proliferation and inhibiting apoptosis, it is considered to be a potentially important therapeutic target [1]. It is thought that survivin over-expression might allow accumulation of mutations in transformed cells and thereby promoting tumor progression. Its expression is associated with increased resistance to cancer therapy-induced apoptosis and with lower patient survival [2]. Survivin contains a single baculoviral inhibitor of apoptosis repeat (BIR) domain and carboxyl terminal α-helix and takes form as a homodimer. Rather than binding directly to caspases, survivin blocks apoptosis by interacting with other partners including XIAP [3,4].
Transcription of the Survivin gene that is prominent in the mitosis phase of the cell cycle is also regulated by various growth factors and cytokines [5,6]. There is evidence that survivin also exists in the extracellular pool in the tumor microenvironment, and may be absorbed by cancer cells for their malignant progression [7]. Survivin’s differential subcellular localization is evidence of its multiple functions. Cytoplasmic/mitochondrial survivin is associated with a protective role against apoptosis, whereas nuclear survivin is proposed to be a regulator of cell division [8]. In normal cells its expression is at its highest in the G2/M phase of the cell cycle, but in tumors, it is reported to be independent of the cell cycle [9,10]. Survivin is a component of the chromosomal passenger complex (CPC), comprised of the Aurora B-kinase, Borealin, and INCENP. The CPC ensures proper attachment between the mitotic spindle and chromosomes and correct sister chromatid segregation, allowing successful cytokinesis [1]. In addition, survivin has been found to stabilize the mitotic spindle and mediate spindle assembly checkpoint [2].
Germline knockout of the survivin gene results in embryonic lethality [11], and its conditional knockout in thymocytes causes impaired cell proliferation, cell cycle arrest, mitotic spindle defects and apoptosis [12]; in neuronal precursors, it causes perinatal lethality and apoptosis [13]; in endothelial cells, it causes embryonic lethality [14]; and in hematopoietic progenitors, lack of survivin causes mortality due to bone marrow ablation, and erythropoiesis defects [15]. The role of survivin in the prostate gland, which primarily develops postnatally and which is a favored site for cancer in aging males, has not been previously investigated.
We have reported a strong expression of survivin protein in prostate cancer [16] of the conditional Pten-deletion mouse model [17–19] and in human prostate cancer specimens [20]. Interestingly, we also documented that certain extracellular signaling proteins, such as bone morphogenetic proteins BMP 2 and BMP7, continue increasing with the progression of prostate cancer in this mouse model, and that there is a direct relationship between BMP/Smad signaling and survivin up-regulation [16,21]. Additionally, we have identified Runx2, the master transcription factor for osteoblast differentiation as a key regulator of survivin transcription in prostate cancer cells, and observed that BMP signaling is also involved in up-regulation of Runx2 protein expression in these cells [16,20]. In this regard, it was interesting to note that in the conditional Pten deletion model of prostate cancer, protein levels of BMPs, Runx2, and survivin all increase with the tumor growth [16,20,21], implicating a potentially central role of survivin in prostate cancer. To determine the extent of survivin contribution to prostate tumor progression in this model system, we first document here that prostatic epithelium-specific deletion of Survivin has no significant effect on prostate organogenesis and function. Based on this finding, we proceeded to delete one or both alleles of Survivin in the Pten deletion model, and through analyses of these new strains we provide direct genetic evidence that loss of survivin expression in the prostate epithelium strongly inhibits the progression of prostatic premalignant lesions to adenocarcinoma in these animals.
Materials and Methods
Generation of prostate-specific Survivin-deleted or both Survivin- and Pten- deleted mice
For prostate epithelium-specific Survivin knockout we used floxed Survivin (S) allelic (S f/f) female mice on a 129sv/Swiss background [22] and bred these with male PB-Cre4 [23] transgenic mice (C57BL/6/DBA2), yielding progenies with heterozygous or homozygous deletion of Survivin (cS +/- and cS -/-, respectively where c depicts Cre). Double deletion of Pten and Survivin in the prostate was generated by mating S f/f female mice with male mice carrying the c transgene and Pten f/f alleles on C57BL/6xDBA2/129 background [17]. All animals generated were of mixed genetic background. More detailed breeding schemes for various mouse genotypes are provided in the Supplementary Figure S1. Four distinct groups were generated: 1) Normal control group: contained the floxed alleles without c, abbreviated as Pten f/f S f/f; 2) Control tumor group: c; Pten f/f ; S wild-type/wild-type or cPten -/- S +/+ ; 3) Experimental group with monoallelic deletion of Survivin: c; Pten f/f ; S wild-type/f or cPten -/- S +/-; and 4) Experimental group with biallelic deletion of Survivin: c; Pten f/f ; S f/f or cPten -/- S -/-. Animals were housed and maintained under identical conditions and animal experimentation was conducted in accordance to the ethical federal guidelines mandated by the University of Southern California Institutional Animal Care and Use Committee (Assurance Number: A-3518-01). Animal Protocols used for this study were approved by the University of Southern California Animal Care and Use Committee.
Mouse genotyping
DNA was extracted from mouse tails and/or prostate tissues and subjected to PCR to determine the genotype. Cre was detected as a 500 bp fragment, while wild type and floxed Survivin was differentiated by the PCR products, 386 bp and 577 bp, respectively, detected using primers Adv 25 and Adv 28 [22]. Deletion of Survivin in the prostate was confirmed by the presence of 420-bp fragment generated with primers Adv17 and Adv28 as described [22]. Other primer sets were: Cre forward primer, GATCCTGGCAATTTCGGCTAT; and Cre reverse primer, TTGCCTGCATTACCGGTCGAT.
Histopathology
Prostate tissues were collected from different age categories and incubated in 4% paraformaldehyde overnight at 4° C and then washed twice in PBS for 30 minutes before storing in 70% ethanol. Fixed tissues were processed by standard procedures, embedded in paraffin, cut to 5 µm sections onto glass microscope slides, and stained with hematoxylin and eosin after deparaffinization and rehydration [24,25]. The histopathology analysis was as thorough as possible as multiple sections of each prostatic lobe of these animals were examined microscopically.
Immunohistochemical analysis
Paraffinized prostate tissue sections were deparaffinized and rehydrated before being subjected to antigen retrieval in 10 mM sodium citrate buffer at 95° C. Slides were then incubated in 1% H2O2 in methanol to block the endogenous peroxidase activity. Sections were blocked with 10% normal goat serum (Vector Laboratories) and 0.3% Triton X-100 in TBS for 1 hour at room temperature and incubated in primary antibody solutions overnight at 4° C. Antibodies used: Androgen receptor 1:200 (Santa Cruz Biotechnology), PTEN 1:400, Phospho-Akt 1:500, Cleaved Caspase-3 1:600, Phospho-H2AX Serine 139 1:1000 (Cell Signaling Technology), Ki67 1:400 (Vector Laboratories), p63 1:100 (Abcam), and Cytokeratin 8 1:50 (Development Studies Hybridoma Bank, IA, U.S.A.). Secondary antibodies used: affinity purified biotinylated rabbit anti rat IgG (H+L) and affinity purified biotinylated goat anti-rabbit IgG (H+L) (Vector Laboratories). Detection reagents: Vectastain Elite ABC kit (Vector Laboratories) and DAB (DAKO). The slides were counterstained with hematoxylin (Sigma), rinsed, and dehydrated before cover slips were placed over the tissue.
Proliferation index
Tissue sections from four samples in each age group were stained with Ki67. Three random areas of each section were photographed at 400x magnification. Ki67 positive cells and total number of cells in each picture were counted using ImageJ software. Proliferation index was calculated as the number of Ki67 positive cells divided by the total number of cells.
Cleaved Caspase-3 quantitation
Tissue sections from at least three samples from each genotype: cPten -/- S +/+, cPten -/- S +/-, and cPten -/- S -/- ranked with different degrees of PINs were stained with cleaved Caspase-3 antibody. Three random areas of each section were photographed at 400x magnification. Cleaved Caspase-3 positive cells and total numbers of cells in each picture were counted using ImageJ software. Extent of apoptosis was calculated as the number of cleaved Caspase-3 positive cells divided by the total number of cells.
Assessment of γ-H2AX staining
Prostate tissue sections from mice at different age groups (10, 21, and 37 weeks) and of various genotypes (cPten -/- S +/+, cPten -/- S +/-, and cPten -/- S -/-) were stained with phosphorylated H2AX (γ-H2AX) antibody. Three random areas of each section were photographed at 400x magnification. γ-H2AX positive cells and total numbers of cells in each picture were counted using ImageJ software. Mean ratio of cells with DNA fragmentation was calculated as the number of γ-H2AX positive cells divided by the total number of cells.
Senescence-associated β-Galactosidase staining
Prostate tissue samples were embedded in OCT, frozen on dry ice, sectioned to 8 µm, set on microscope glass slides and air-dried. The sections were then fixed in 0.5% glutaraldehyde in PBS for 15 minutes at room temperature and washed twice in PBS at room temperature. The slides were stained in β-Galactosidase staining solution (X-Gal, NaCl, MgCl2, Fe II, and Fe III in phosphate citrate buffer pH 6.0) for 8 hours in a 37° C incubator [26]. The samples were then washed three times in PBS until no longer yellow and counter-stained with 0.1% Nuclear Fast Red (Sigma) in 5% aluminum sulfate, rinsed, and dehydrated before mounting medium was added to the slides to hold the cover slips.
Western blot analysis
Prostate tissues were pulverized and then lysed using RIPA buffer (Sigma). Protein concentration was determined by the BCA protein assay method (Pierce). 5 µg of protein was loaded in each lane and subjected to Western blot analysis. Antibodies used: Survivin, XIAP, and Livin 1:2000 (Cell Signaling Technology). β-Actin 1:1000 (Santa Cruz Biotechnology, Inc.) was used as loading control.
Statistical analysis
Statistical comparisons were established using an unpaired, two-tailed t test. A minimum of three independent analyses were carried out for each experiment. Statistical significance is determined by p-value < 0.05.
Results
Murine prostate organogenesis and growth are not impaired by the loss of survivin in the prostatic epithelium
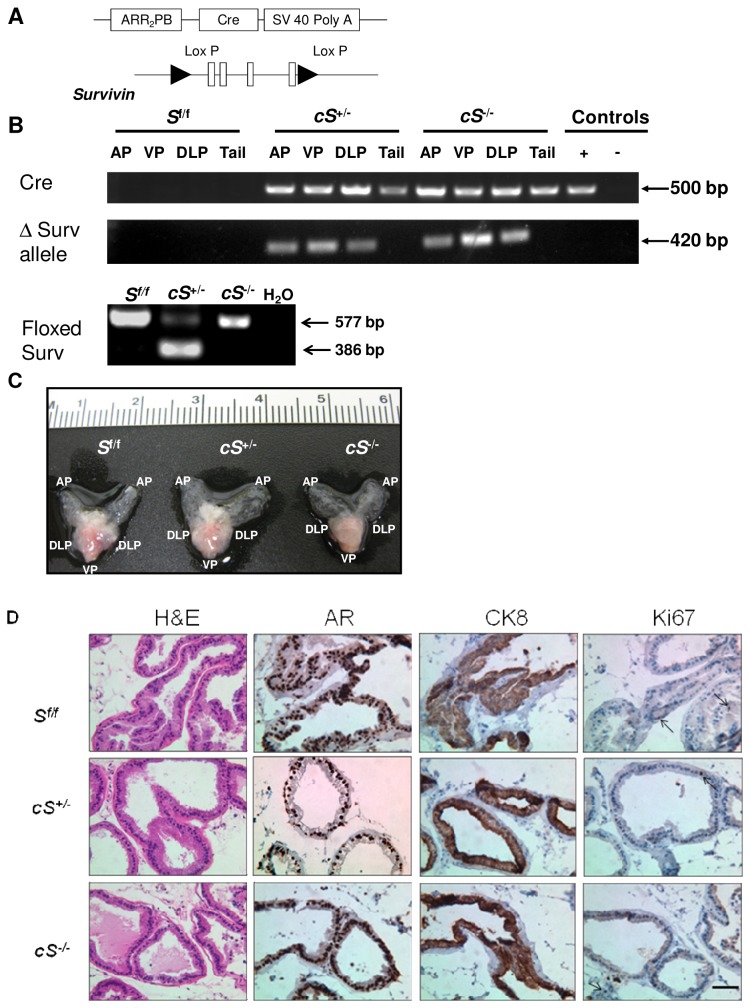
Survivin gene deletion in the prostate of male mice was accomplished using the Cre-Lox P system. Male mice expressing the PB-Cre4 transgene [23] driven by the prostate-epithelium-specific rat ARR 2 PB promoter [27] were crossed with female Survivinfloxed (S f/f) mice to yield progeny with S f/f , cS +/-, and cS -/- genotypes (Figure 1A, Figure S1A). The genotype of each mouse was determined by PCR as described in Methods. Deletion of survivin was confirmed by the presence of a 420 bp band on the gel (Figure 1B). Murine prostates were collected at various time points of about 8, 20, 36, and 52 weeks, and there was no significant difference observed in the gross morphology of the prostates between S f/f , cS +/-, and cS -/- mice in all age groups. All prostates appeared normal and were similar in size (Figure 1C). Furthermore, histopathological and immunohistochemical analyses did not detect any abnormalities in the morphology and marker protein expression pattern of prostatic glandular structures of mice with single and double Survivin allelic deletion (Figure 1D). The proliferation marker Ki67 expression level also did not deviate from normal upon Survivin deletion (Figure 1D). Thus, Survivin deletion appeared not to affect normal prostate organogenesis and growth. A number of mature male cS -/- were used for breeding and were determined to be fertile, with the size of litters produced falling within the normal range.
Figure 1. Survivin deletion has no effect on normal prostate development.
(A) Prostate epithelium-specific Survivin deletion was produced by homologous recombination via ARR2PB promoter-driven Cre expression and Lox P sites flanking all four exons of Survivin. (B) Illustration of PCR analysis for ascertaining genotypes, in this case, using tissues from 20-week old mice. Tail DNA extracts from cPten-/- and Pten f/f mice were used as positive (+) and negative (-) controls for Cre. H2O, water; AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral prostate. (C) Ventral view of prostate showing generally normal gross morphology whether with either single or biallelic inactivation of Survivin. (D) Examples of H&E or immunostaining for androgen receptor (AR), luminal epithelial cell marker cytokeratin 8 (CK8), and proliferation marker Ki67, using ventral prostate lobes collected from 20-week old mice. Positive expression is indicated by the brown staining in the cytoplasm (CK8) or nucleus (AR and Ki67). The results illustrate retention of normal tissue morphology, protein marker expression pattern, and proliferation rate in prostates with heterozygous or homozygous deletion of Survivin. Bar, 50 µm.
Loss of survivin in prostate of the conditional Pten-deletion mouse model inhibits tumor progression
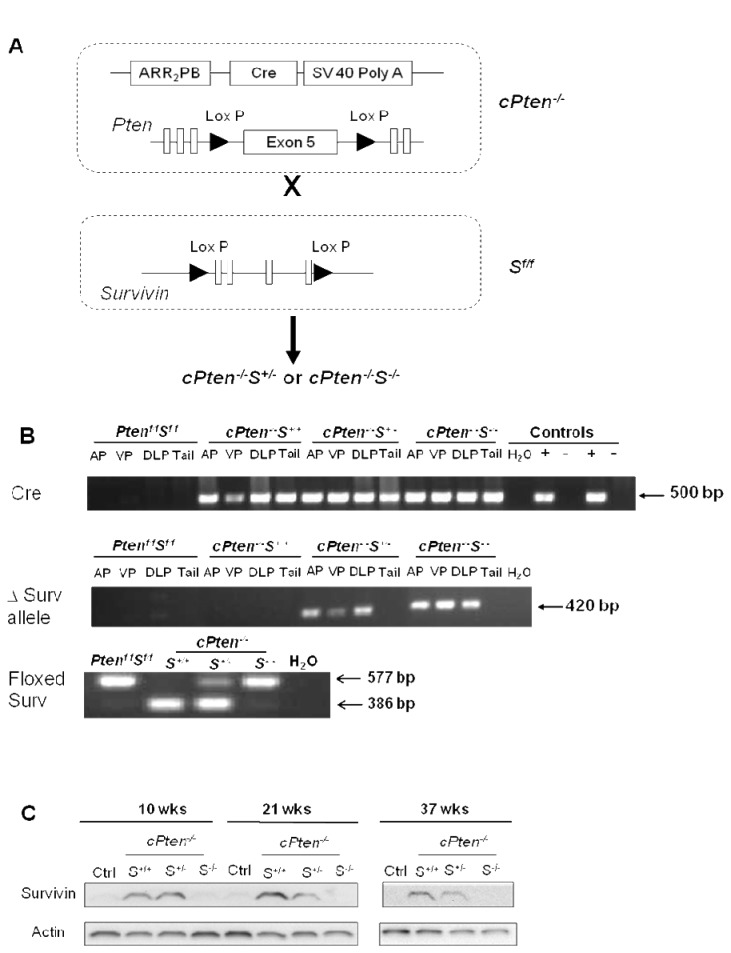
For this study we established a prostate-specific Pten and Survivin double knockout mouse strain as illustrated in Figure 2A. The complete breeding schematic is outlined in Figure S1B. Mice with the following genotypes, confirmed with PCR analysis, were included in our study: Pten f/f S f/f, cPten -/- S +/+, cPten -/- S +/-, and cPten -/- S -/-. The presence of Cre was ascertained by a 500 bp fragment, and by a 386 bp product for wild type Survivin and a 577 bp fragment representing floxed Survivin (Figure 2B). Deletion of Survivin alleles was assessed by the presence of a 420 bp band on the gel as well as significant lack of detection of the corresponding protein on the Western blots (Figure 2B).
Figure 2. Generation of double conditional knockout mice lacking alleles of Pten and Survivin.
(A) Mouse genotype of interest was obtained by crossing mice carrying Cre transgene and floxed phosphatase region of Pten (exon V) with floxed Survivin mice. (B) Genotypes of mice (shown: 10 weeks old) were determined by PCR analysis of the tissue samples. Tail DNA extracts from cPten-/- and Pten f/f mice were used as positive (+) and negative (-) controls for Cre, H2O, water. (C) Status of Survivin deletion was also confirmed at the protein level. This is illustrated by a representative Western blot from the ventral prostate of mice from the 10-, 21-, and 37-week groups, with β-actin serving as loading control. Prostate tissue lysates from Pten f/f S f/f mice were used as normal control (ctrl).
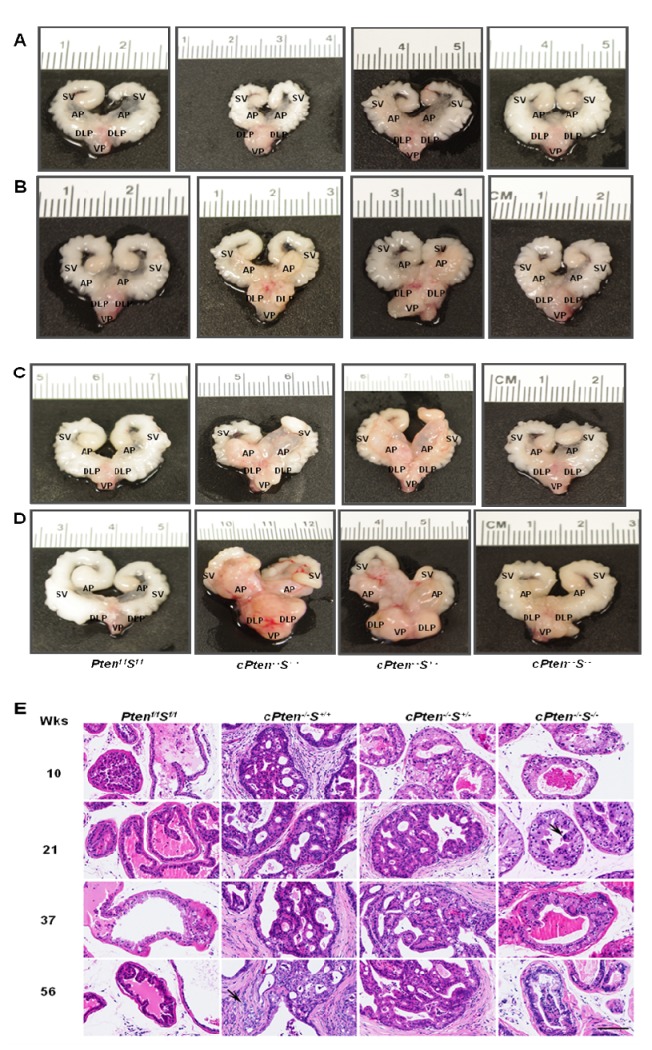
The gross morphology of the prostate glands of the Pten f/f S f/f , cPten -/- S +/+ , cPten -/- S +/-, and cPten -/- S -/- mice was examined. Starting at 10 weeks (range 9-11 weeks), prostates collected from cPten -/-S+/+ mice already exhibited abnormality by their whitish, denser appearance compared to the translucent, smaller normal prostate. While cPten -/- S +/- seemed to exhibit a gross morphology similar to the cPten -/- S +/+ animals, the gland of the cPten -/-S-/- mice displayed a normal morphology (Figure 3A). Although by 21 weeks (range 19-23 weeks) of age cPten -/-S-/- prostates no longer looked normal, still the gross appearance reflected a smaller size and less denser glands relative to those of the cPten -/- S +/+ and cPten -/- S +/- animals at the corresponding age (Figure 3B). This pattern continued through 37 weeks (range 34-41 weeks) to the last time point of observation, 56 weeks (range 51-62 weeks), with cPten -/-S+/+ and cPten -/- S +/- displaying a similar extent of enlargement of the prostate, much more so than that of cPten -/-S-/- (Figure 3C, 3D).
Figure 3. Loss of Survivin in conditional Pten deletion mouse model delays prostate tumor progression.
Representative ventral view of prostate of various genotypes at different time points of aging, (A), (B), (C), (D) indicating animals from the 10-, 21-, 37-, 56-week groups, respectively. Deletion of both Survivin alleles yielded a significantly smaller prostate relative to the sizes seen in the other groups. AP, VP, DLP as in Figure 1; SV, seminal vesicles. (E) Representative depiction of histological analysis of H&E staining of paraffin-embedded prostate tissue sections of mice from 10, 21, 37, and 56 week-old groups: cPten -/- S +/+ mice appeared to develop high grade PINs [28–30] in the majority of glands as early as 9 weeks of age. At later time points invasive adenocarcinomas (arrow, 56 weeks) could be detected. cPten -/- S +/- mice of the 10-week group displayed PINs 1-2, followed by detection of PINs 3-4 in the 21-week age group. Prostate epithelium of cPten -/- S -/- mice encompasses single atypical cells containing large hyperchromatic nuclei (arrow, 20 weeks) and large cytoplasm between 8 and 20 weeks of age. PINs 2-3 were observed in some of the glands from the 37- and 56-week age groups. Note that desmoplastic reaction was absent or minimal in lesions of cPten -/- S -/- mice. Dorsolateral lobes. Bar, 50 µm.
Histological and immunohistochemical analyses indicated the nature of the lesions formed in the presence or absence of survivin expression in the conditional Pten deletion model. By 10 weeks, 3 of 4 of the cPten -/-S+/+ mice displayed high grade prostatic intraepithelial neoplasia (PIN), which could be identified as PIN3 or 4, while only one out of 4 of the cPten -/- S +/- and none of cPten -/-S-/- mice exhibited this phenotype at this time point (Figure 3E, Table 1). At 21 weeks, however, high grade PIN lesions were observed in all prostate glands of cPten -/- S +/- (Figure 3E). Single atypical cells with large heterochromatic nuclei and large cytoplasm were found in prostate specimens of cPten -/-S-/- mice between 10 and 21 weeks of age, and PIN 2 (low grade PIN) and PIN 3 were detected in some glands after further aging (Figure 3E, Table 2). As early as 10 weeks, one out of 4 cPten -/- S +/+ mice developed early carcinoma characterized by microinvasion, while none such lesions were observed in the cPten -/- S +/- or cPten -/-S-/- group at this age. Appearance of early carcinoma lesions was detected in the single Survivin allelic deletion in the Pten null prostate at 21-week time point. The incidence and severity of carcinoma (early to adenocarcinoma) progressively increased up to 56 weeks in both the cPten -/- S +/+ and cPten -/-S+/- mice (Table 1, Tables S1 and S2). In contrast, no indications of early carcinoma or adenocarcinoma were found in the prostates of the cPten -/-S-/- mice at any of the time points analyzed. The most severe diagnosis that could be assigned to these mice at 56 weeks was high grade PINs (Tables 1, 2). Desmoplastic reaction, characterized by the presence of larger stromal cells with increased formation of collagenous extracellular matrix, was detectable in prostate lesions of the cPten -/- S +/+ and cPten -/-S+/- mice by 10 weeks and on, while it was practically absent in cPten -/-S-/- group until the 56-week time point. Analysis of normal Ptenfloxed / Survivinfloxed (Pten f/f S f/f) control tissue sections at various time points consistently showed the presence of regular prostatic glandular structures (Table S3).
Table 1. Prostate pathology of cPten -/- mice with monoallelic or biallelic deletion of survivin.
| Genotype | Age group (weeks) | Total no. of animals | No. of animals with PIN | No. of animals with early Ca to AdCa |
|---|---|---|---|---|
| cPten -/- | 10 (9-11) | 4 | 4 (1 LG, 3 HG) | 1 |
| 21 (19-23) | 6 | 6 (HG) | 1 | |
| 37 (34-41) | 6 | 6 (HG) | 3 | |
| 56 (51-62) | 4 | 4 (HG) | 4 | |
| cPten -/- S +/- | 10 (9-11) | 4 | 4 (3 LG, 1 HG) | 0 |
| 21 (19-23) | 5 | 5 (HG) | 2 | |
| 37 (34-41) | 5 | 5 (HG) | 4 | |
| 56 (51-62) | 5 | 5 (HG) | 4 | |
| cPten -/- S -/- | 10 (9-11) | 5 | 5 (LG) | 0 |
| 21 (19-23) | 5 | 5 (HG) | 0 | |
| 37 (34-41) | 5 | 5 (HG) | 0 | |
| 56 (51-62) | 5 | 5 (HG) | 0 |
Abbreviations: PIN, prostatic intraepithelial neoplasm PIN 1 and PIN2, low grade (LG) PINs; PIN3 and PIN4, high grade (HG) PINs; Early cancer, microscopic cancer; Adca, adenocarcinoma. Lesion classifications were as described [28-30]
Table 2. Details of prostate pathology observed in the cPten -/- S -/-group.
| Animal No. | Age (wks) |
Pathology
|
Remarks | ||
|---|---|---|---|---|---|
| AP | VP | DLP | |||
| 5496 | 9.4 | PIN1 | PIN1 | PIN1 | Few atypical cells (AP), single atypical (hypertrophic, polyploid) |
| cells, some exfoliated (VP, DLP) | |||||
| 5912 | 9.6 | PIN1 | PIN1 | PIN1 | Exfoliation, single atypical cells, focal hyperplasia (AP), single |
| atypical cells, exfoliation (VP), single atypical cells, hypertrophy, | |||||
| exfoliation (DLP) | |||||
| 5927 | 10 | PIN1 | PIN1 | PIN2 | Mainly RS, few PIN1, single atypical cell (AP), single atypical |
| cells (VP), small PIN2 with large atypical cells, no desmoplastic | |||||
| stroma, exfoliation (DLP) | |||||
| 5928 | 10 | RS | PIN1 | PIN2 | Areas of exfoliation, single atypical cells (AP), few atypical cells, |
| exfoliation (DLP) | |||||
| 5812 | 11 | RS | PIN1 | PIN2 | Some exfoliation (AP), few atypical/apoptotic/exfoliated cells |
| (VP), small areas of enlarged (nucleus and cytoplasm) cells, | |||||
| some exfoliation, rare PIN2 (DLP) | |||||
| 5498 | 18.6 | PIN1 | PIN1 | PIN3 | Few atypical cells, exfoliation (AP), few large atypical cells, |
| hypertrophy, exfoliation (VP), PINs with large atypical and | |||||
| apoptotic cells (DLP) | |||||
| 5753 | 19.7 | PIN2 | PIN1 | PIN3 | Small PIN2 (AP), large vacuolized cells filling up the ducts- |
| hypertrophy (AP, DLP), few atypical cells (AP, VP) | |||||
| 5754 | 19.7 | PIN1 | PIN1 | PIN3 | Modest hyperplasia, some exfoliation (AP), few atypical cells, as |
| in other cases such cells can be hyperploid (VP), large atypical | |||||
| cells, some of them are exfoliated, moderate hyperlasia (DLP) | |||||
| 5757 | 19.7 | PIN3 | PIN2 | PIN3 | Few large atypical cells (AP, VP), hyperplasia/hypertrophy (AP, |
| VP, DLP), some apoptosis (DLP) | |||||
| 5774 | 19.7 | PIN1 | PIN1 | PIN3 | Single atypical cells, exfoliation (AP), large atypical cells but no |
| no stratification/piling up (VP), hyperplasia, PIN3, single atypical | |||||
| cells, no desmoplastic reaction (DLP) | |||||
| 5675 | 39.3 | PIN1 | PIN2 | PIN3 | Single atypical cells (AP, VP), areas of hypertrophy (VP), |
| hypertrophy/hyperplasia, some apoptosis, no desmoplasia (DLP) | |||||
| 5678 | 39.3 | PIN1 | PIN2 | PIN3 | Hypertrophy (AP), few large atypical cells (VP), hyperplasia/ |
| hypertrophy (VP, DLP), some apoptosis (DLP) | |||||
| 5679 | 39.3 | PIN1 | PIN1 | PIN3 | Few atypical cells (AP), single atypical and hypertrophic cells |
| (VP), hypertrophy/hyperplasia, some apoptosis (DLP) | |||||
| 5683 | 39.3 | PIN3 | PIN4 | PIN3 | Few large atypical cells/hypertrophy (AP, VP), hyperplasia (VP), |
| apoptosis (VP, DLP) | |||||
| 5681 | 39.4 | PIN3 | PIN4 | PIN3 | Massive exfoliation (AP) |
| 5628 | 54 | PIN3 | PIN3 | PIN4 | Hypertrophy/hyperplasia, diffusely located atypical cells (VP) |
| 5491 | 54.6 | PIN3 | PIN4 | PIN3 | Areas of hyperplasia (AP), apoptosis (VP) |
| 5584 | 54.6 | PIN2 | PIN4 | PIN4 | Hypertrophy/hyperplasia, few atypical cells (AP) |
| 5580 | 54.9 | PIN3 | PIN4 | PIN4 | Intraglandular focal necrosis (DLP) |
| 5581 | 54.9 | PIN4 | PIN4 | ||
Abbreviations: RS, regular structures as seen in the normal mouse prostate; PIN, prostatic intraepithelial neoplasm PIN 1 and PIN2, low grade (LG) PINs; PIN3 and PIN4, high grade (HG) PINs; Early cancer, microscopic cancer; Adca, adenocarcinoma. Lesion classifications were as described (28-30)
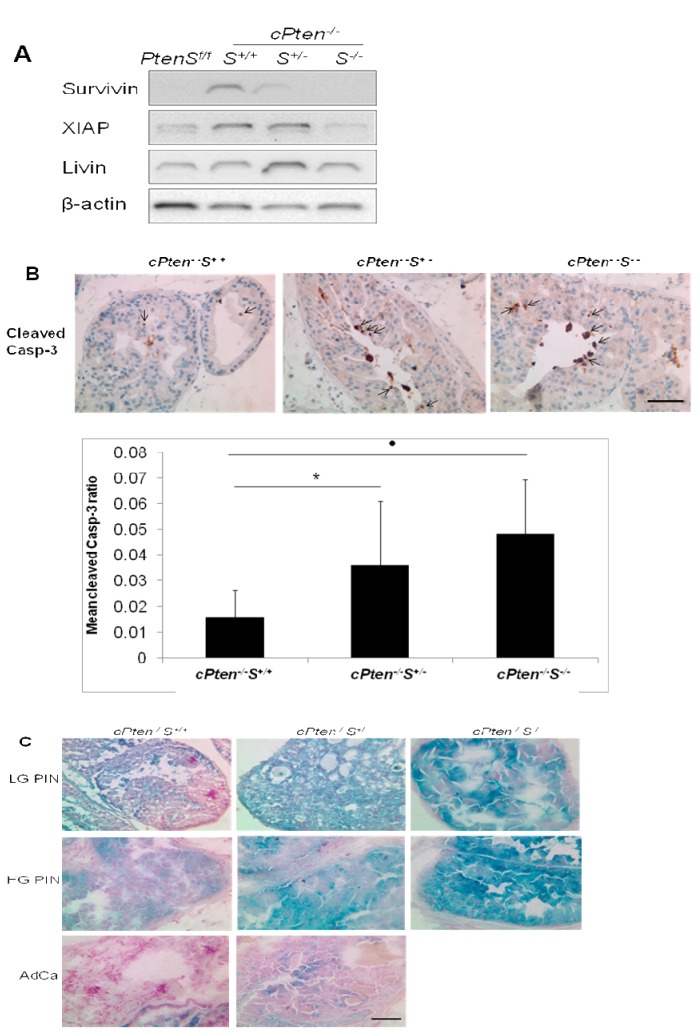
Characteristics of expression of cellular markers in the prostate tumors formed in the conditional Pten deletion mice with heterozygous or homozygous deletion of Survivin gene
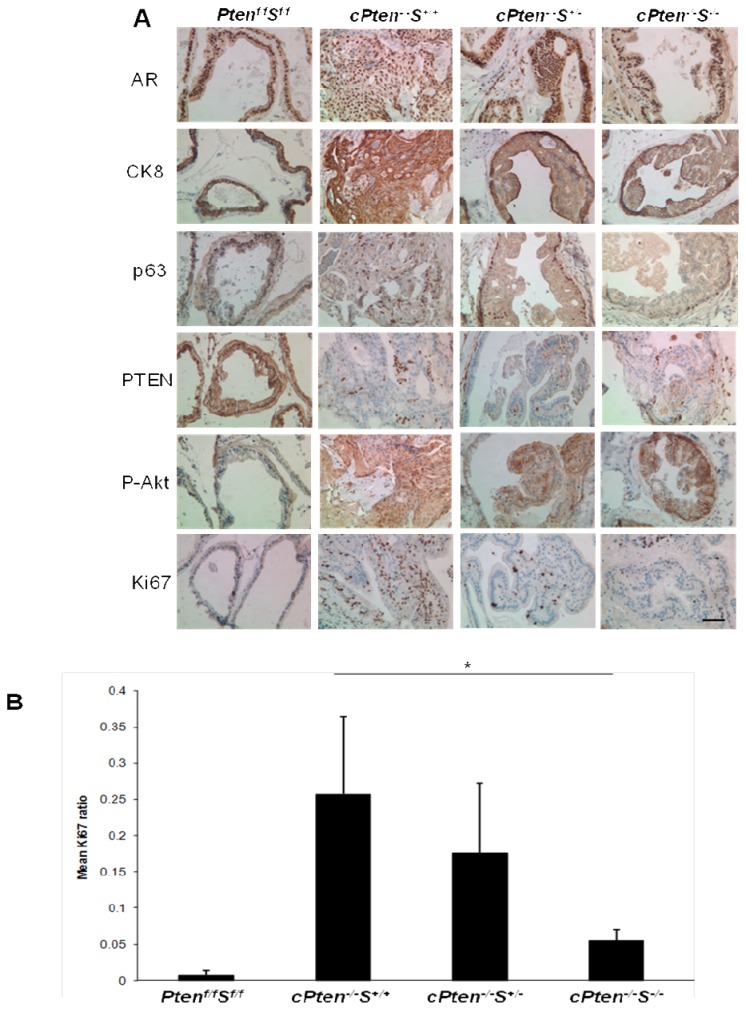
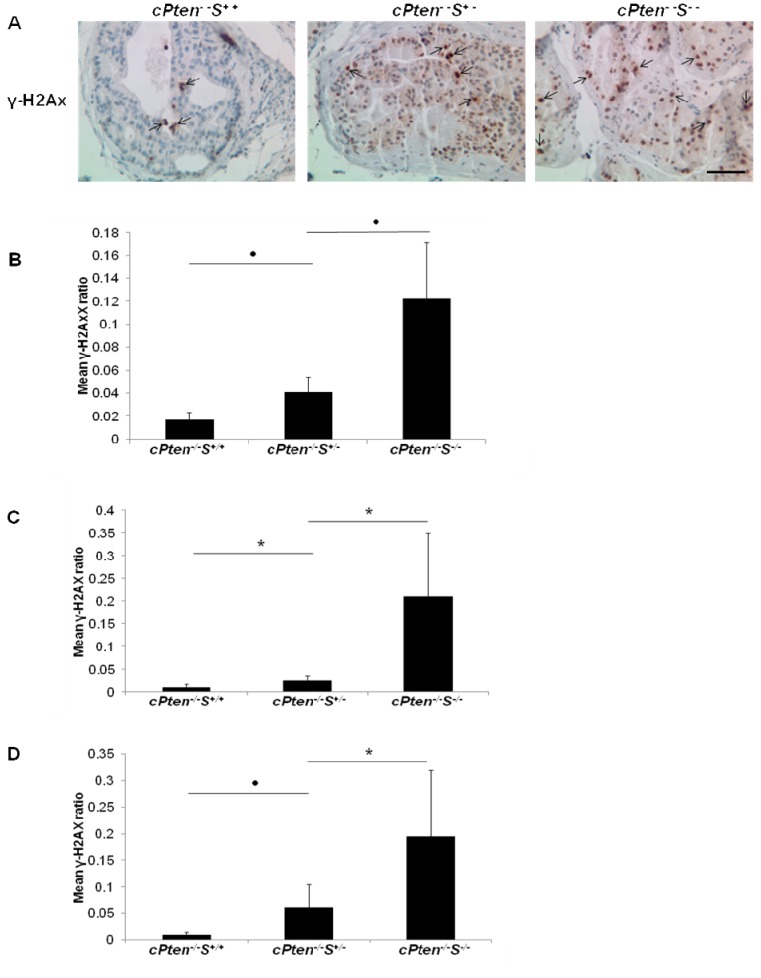
Immunohistochemistry results (Figure 4A) showed that the prostate cells making up glandular structures in all four groups of mice stained positive for androgen receptor (AR), luminal epithelial marker cytokeratin 8 (CK8), and basal epithelial cell marker p63. Knock-down of Pten specifically in the prostate epithelium was confirmed by the distinctive lack of PTEN protein staining in that area compared to the abundance of PTEN expression in the surrounding stroma. Correspondingly, the level of detection of phosphorylated AKT was elevated in all mouse prostate tissue sections with conditional Pten deletion, regardless of the status of survivin expression. A significant downregulation in the expression of Ki67 was observed in prostate tissues of cPten -/-S-/- compared to cPten -/- S +/+ or cPten -/- S +/-. Representative results illustrating these observations are shown in Figure 4A, B. The results of Ki67 staining suggested a role of survivin in cell proliferation. This observation was also consistent with the finding that the tumor size in cPten -/-S-/- being strikingly smaller in comparison to those of either cPten -/- S +/+ and cPten -/- S +/- (Figure 3A–D). Considering that an increased occurrence of apoptotic cells was noted in the histological analyses of samples from cPten -/-S-/- group (Table 2), we undertook a further assessment by immunohistochemistry using an antibody against cleaved caspase-3. We found that the activated caspase-3 expression level was indeed higher in the prostates of cPten-/-S-/- compared to those of the other groups (Figure 5B). This is illustrated with PIN2 lesions where the increase was most pronounced. Another noteworthy histologic observation was that the prostatic lesions in the cPten -/-S-/- mice frequently contained, in addition of apoptotic cells, other enlarged, atypical cells indicative of increased incidence of cellular senescence (Table 2, as compared to Supplemental Tables 1-3). This assumption was then tested by an in situ assay of senescence-associated β-galactosidase enzyme activity on the frozen tissue sections, as described [26]. It appeared that a higher proportion of cells exhibiting stronger staining (blue color) for β-galactosidase was present in the cPten -/-S-/- tissues relative to either cPten+/- or cPten -/- S +/+ tissues, especially in high grade PIN stage (Figure 5C). Cells in the adenocarcinoma samples showed only minimal staining for senescence. A significant increase of phosphorylated H2AX, or γ-H2AX, was also detected by immunohistochemical analysis of the prostate samples of mice lacking a single or both alleles of Survivin compared to those with intact Survivin (Figure 6A). The extent of phosphorylation was positively correlated with the degree of Survivin deletion and was consistently observed throughout various time points (Figure 6B–D).
Figure 4. Representative pattern of expression of cellular markers in prostates of conditional Pten knockout mice with heterozygous and homozygous deletion of Survivin.
(A) Immunostaining of dorsolateral prostate lobes of animals from the 56-week old group of different genotypes using antibodies against androgen receptor (AR), cytokeratin 8 (CK8), basal epithelial marker p63, PTEN, phosphorylated Akt (P-Akt), and Ki67. All images were taken at 400x magnification. Bar, 50 µm. (B) Comparison of proliferation index as assessed by Ki67 staining of dorsolateral lobes of the 56-week old group. * P < 0.05.
Figure 5. Effects of Survivin deletion on other molecular and cellular parameters.
(A) Representative Western blot analysis of dorsolateral prostates from the 37-week old group showed that XIAP level was down-regulated in prostate tissues lacking both alleles of Survivin, while Livin was relatively unaffected. A similar pattern of XIAP expression was also observed when samples were obtained from in 10 and 21 week- old groups. (B) Detection of higher levels of cleaved-caspase 3 expression, indicated by arrows, in the PIN lesions from the conditional Pten deleted prostate tumors lacking single or both alleles of Survivin compared to tumors with intact Survivin. * P < 0.05; · P <0.01. (C) Illustration of senescence-activated β-galactosidase staining results. An increase in senescence was indicated in the conditional Pten deleted prostate samples with complete inactivation of Survivin especially at the high-grade PIN stage compared to samples with intact and monoallelic deletion of Survivin. Note only low level of senescence in prostate adenocarcinoma tissues of conditional Pten knockout mice with either with intact and single deletion of Survivin. PINs are denoted as low-grade (LG) or high-grade (HG), and adenocarcinoma as AdCa. All images were taken at 400x magnification. Bar, 50 µm.
Figure 6. Assessment of γ-H2AX immunostaining in prostate tissue sections.
(A) Representative view of γ-H2AX expression, indicated by arrows, in the prostate samples at 10 weeks from the conditional Pten knockout mice with single or double deletion of Survivin as compared to corresponding samples with intact Survivin. Images were taken at 400x magnification. Bar, 50 µm. (B) (C), and (D) show the results of quantitation of γ-H2AX positive cells in samples at 10, 21 and 37 weeks, respectively. * P < 0.05; · P <0.01.
Effects of Survivin knockout on the expression levels of other IAP members in the prostate tumor tissues
Western blot analysis revealed that XIAP, a member of the IAP family, displayed a similar expression level as survivin. Although, like survivin, its expression increased in the Pten deleted tumor tissues, XIAP appeared to be down-regulated with the loss of survivin expression. This type of corollary expression, however, was not observed with Livin, another IAP family member. Livin protein expression did increase in the lesions of Pten- deleted prostates, but there was no significant alteration in its expression in the cases when Survivin was deleted (Figure 5A).
Discussion
In the cancer field, survivin stands as a unique member of the IAP family with essential roles in mitosis, cellular stress response and inhibition of cell death. It was, however, not known whether survivin plays a role in the development of the normal prostate and how this multi-functional protein might be relevant to its role in prostate carcinogenesis. For this purpose, we first determined if the organogenesis and growth of the prostate gland might be influenced by survivin. Mice with conditional inactivation of Survivin in prostate epithelium were generated by crossing mice of our PB-Cre4 line [23] with floxed Survivin mice [22]. Through breeding and analyses of these mice at various ages up to one year, we demonstrate that homozygous inactivation of Survivin alleles in the epithelial cells of the prostate does not interfere with the development of fertile males harboring prostate gland with generally normal gross and microscopic anatomy. The role of survivin in prostate cancer genesis and progression was then investigated using the conditional biallelic Pten deletion model [17–19] by crossing the tumor model with the floxed Survivin allelic mice. Our contention was that prostate epithelium-specific Survivin nullizygous condition would likely enhance cellular apoptosis to counter prostate tumorigenesis. Here, using this combined model we provide evidence that loss of survivin inhibits progression of premalignant lesions to adenocarcinoma, and that the premalignant lesions exhibiting decreased proliferation index are composed of atypical cells, many of which exhibit increased hypertrophy and senescence.
Survivin expression was reported to be up-regulated in the early prostate tumor growth in both the conditional Pten knockout [16] and the TRAMP [31] mouse models. This implication of survivin in early pathogenesis is corroborated by our observation that deletion of either single or both alleles of Survivin can influence the phenotype of the PIN formed as early as 9 weeks of age. While all groups displayed low grade PIN formation, none of the four cPten -/-S-/- and only 1 of 4 cPten -/-S+/- mice was found to develop high grade PIN lesions at this age, compared to 3 out of 4 cPten -/-S+/+ mice. In the age group of 56 weeks, 80-100% of the mice with intact or singly deleted Survivin alleles harbored adenocarcinoma, while none (0/5) of the mice with the homozygous Survivin deletion developed adenocarcinoma, although high grade PINs were detected. In relation to the known influence of the genetic background and modifier genes on susceptibility to tumorigenesis, we believe that it is unlikely that the suppression of the progression to adenocarcinoma in our test model is due to variation in the mixed genetic background of the animals, because some of the cPten -/-S+/- mice developed adenocarcinoma at age as early as 21 weeks, whereas cPten -/-S-/- littermates with similar mixed background were free of cancer even at over one year age. Still, to increase the validity of the study, it will be necessary to breed all of the three pertinent strains, namely PBCre-4, floxed Pten, and the floxed Survivin, into a single genetic background that is also conducive for tumorigenesis. This major task, however, remains to be initiated. The question of whether the cPten -/-S-/- mice might manifest cancer on further aging was examined very recently. Of the three cPten -/-S-/- 72 week-old males examined subsequently, only one was found to display development of adenocarcinoma in the prostate (data not shown).
With respect to other histopathology parameters, hyperplasia was abundantly detected in the cPten -/-S-/- mice, but only rarely in cPten -/-S+/- or the control cPten -/-S+/+ group. Furthermore, hypertrophy or enlargement of cells, and polyploidy or hyperploidy were relatively more frequent in the prostate samples from cPten -/-S-/- compared to the other groups. Survivin deficient cells have been reported to exhibit multiple nuclei in vitro and in vivo, consistent with the known role of survivin in the regulation of cytokinesis and cell division [11,22]. Another striking difference between single and biallelic deletion of Survivin was the lack of desmoplasia observed in the prostate tissue of cPten -/-S-/- mice younger than the 56 weeks age group that was prominently present in cPten -/-S+/+ and cPten -/-S+/- samples from all four age groups tested. A reduction in the rate of cell proliferation was evident from the significant down-regulation of Ki67 expression in the specimens from the cPten -/-S-/- animals. This effect was the most striking with the 10 weeks age group, whether the samples were from the monoallelic or biallelic Survivin knockout animals. However, with time, the degree of proliferation became somewhat parallel with the extent of Survivin insufficiency.
Another noteworthy point is that we found that the degree of Survivin deletion in the tumors differentially affected the detectable levels of expression of some other IAP family members. For example, the expression of XIAP, but not Livin was reduced. This phenomenon could probably be attributed to the function of the complex known to form between survivin and XIAP that stabilizes XIAP and protects it from ubiquitin-dependent degradation [3]. The survivin-XIAP complex was also described to enhance XIAP’s capacity to inhibit caspases and to facilitate tumor growth in vivo [3,32]. The reduction of XIAP in the setting of Survivin deficiency may therefore further protect against tumorigenesis.
Consistent with previous findings in various other study systems [33–35], we observed an increase in apoptosis as assessed by the expression level of activated caspase-3 in the prostatic lesions of the conditional Pten knockout mice lacking both alleles of Survivin. This effect was most pronounced and significant at the low grade PIN stage. We also detected a correlation between loss of survivin expression and senescence in Pten-deleted prostate tumor tissues. By using the simplified method of staining for senescence-associated β-galactosidase in the tissue sections [36], we attempted to measure the extent of senescence induction in the prostate of the various groups of mice. Presence of senescent cells was readily detected in conditional Pten deleted mice with intact Survivin at low grade and high grade PIN stages but minimally when the tumor had advanced to adenocarcinoma. This finding is consistent with previous reports that senescence may be an initial barrier in cancer development [37–39], and that senescent cells exist in premalignant tumors but not in malignant ones [39]. The pattern of senescence observed in the group with monoallelic and biallelic Survivin loss was not detectably different from the control tumor group at the low grade PIN stage. However, at the high grade PIN stage, lesions of the group with single and especially double deletion of Survivin loss exhibited a higher proportion of senescent cells and greater intensity of staining. Since γ-H2AX, whose expression is induced in cells following initial DNA fragmentation, is considered as a cellular senescence marker [40–42] our data on β-galactosidase criterion appears to be supported by the results of γ-H2AX staining. Clearly, the increase in the detection of γ-H2AX-positive cells is found to be associated with the degree of Survivin deletion in the conditional Pten knockout mice. However, it remains unknown to what extent this increased staining may be subscribed to senescence vs. DNA double strand breaks and induced γ-H2AX that occurs during apoptosis [43]. Although an association of survivin loss with senescence in the lesions is indicated, not clear is whether this relationship is direct or secondary to the loss of the multi-functionality of the survivin protein that may be critical for the progression of the preneoplastic lesions to cancer. It is also possible that the defects in microtubule assembly, loss of mitotic spindles, and formation of multinucleated cells, the abnormalities that are triggered by the loss of survivin [2,9,11] could make the cells prone for senescence. Further studies into the effect of survivin on cellular senescence would be important.
In summary, the results of our investigation on the role of survivin in prostate cancer progression using a double conditional Pten and Survivin mouse model is particularly significant because it led to insights of the direct impact of Survivin deletion occurring simultaneously with that of Pten deletion in the process of tumorigenesis. Evidence is obtained to link a supporting role of survivin in the progression of PIN lesions to adenocarcinoma of the prostate in the model system. Additionally, it is apparent that lesions formed in the absence of survivin are variant in microscopic phenotypes with hallmarks of hypertrophy, exfoliation, apoptosis and senescence. These findings offer a potential for future pre-clinical or clinical investigation for the control of PIN lesions. It is projected from the findings of this study that inhibition of survivin activity may retard or block progression of the PIN lesions, and, thereby extending the therapeutic window for prostate cancer.
Supporting Information
(A) Conditional deletion of Survivin in murine prostate. (B) Double conditional knockout of Pten and Survivin in murine prostate. The genotypes of the mice outlined with the red boxes were used for the study.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; PIN, prostatic intraepithelial neoplasms; PIN1 and PIN2, low grade PINs; PIN3 and PIN4, high grade PINs; Early cancer, microscopic cancer, AdCa, adenocarcinoma.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; PIN, prostatic intraepithelial neoplasms; PIN1 and PIN2, low grade PINs; PIN3 and PIN4, high grade PINs; Early cancer, microscopic cancer, AdCa, adenocarcinoma.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; RS, regular structures as seen in the normal mouse prostate.
(PPTX)
Acknowledgments
We would like to thank Lora Barsky and Emily Zamalea for their assistance with FACS experiments, and Man-Chung Ting, Florence M. Hofman, Niyati Jhaveri, members of Stiles and Dubeau laboratories, and all members of the Roy-Burman laboratory for helpful technical discussions and for sharing reagents and equipments.
Funding Statement
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers R01CA059705 and R01CA113392 (to PRB), and number R01CA096823 (to AYN), and, in part, by California Institute for Regenerative Medicine training grant TG2-01161 (HA). EMC is supported by the Canadian Institutes of Health Research and the Canada Foundations for Innovation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Altieri DC (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8: 61-70. doi:10.1038/nrc2293. PubMed: 18075512. [DOI] [PubMed] [Google Scholar]
- 2. Altieri DC (2006) The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol 18: 609-615. doi:10.1016/j.ceb.2006.08.015. PubMed: 16934447. [DOI] [PubMed] [Google Scholar]
- 3. Srinivasula SM, Ashwell JD (2008) IAPs: what’s in a name? Mol Cell 30: 123-135. doi:10.1016/j.molcel.2008.03.008. PubMed: 18439892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dohi T, Okada K, Xia F, Wilford CE, Samuel T et al. (2004) An IAP-IAP complex inhibits apoptosis. J Biol Chem 279: 34087-34090. doi:10.1074/jbc.C400236200. PubMed: 15218035. [DOI] [PubMed] [Google Scholar]
- 5. Aoki Y, Feldman GM, Tosato G (2003) Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101: 1535-1542. doi:10.1182/blood-2002-07-2130. PubMed: 12393476. [DOI] [PubMed] [Google Scholar]
- 6. Roca H, Varsos ZS, Mizutani K, Pienta KJ (2008) CCL2, survivin and autophagy: new links with implications in human cancer. Autophagy 4: 969-971. PubMed: 18758234. [DOI] [PubMed] [Google Scholar]
- 7. Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O et al. (2009) Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 100: 1073-1086. doi:10.1038/sj.bjc.6604978. PubMed: 19293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi K, Hatano M, Otaki M, Ogasawara T, Tokuhisa T (1999) Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc Natl Acad Sci U S A 96: 1457-1462. doi:10.1073/pnas.96.4.1457. PubMed: 9990045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrasco RA, Stamm NB, Marcusson E, Sandusky G, Iversen P et al. (2011) Antisense inhibition of survivin expression as a cancer theurapeutic. Mol Cancer Ther 10: 221-232. doi:10.1158/1535-7163.TARG-11-A221. PubMed: 21216939. [DOI] [PubMed] [Google Scholar]
- 10. Stauber RH, Mann W, Knauer SK (2007) Nuclear and cytoplasmic survivin: Molecular mechanism, prognostic, and therapeutic potential. Cancer Res 67: 5999-6002. doi:10.1158/0008-5472.CAN-07-0494. PubMed: 17616652. [DOI] [PubMed] [Google Scholar]
- 11. Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ et al. (2000) Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol 10: 1319-1328. doi:10.1016/S0960-9822(00)00769-7. PubMed: 11084331. [DOI] [PubMed] [Google Scholar]
- 12. Okada H, Bakal C, Shahinian A, Elia A, Wakeham A et al. (2004) Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med 199: 399-410. doi:10.1084/jem.20032092. PubMed: 14757745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J et al. (2005) Essential role for survivin in early brain development. J Neurosci 25: 6962-6970. doi:10.1523/JNEUROSCI.1446-05.2005. PubMed: 16049172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zwerts F, Lupu F, De Vriese A, Pollefeyt S, Moons L et al. (2007) Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood 109: 4742-4752. PubMed: 17299096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leung CG, Xu Y, Mularski B, Liu H, Gurbuxani S et al. (2007) Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med 204: 1603-1611. PubMed: 17576776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang S, Lim M, Pham LK, Kendall SE, Reddi AH et al. (2006) Bone Morphogenetic Protein 7 protects prostate cancer cells from stress-induced apoptosis via both Smad and c-Jun NH2-terminal kinase pathways. Cancer Res 66: 4285-4290. doi:10.1158/0008-5472.CAN-05-4456. PubMed: 16618753. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C et al. (2003) Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4: 209-221. doi:10.1016/S1535-6108(03)00215-0. PubMed: 14522255. [DOI] [PubMed] [Google Scholar]
- 18. Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A et al. (2003) Pten dose dictates cancer progression in the prostate. PLOS Biol 1: 385-396. PubMed: 14691534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liao CP, Zhong C, Saribekyan G, Bading J, Park R et al. (2007) Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res 67: 7525-7533. doi:10.1158/0008-5472.CAN-07-0668. PubMed: 17671224. [DOI] [PubMed] [Google Scholar]
- 20. Lim M, Zhong C, Yang S, Bell AM, Cohen MB et al. (2010) Runx2 regulates survivin expression in prostate cancer cells. Lab Invest 90: 222-233. doi:10.1038/labinvest.2009.128. PubMed: 19949374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P (2005) Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res 65: 5769-5777. doi:10.1158/0008-5472.CAN-05-0289. PubMed: 15994952. [DOI] [PubMed] [Google Scholar]
- 22. Xing Z, Conway EM, Kang C, Winoto A (2003) Essential role of survivin, an inhibitor of apoptosis protein. T Cell Dev Maturation And Homeostasis J Exp Med 99: 69-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Wu J, Huang J, Powell WC, Zhang J et al. (2001) Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 101: 61-69. doi:10.1016/S0925-4773(00)00551-7. PubMed: 11231059. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Z, Flesken-Nikitin A, Nikitin AY (2007) Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res 67: 5683-5690. doi:10.1158/0008-5472.CAN-07-0768. PubMed: 17553900. [DOI] [PubMed] [Google Scholar]
- 25. Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW et al. (2006) Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res 66: 7889-7898. doi:10.1158/0008-5472.CAN-06-0486. PubMed: 16912162. [DOI] [PubMed] [Google Scholar]
- 26. Bandyopadhyay D, Gatza C, Donehower LA, Medrano EE (2005) Analysis of cellular senescence in culture in vivo: The senescence-associated β-galactosidase assay. Current Protoc Cell Biol 189: 1-18.9.9 [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Thomas TZ, Kasper S, Matusik RJ (2000) A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo . Endocrinology 141: 4698-4710. doi:10.1210/en.141.12.4698. PubMed: 11108285. [DOI] [PubMed] [Google Scholar]
- 28. Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C et al. (2002) Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol 161: 725-735. PubMed: 12163397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Couto SS, Cao M, Duarte PC, Banach-Petrosky W, Wang S et al. (2009) Simultaneous haploinsufficiency of Pten and Trp53 tumor suppressor genes accelerates tumorigenesis in a mouse model of prostate cancer. Differentiation 77: 103-111. doi:10.1016/j.diff.2008.09.010. PubMed: 19281769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi N, Zhang B, Zhang L, Ittmann M, Xin L (2012) Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21: 253-265. doi:10.1016/j.ccr.2012.01.005. PubMed: 22340597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krajewska M, Krajewski S, Banares S, Huang X, Turner B et al. (2003) Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res 9: 4914-4925. PubMed: 14581366. [PubMed] [Google Scholar]
- 32. Dohi T, Xia F, Altieri DC (2007) Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell 27: 17-28. doi:10.1016/j.molcel.2007.06.004. PubMed: 17612487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheung CH, Sun X, Kanwar JR, Bai JZ, Cheng L et al. (2010) A cell-permeable dominant-negative survivin protein induces apoptosis and sensitizes prostate cancer cells to TNF-α therapy. Cancer Cell Int 10: 36. doi:10.1186/1475-2867-10-36. PubMed: 20920299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanwar JR, Shen WP, Kanwar RK, Berg RW, Krissansen GW (2001) Effects of survivin antagonists on growth of established tumors and B7-1 immunogene therapy. J Natl Cancer Inst 93: 1541-1552. doi:10.1093/jnci/93.20.1541. PubMed: 11604477. [DOI] [PubMed] [Google Scholar]
- 35. Ambrosini G, Adida C, Sirugo G, Altieri DC (1998) Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem 273: 11177-11182. doi:10.1074/jbc.273.18.11177. PubMed: 9556606. [DOI] [PubMed] [Google Scholar]
- 36. De Jesus BB, Blasco MA (2012) Assessing cell and organ senescence biomarkers. Circ Res 111: 97-109. doi:10.1161/CIRCRESAHA.111.247866. PubMed: 22723221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mooi WJ, Peeper DS (2006) Oncogene-induced cell senescence—halting on the road to cancer. N. Engl. J. Med. 355: 1037-1046. doi:10.1056/NEJMra062285. PubMed: 16957149. [DOI] [PubMed] [Google Scholar]
- 38. Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA et al. (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725-730. doi:10.1038/nature03918. PubMed: 16079851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ et al. (2005) Tumour biology: senescence in premalignant tumours. Nature 436: 642. doi:10.1038/436642a. PubMed: 16079833. [DOI] [PubMed] [Google Scholar]
- 40. Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P et al. (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444: 638-642. doi:10.1038/nature05327. PubMed: 17136094. [DOI] [PubMed] [Google Scholar]
- 41. Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D et al. (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444: 633-637. doi:10.1038/nature05268. PubMed: 17136093. [DOI] [PubMed] [Google Scholar]
- 42. Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A et al. (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907-913. doi:10.1038/nature03485. PubMed: 15829965. [DOI] [PubMed] [Google Scholar]
- 43. Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM (2000) Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 275: 9390-9395. doi:10.1074/jbc.275.13.9390. PubMed: 10734083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Conditional deletion of Survivin in murine prostate. (B) Double conditional knockout of Pten and Survivin in murine prostate. The genotypes of the mice outlined with the red boxes were used for the study.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; PIN, prostatic intraepithelial neoplasms; PIN1 and PIN2, low grade PINs; PIN3 and PIN4, high grade PINs; Early cancer, microscopic cancer, AdCa, adenocarcinoma.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; PIN, prostatic intraepithelial neoplasms; PIN1 and PIN2, low grade PINs; PIN3 and PIN4, high grade PINs; Early cancer, microscopic cancer, AdCa, adenocarcinoma.
(PPTX)
Abbreviations: AP, VP, DLP, anterior, ventral and dorsolateral prostates, respectively; RS, regular structures as seen in the normal mouse prostate.
(PPTX)