Abstract
The high prevalence of bladder cancer and its recurrence make it an important target for chemoprevention. About half of invasive urothelial tumors have mutations in p53. We determined the chemopreventive efficacy of a p53-stabilizing agent, CP-31398, in a transgenic UPII-SV40T mouse model of bladder transitional cell carcinoma (TCC) that strongly resembles human TCC. After genotyping, six-week-old UPII-SV40T mice (n = 30/group) were fed control (AIN-76A) or experimental diets containing 150 or 300 ppm of CP-31398 for 34 weeks. Progression of bladder cancer growth was monitored by magnetic resonance imaging. At 40 weeks of age, all mice were killed; urinary bladders were collected to determine weights, tumor incidence, and histopathology. There was a significant increase in bladder weights of transgenic versus wild-type mice (male: 140.2 mg vs 27.3 mg, P < .0001; female: 34.2 mg vs 14.8 mg, P < .0001). A significant decrease in the bladder tumor weights (by 68.6–80.2%, P < .0001 in males and by 36.9–55.3%, P < .0001 in females) was observed in CP-31398-treated mice. Invasive papillary TCC incidence was 100% in transgenic mice fed control diet. Both male and female mice exposed to CP-31398 showed inhibition of invasive TCC. CP-31398 (300 ppm) completely blocked invasion in female mice. Molecular analysis of the bladder tumors showed an increase in apoptosis markers (p53, p21, Bax, and Annexin V) with a decrease in vascular endothelial growth factor in transgenic mice fed CP-31398. These results suggest that p53-modulating agents can serve as potential chemopreventive agents for bladder TCC.
Introduction
Urinary bladder cancer is one of the common cancers worldwide, with the highest incidence rates in industrialized countries. In 2013, about 72,570 new cases of bladder cancer are expected to be diagnosed (about 54,610 in men and 17,960 in women), of which approximately 15,210 will die because of this cancer [1]. Smoking tobacco and exposure to arsenic are known risk factors for development of urothelial neoplasms. Urothelial cancers of the bladder generally are classified into noninvasive and invasive types, which are thought to originate independently [2,3]. A majority of the urothelial tumors are noninvasive, low-grade, papillary transitional cell carcinoma (TCC), but up to 30% of all diagnosed tumors are classified as invasive TCC that pose a very high risk for distant metastases. In addition, 10% to 15% of the noninvasive TCC progress to the invasive form with recurrence after surgery, having acquired additional genetic mutations. Noninvasive forms of TCC have better treatment options and 5-year survival (88–98%). However, the management of invasive urothelial cancers is a major challenge, with a very low 5-year survival rate of only 6% in distantly metastasized cases [1]. Molecular analysis of these two types of tumors clearly indicates that more than half of the invasive tumors have mutations in the tumor suppressor genes p53 and pRB [4–6]. The high prevalence, progression to invasive carcinoma, and frequent tumor recurrence make bladder cancer an important neoplastic disease for exploration of prevention strategies.
In normal cells, the p53 tumor suppressor gene plays a critical role in induction of programmed cell death during cellular stress and DNA damage. In many cancers, p53 is mutated, leading to underexpression or loss of function; hence, p53 has emerged as an important target for chemoprevention and therapy. CP-31398, a synthetic styrylquinazoline, (N′-[2-[(E)-2-(4-methoxyphenyl)ethenyl]-quinazolin-4-yl]-N,N-dimethylpropane-1,3-diamine hydrochloride)], was found to restore the DNA-binding activity of mutant p53 protein by stabilizing the conformation of the DNA-binding domain [7–9]. The chemopreventive potential of this agent has been demonstrated successfully in various in vitro and in vivo tumor efficacy studies [10–12].
Among the preclinical models for urothelial cancer, UPII-SV40T transgenic mice develop invasive urothelial tumors, similar to those observed in humans, owing to the expression of Simian virus large T antigen driven by the urothelium-specific uroplakin II promoter [13]. Simian virus large T antigen inactivates p53/pRB pathways [14] and its expression in urothelium was found to drive development of bladder tumors in these mice that replicate the invasive urothelial tumors of humans, both genetically and histopathologically [13]. Hence, in the present study, we determined the chemopreventive efficacy of the p53-stabilizing agent CP-31398 using the UPII-SV40T transgenic mouse model for invasive urothelial TCC.
Materials and Methods
Animals, Diet, and Care
All animal experiments were done in accordance with the guidelines of the Institutional Animal Care and Use Committee. UPII-SV40T transgenic mice were obtained from Xue-Re Wu at the NYU Medical Center (New York, NY). The required number of UPII-SV40T transgenic mice was generated by breeding as described below. Animals were housed in ventilated cages under standardized conditions (21°C, 60% humidity, 12-hour light/12-hour dark cycle, 20 air changes per hour) in the University of Oklahoma Health Sciences Center Rodent Barrier Facility. Semipurified modified AIN-76A diet ingredients were purchased from Bioserv, Inc (Frenchtown, NJ). CP-31398 (Figure 1A) was procured from the National Cancer Institute chemoprevention drug repository. CP-31398 (150 and 300 ppm) was premixed with small quantities of casein and then blended into the diet using a Hobart mixer. Both control and experimental diets were prepared weekly and stored in a cold room. Agent content in the experimental diets was determined periodically in multiple samples taken from the top, middle, and bottom portions of individual diet preparations to verify uniform distribution. Mice were allowed ad libitum access to the respective diets and to automated tap water purified by reverse osmosis.
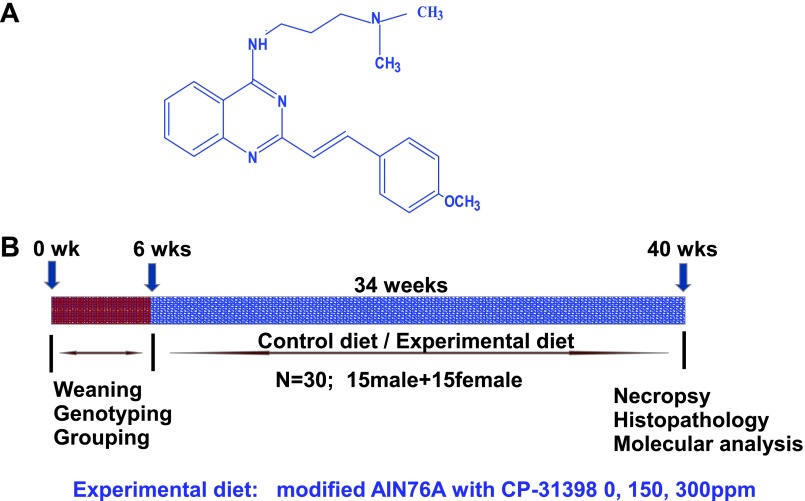
Figure 1.
(A) Structure of p53-stabilizing agent, CP-31398. (B) Experimental design for chemopreventive efficacy evaluation of CP-31398 in UPII-SV40T transgenic mice. At 6 weeks of age, groups (30 per group UPII-SV40T or 12 per group wild type) of mice were fed experimental diets containing 0, 150, or 300 ppm of CP-31398 continuously for 34 weeks and bladders from each mouse were evaluated histopathologically and also for expression of various markers as described in the text.
Breeding and Genotyping
Heterozygous UPII-SV40T and wild-type mice were maintained in an FVB genetic background. Male UPII-SV40T mice were crossed to wild-type females and offspring were generated at required quantities. Transgenic pups were confirmed by tail DNA extraction and polymerase chain reaction (PCR). Briefly, genomic DNA was extracted from snap-frozen tail tissue samples using the mini-prep kit (Invitrogen, Carlsbad, CA). PCR for the SV40T gene was done using oligonucleotide primer sequences 5′-CTTTGGAGGCTTCTGGGATGCAACT-3′ (sense) and 5′-GCATGACTCAAAAAACTTAGCAATTCTG-3′ (antisense) and amplifying under the following PCR conditions: denaturation at 95°C for 5 minutes, followed by 35 cycles at 95°C for 1 minute, 58°C for 45 seconds, and 72°C for 45 seconds. The PCR products, when separated on a 2% agarose gel, showed a 550-bp band.
Bioassay
Genotyped UPII-SV40T transgenic mice were used in the efficacy study. The experimental protocol is summarized in Figure 1B. Five-week-old mice were selected and randomized so that the average body weights in each group were equal (n = 30 UPII-SV40T mice per group and n = 12 wild-type mice per group) and were fed AIN-76A diet for 1 week. At 6 weeks of age, mice were fed control or experimental diets containing 0, 150, or 300 ppm of CP-31398 until termination of the study. Mice were checked routinely for signs of weight loss, toxicity, or any abnormalities. The food intake and body weight of each animal were measured once weekly for the first 6 weeks and then once a month until termination. After 34 weeks on experimental diets, all mice were killed (at 40 weeks of age) by CO2 asphyxiation and necropsied; urinary bladders were collected and weighed. To determine the tumor weight, the average of wild-type (tumor-free) bladder was subtracted from the weight of tumor-bearing transgenic mouse bladders. A portion of the urinary bladder was fixed in 10% neutral buffered formalin for histopathologic evaluation and the rest was snap frozen in liquid nitrogen for further analysis.
Magnetic Resonance Imaging of Urothelial Tumors
Magnetic resonance imaging (MRI) was performed using a 30-cm, horizontal bore, 7-T magnet (Bruker BioSpin MRI GmbH, Ettlingen, Germany) at 40 weeks of age on mice fed the control and experimental diets. For imaging, the mice were anesthetized with 2% isoflurane and restrained in a cradle for MRI scans. During MRI scans, the animals' respiratory rate was monitored (SA Instruments, Stony Brook, NY), and body temperature was maintained at 37°C with a water heating system (Gaymar T/Pump). Morphologic and contrast-enhanced imaging with gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA; 0.5 mmol/kg body weight, 50-µl volume in sterile saline) were performed on these mice. Gd-DTPA was administered through an i.v. tail vein catheter.
Tissue Processing and Histologic Analysis
Formalin-fixed, paraffin-embedded tissues were sectioned (4 µm) and stained with hematoxylin and eosin. Sections of each urothelial tumor were evaluated histologically by a pathologist blinded to the experimental groups. Carcinomas were classified into noninvasive carcinoma in situ (CIS) and invasive carcinomas (lamina propria-invasive and muscularis propria-invasive types; Figure 2, E–H) according to histopathologic criteria.
Figure 2.
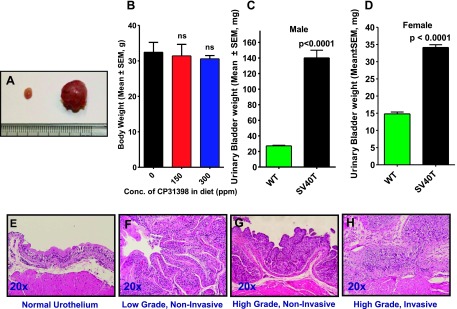
Upper panels: (A) Urinary bladders of the UPII-SV40T mice increase in both size and weight compared with the wild-type mice. (B) Terminal body weights of the transgenic mice fed control or experimental diets. (C) Male and (D) female bladder weights (means ± standard error) at the termination of the experiment. The differences between the wild-type and UPII-SV40T animals fed control diet were analyzed by unpaired t test with Welch correction. Lower panels: Typical histologic characterization of urothelium showing (E) normal wild-type or (F) low-grade noninvasive, (G) high-grade noninvasive, and (H) high-grade invasive TCC in UPII-SV40T mice upon feeding with control AIN-76A diet. No evidence of TCC was seen in the bladders of wild-type mice.
Real-time PCR
Total RNA from urothelial tissue and tumor samples of male mice was extracted using the Totally RNA Kit (Ambion: Life Technologies, Grand Island, NY) as per the manufacturer's instructions. Equal quantities of DNA-free RNA were used in reverse transcription reactions for making cDNA using SuperScript reverse transcriptase (Invitrogen). Real-time PCRs were done for p53, Bax, and vascular endothelial growth factor (VEGF) using SYBR Green and the following primers: for p53, 5′-TGAAACGCCGACCTATCCTTA-3′ (sense) and 5′-GGCACAAACACGAACCTCAAA-3′ (antisense), Tm 60; for Bax, 5′-CAGGATGCGTCCACCAAGAA-3′ (sense) and 5′-GCAAAGTAGAAGAGGGCAACCA-3′ (antisense), Tm 60; for VEGF, 5′-GGAGATCCTTCGAGGAGCACTT-3′ (sense) and 5′-GGCGATTTAGCAGCAGATATAAGAA-3′ (antisense), Tm 60; for glyceraldehyde phosphate dehydrogenase, 5′-CCTCGTCCCGTAGACAAAATG-3′ (sense) and 5′-TGAAGGGGTCGTTGATGGC-3′ (antisense), Tm 55. Relative gene expression was calculated using the 2-ΔΔCT formula [15]. All experiments were performed in at least triplicates.
Immunohistochemistry
The effects of CP-31398 on the expression of p21 and Annexin V were evaluated by immunohistochemistry. Briefly, sections of paraffin-embedded tissues were deparaffinized in xylene, rehydrated through graded ethanol solutions, and washed in phosphate-buffered saline (PBS). Antigen retrieval was carried out by heating the sections in 0.01 M citrate buffer (pH 6.0) for 30 minutes in a boiling water bath. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in PBS for 5 minutes. Nonspecific binding sites were blocked using Protein Block for 20 minutes. Then, sections were incubated overnight at 4°C with 1:300 dilutions of monoclonal antibodies against p21 and Annexin V (Santa Cruz Biotechnology, Santa Cruz, CA). After several washes with PBS, they were incubated with appropriate secondary antibody for 2 hours, then exposed to avidin-biotin complex reagent (Invitrogen). After rinsing with PBS, the slides were incubated with the chromogen 3,3′-diaminobenzidine for 3 minutes, then counterstained with hematoxylin. Nonimmune rabbit Igs were substituted for primary antibodies as negative controls. Specimens were observed using an Olympus microscope IX71, and digital computer images were recorded with an Olympus DP70 camera. Scoring was done according to the intensity of the nucleic or cytoplasmic staining (no staining = 0, weak staining = 1, moderate staining = 2, strong staining = 3) and prevalence of stained cells (1–10% = 1, 11–0% = 2, 21–0% = 3, and >50% = 4). The final immunoreactive score was determined by multiplying the intensity scores by the prevalence of positivity scores of stained cells, yielding a minimum score of 0 and a maximum score of 12.
Statistical Analysis
The data are presented as means ± standard errors. Differences in body weights were analyzed by analysis of variance. Statistical differences between bladder and tumor weights of the control and treated groups were evaluated using unpaired t test with Welch correction. Tumor incidences (percentage of mice with urothelial tumors) were analyzed by Fisher exact test. Differences between control and treatment groups were considered significant at P < .05. All statistical analyses were performed using Graphpad Prism 5.0 Software.
Results
Weight, Necropsy, and Histopathology
All of the transgenic and wild-type mice of both control and experimental groups were weighed weekly and monitored throughout the study. There was no significant difference in the body weights of the control and experimental groups (Figure 2B). Gross necropsy observations also revealed that organs such as stomach, liver, spleen, pancreas, small intestine, colon, and testes were normal in all animals, while the urinary bladders (Figure 2A) and kidneys were visibly abnormal and because of tumor formation. Urinary bladders from all of the transgenic mice weighed significantly more [∼5.4-fold more for males (147.2 mg vs 27.3 mg, P < .0001; Figure 2C) and ∼2.4-fold more for females (34.1 g vs 14.8 g, P < .0001; Figure 2D)] than did those from wild-type mice. Histopathologic evaluation of these bladders identified that all the tumors were high-grade papillary invasive type TCC (Table W1).
Magnetic Resonance Imaging
At the end of the study, MRI was performed on experimental mice fed control or CP-31398 diets. Images from wild-type mice showed normal urinary bladder,whereas those from transgenic mice fed control diet clearly show large, dense tumor formation inside the urinary bladders. In transgenic mice fed the experimental diet, the bladders showed significantly reduced tumor formation (Figure 3A). We observed a good correlation between the bladder weights and the MRI of the bladders.
Figure 3.
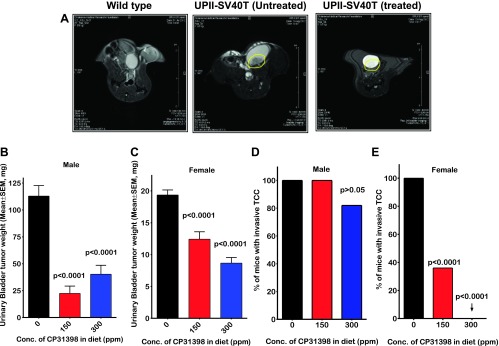
(A) MRI images of the urinary bladders from wild-type and UPII-SV40T transgenic mice. Transgenic mice were given either control diet or diet containing CP-31398 for 36 weeks. Morphologic and contrast-enhanced imaging (Gd-DTPA administered through an i.v. tail vein catheter) MRI was performed on these mice at 40 weeks of age. Wild-type urinary bladders do not show any tumors. Untreated UPII-SV40T mice have large, dense tumor inside the bladder, while the treated transgenic mice have significantly reduced and barely visible tumor. (B and C) Effect of CP-31398 on bladder tumor weights in male (B) and female (C) mice, respectively, at the termination of the experiment. CP-31398 treatment led to a significant reduction of tumor weight in both groups. (D and E) CP-31398 treatment had an inhibitory effect on the incidence of invasive TCC in male (D) and female (E) mice, respectively.
Inhibition of Tumor Growth by CP-31398
Urinary bladders of the transgenic and wild-type mice fed control and experimental diets were weighed at the end of the study. Wild-type mice fed control and CP-31398 diets showed no significant difference in bladder weights (27.25 mg vs 25.34 mg, P > .05 for males and 14.8 mg vs 16.9 mg, P > .05 for females). However, a comparison of the bladder weights from the transgenic mice revealed that CP-31398 treatment lead to marked inhibition (P < .0001) of the tumor growth. The average tumor mass in the male transgenic mice was 119.9 mg (control) vs 22.41 mg (150 ppm of CP-31398) vs 40.25 mg (300 ppm of CP-31398; Figure 3B). Similarly, the average tumor mass in the female transgenic mice was 19.3 mg (control), 12.4 mg (150 ppm of CP-31398), and 8.7 mg (300 ppm of CP-31398; Figure 3C). Therefore, treatment of mice with the p53-stabilizing agent CP-31398 led to 68.5% to 80.2% inhibition of tumor mass in males with the lower dose showing better effects than the higher dose but no statistical significance (P > .05) between the groups. In female mice, there was a dose-dependent 36.9% to 55.3% inhibition of bladder tumor weights (P < .05).
Inhibition of Tumor Invasion by CP-31398
There was a significant inhibition of urothelial tumor invasion into the lamina propria and muscularis in CP-31398-treated mice as determined by histopathology. High-grade TCC with invasion into lamina propria or muscularis was observed in 100% of the urinary bladders from mice fed control diet (Table W1). The low dose of CP-31398 had no effect on the invasion in male mice, but the high-dose treatment showed 18% inhibition of tumor invasion (Figure 3D; P > .05). The effect was more profound in the female mice where only 36% (Figure 3E; P < .0001) of the mice fed low dose and none of mice fed the high dose showed tumor invasion (Figure 3E; P < .0001). In all of the mice in which invasion was inhibited, the urothelium showed noninvasive CIS. Thus, treatment of mice with CP-31398 at the higher dose suppressed tumor invasion and limited lesions to CIS.
Modulation of p53, p21, Bax, Annexin V, and VEGF Expression by CP-31398
Molecular analysis of the urothelium from control and experimental mice was done using quantitative real-time PCR and immunohistochemistry. Compared with normal urothelium, urinary bladder tumors exhibited a significant inhibition of proapoptotic proteins p21 (Figure 5A) and Bax (Figure 4C), whereas VEGF was elevated (Figure 4E). Treatment of mice with CP-31398 led to an increase in the p53 and Bax by about three-fold (P < .0001; Figure 4B) and two-fold (P < .0001; Figure 4D), respectively, with clear accumulation of nuclear p21 protein (Figure 5, B–D). There was also an increase in Annexin V expression, suggesting an increase in apoptotic cells upon CP-31398 treatment (Figure 5, E–H). CP-31398 treatment also caused a significant inhibition of expression of the angiogenic factor VEGF (P < .0001; Figure 4F). These observations suggest that CP-31398 could successfully induce p53, leading to apoptosis and inhibition of tumor growth.
Figure 5.
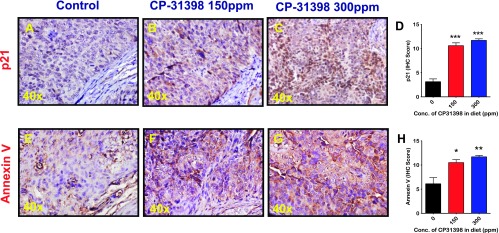
Effect of CP-31398 on p21 (A–D) and Annexin V (E–H) expression in bladder tumors. Immunohistochemical analysis was performed with paraffin-embedded and microsectioned bladder tissues from male mice as described in Materials and Methods section. A significantly increased expression of p21 (A–D) and Annexin V (E–H) was seen in the treatment groups.
Figure 4.
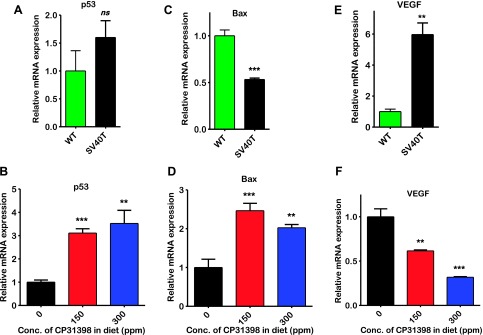
Effect of CP-31398 on expression of p53, Bax, and VEGF markers. Quantitative real-time PCR analysis was done on bladder tumors of male mice as described in Materials and Methods section. (A, C, E) Relative expression of p53, Bax, and VEGF mRNA, respectively, in UPII-SV40T urothelium compared with wild type. (B, D, F) CP-31398 treatment led to an increase in p53 and Bax, while VEGF mRNA levels were inhibited.
Discussion
TCC of the urothelium, which constitutes 95% of all urinary bladder cancers, causes significant morbidity and mortality all over the world. Cessation of tobacco smoking by active smokers may decrease the risk for bladder cancer; however, the risk in former smokers does not fall to the level of those who never smoked [16,17]. Reported data suggest that almost 70% of the bladder cancer cases are still at a noninvasive stage at the time of diagnosis; however, if the noninvasive CIS is left untreated, it will progress to high-risk, invasive TCC, and even if treated, invasive TCC may recur [18].
Various transgenic mouse models with invasive bladder tumors have been developed [13,19,20] and used to understand tumor initiation and progression. In addition, bladder cancer induced by the carcinogen N-butyl-N-(4-hydroxybutyl)-nitrosamine (OH-BBN) [21] has been used extensively to test drug efficacy [22–27]. Genetically engineered mice with tissue-specific expression of SV40 T antigen have been instrumental in elucidating the biology of many cancers. These mice develop invasive carcinomas in the tissue of expression, e.g., breast [28], prostate [29], liver [30], pancreas [31], and bladder [13,19], and have been valuable models for drug development [32,33]. In a recent study, Liu et al. [34] showed the usefulness of UPII-SV40 T transgenic mice for urothelial cancer chemoprevention studies using Flavokawain A as a test agent. Here, we have tested the p53-modulating agent CP-31398 for chemoprevention of invasive urothelial cancer in UPII-SV40T transgenic mice.
Chronic administration of 150 and 300 ppm of CP-31398 in both male and female transgenic mice and wild-type mice did not elicit any overt toxicities or body weight loss. Dietary CP-31398 administration significantly suppressed growth of urothelial tumors as evident by decreased bladder weights in both sexes. That the percent reduction in tumor weights from male mice was greater than that in female mice can be explained by the difference in tumor growth rate between two sexes. In male mice, tumor growth progression seems to be very aggressive, leading to at least a five-fold increase in bladder weights as compared with bladder weights from female mice. It is important to note that CP-31398 showed dose-dependent suppression of invasive TCC inhibition in female mice, with the higher dose showing 100% inhibition of invasive TCC (Table W1). The mechanisms for the difference in SV40 T antigen-induced tumor progression in male versus female mice are not clear, but they may be due, in part, to the influence of sex hormones on tumor growth [35,36]. Epidemiological data indicate that men also have a three to four times higher chance of developing bladder cancer compared with women; however, differences between men and women in the rate of tumor progression have not been studied extensively.
Unlike the dose-dependent effects of CP-31398 in female mice, the inhibition of bladder tumor weight in male mice did not show dose dependence. However, CP-31398 administration appears to be effective in preventing tumor invasion in both male and female mice in a dose-response manner. Antitumor effects of CP-31398 have been demonstrated in various in vitro studies using cancer cells with a mutated p53 gene [37–42]. Chemopreventive efficacy of CP-31398 was also shown in several preclinical models [10–12]. Earlier studies have demonstrated that CP-31398 blocked UVB-induced skin carcinogenesis and was associated with increases in p53 [10]. Our laboratory has shown that dietary administration of CP-31398 significantly inhibited intestinal tumor formation in a dose-dependent manner in APCmin/+ mice [11]. In a rat model of colon cancer, CP-31398 was found to inhibit 63% of multicrypt aberrant crypt foci and up to 65% of adenocarcinoma multiplicity induced by a chemical carcinogen [12]. Inhibition of colon adenocarcinoma incidence and multiplicity was also observed when CP-31398 was combined with the low-dose cyclooxygenase inhibitor celecoxib in rats [12]. The present study results further support previous in vivo observations of the chemopreventive effects of CP-31398 [10–12]. The difference in efficacy of CP-31398 between male and female mice was not noted in previous studies because CP-31398 had not been tested in both male and female mice simultaneously.
Mutations in p53 protein lead to down-regulation of p21 protein expression and other downstream molecules involved in p53-dependent cell proliferation and apoptosis pathways. In the mice fed control diet, we have seen loss of Bax and p21 expression in urothelial tumors, which indicates loss of p53 function in these tumors. It is well established that nuclear localization of p53 activates p21, which blocks cell cycle progression, both by acting as an inhibitor of cyclin-dependent kinase/cyclin complexes and by inhibiting DNA replication by binding to proliferating cell nuclear antigen [43]. We observed a significant increase in p53, p21, and Bax expression in the tumors from CP-31398-treated mice, with significant nuclear localization of p53 and increase in apoptosis. Loss of p21 expression was observed in human bladder tumors and that loss was identified as a predictor of cancer progression [43], while induction of p21 was shown to increase apoptosis and antitumor effects in bladder cancer in vitro and in vivo [44]. Consistent with these observations, we have observed a loss of p21 expression in the bladder tumors of mice fed control diet and an induction of this protein and apoptosis in tumors from animals treated with CP-31398.
Urothelial tumors also overexpress the angiogenesis stimulator VEGF, which is important for tumor growth and metastasis [45]. Evidence from both patient tumors and preclinical models supports the role of VEGF as a key mediator of angiogenesis in bladder cancer [46,47]. Disruption of VEGF signaling was found to impair tumor growth and vascularity, as well to prevent metastatic spread and to prolong survival [48]. In the present study, we found approximately six-fold overexpression of VEGF in tumors, with significant inhibition in urothelial tumors from the CP-31398-treated mice, suggesting antiangiogenic effects of CP-31398. It is not clearly understood how p53 mutations or loss of functional activity lead to increased VEGF and/or proangiogenic effects in urothelial tumors. It is possible that tumors with inactivated p53 would have much greater angiogenic potential because of both increased production of proangiogenic factors such as VEGF and decreased synthesis of antiangiogenic factors. In support of this possibility, studies by Zhang et al. [49] showed that introduction of wild-type p53 into sarcoma cells containing mutant p53 significantly reduced the expression of VEGF by transcriptional repression. In addition, loss of p53 in tumor cells enhances hypoxia-inducible factor (HIF-1α) levels and augments HIF-1-dependent transcriptional activation of the VEGF gene in response to hypoxia, suggesting that hypoxia-induced apoptosis and activation of VEGF are partially dependent on p53 functional activity. Thus, the p53-stabilizing agent CP-31398 may induce tumor cell inhibition in part by inducing p53/p21 and inhibiting VEGF expression.
Conclusion
Invasive carcinomas can be prevented if intervention occurs early enough to block progression by targeting appropriate molecules that play key roles in this process. Our results suggest that p53-modulating agents can serve as potential chemopreventive agents for invasive urothelial cancers. CP-31398 emerged as a promising chemopreventive agent for various cancers with frequent p53 mutations. Several other molecules and pathways are also deregulated in bladder cancer. A combination of CP-31398 and other selective inhibitors such as anti-VEGF and HIF-1α inhibitors may result in better efficacy and better management of the disease.
Supplementary Material
Acknowledgments
We thank Julie Sando for valuable suggestions and editorial help.
Footnotes
We thank the National Institute of Health/National Cancer Institute (NIH/NCI) for funding (NCI-CN53300) and the University of Oklahoma Health Sciences Center Rodent Barrier Facility and Dr Rheal Towner from the Small Rodent Imaging Facility, Oklahoma Medical Research Foundation.
This article refers to supplementary material, which is designated by Table W1 and is available online at www.neoplasia.com.
References
- 1.American Cancer Society (ACS), author Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Schalken JA, van Moorselaar RJ, Bringuier PP, Debruyne FM. Critical review of the models to study the biologic progression of bladder cancer. Semin Surg Oncol. 1992;8:274–278. doi: 10.1002/ssu.2980080505. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg GD, Trump DL, Cummings KB. Metastatic bladder cancer. Natural history, clinical course, and consideration for treatment. Urol Clin North Am. 1992;19:735–746. [PubMed] [Google Scholar]
- 4.Dalbagni G, Presti J, Reuter V, Fair WR, Cordon-Cardo C. Genetic alterations in bladder cancer. Lancet. 1993;342:469–471. doi: 10.1016/0140-6736(93)91595-d. [DOI] [PubMed] [Google Scholar]
- 5.Spruck CH, Ohneseit PF, Gonzalez-Zulueta M, Esrig D, Miyao N, Tsai YC, Lerner SP, Schmutte C, Yang AS, Cote R, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994;54:784–788. [PubMed] [Google Scholar]
- 6.Wagner U, Sauter G, Moch H, Novotna H, Epper R, Mihatsch MJ, Waldman FM. Patterns of p53, erbB-2, and EGF-r expression in premalignant lesions of the urinary bladder. Hum Pathol. 1995;26:970–978. doi: 10.1016/0046-8177(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 7.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, El-Deiry WS. Restoration of p53 to limit tumor growth. Curr Opin Oncol. 2008;20:90–96. doi: 10.1097/CCO.0b013e3282f31d6f. [DOI] [PubMed] [Google Scholar]
- 9.Wiman KG. Restoration of wild-type p53 function in human tumors: strategies for efficient cancer therapy. Adv Cancer Res. 2007;97:321–338. doi: 10.1016/S0065-230X(06)97014-6. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao CV, Swamy MV, Patlolla JM, Kopelovich L. Suppression of familial adenomatous polyposis by CP-31398, a TP53 modulator, in APCmin/+ mice. Cancer Res. 2008;68:7670–7675. doi: 10.1158/0008-5472.CAN-08-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao CV, Steele VE, Swamy MV, Patlolla JM, Guruswamy S, Kopelovich L. Inhibition of azoxymethane-induced colorectal cancer by CP-31398, a TP53 modulator, alone or in combination with low doses of celecoxib in male F344 rats. Cancer Res. 2009;69:8175–8182. doi: 10.1158/0008-5472.CAN-09-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Pak J, Shapiro E, Sun T, Wu X. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999;59(14):3512–3517. [PubMed] [Google Scholar]
- 14.Ahuja D, Sáenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 17.Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, Lotan Y, Karakiewicz PI, Novara G, Robinson BD, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol. 2012;63(4):724–732. doi: 10.1016/j.eururo.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prout GR. Bladder carcinoma and a TNM system of classification. J Urol. 1997;117:583–588. doi: 10.1016/s0022-5347(17)58544-4. [DOI] [PubMed] [Google Scholar]
- 19.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157:805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J-W, Kim Y, Lee J-H, Kim Y-S. CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int J Urol. 2013;20:251–255. doi: 10.1111/j.1442-2042.2012.03128.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SM. Urinary bladder carcinogenesis. Toxicol Pathol. 1998;26:121–127. doi: 10.1177/019262339802600114. [DOI] [PubMed] [Google Scholar]
- 22.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60(20):5599–5602. [PubMed] [Google Scholar]
- 23.Vinh PQ, Sugie S, Tanaka T, Hara A, Yamada Y, Katayama M, Deguchi T, Mori H. Chemopreventive effects of a flavonoid antioxidant silymarin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Jpn J Cancer Res. 2002;93(1):42–49. doi: 10.1111/j.1349-7006.2002.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato D, Matsushima M. Preventive effects of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves. Int J Urol. 2003;10(3):160–166. doi: 10.1046/j.1442-2042.2003.00587.x. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, Glode LM, Agarwal R. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007;6(12 pt 1):3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 26.Lubet RA, Fischer SM, Steele VE, Juliana MM, Desmond R, Grubbs CJ. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl-(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer. 2008;123(10):2254–2259. doi: 10.1002/ijc.23765. [DOI] [PubMed] [Google Scholar]
- 27.Fragoso MF, Prado MG, Barbosa L, Rocha NS, Barbisan LF. Inhibition of mouse urinary bladder carcinogenesis by acai fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum Nutr. 2012;67(3):235–241. doi: 10.1007/s11130-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 28.Green JE, Shibata MA, Shibata E, Moon RC, Anver MR, Kelloff G, Lubet R. 2-Difluoromethylornithine and dehydroepiandrosterone inhibit mammary tumor progression but not mammary or prostate tumor initiation in C3(1)/SV40 T/t-antigen transgenic mice. Cancer Res. 2001;61:7449–7455. [PubMed] [Google Scholar]
- 29.Perez-Stable CM, Schwartz GG, Farinas A, Finegold M, Binderup L, Howard GA, Roos BA. The Gγ/T-15 transgenic mouse model of androgen-independent prostate cancer: target cells of carcinogenesis and the effect of the vitamin D analogue EB 1089. Cancer Epidemiol Biomarkers Prev. 2002;11:555–563. [PubMed] [Google Scholar]
- 30.Baratin M, Ziol M, Romieu R, Kayibanda M, Gouilleux F, Briand P, Leroy P, Haddada H, Rénia L, Viguier M, et al. Regression of primary hepatocarcinoma in cancer-prone transgenic mice by local interferon-γ delivery is associated with macrophages recruitment and nitric oxide production. Cancer Gene Ther. 2001;8:193–202. doi: 10.1038/sj.cgt.7700285. [DOI] [PubMed] [Google Scholar]
- 31.Parangi S, O'Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, Hanahan D. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci USA. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degraff DJ, Robinson VL, Shah JB, Brandt WD, Sonpavde G, Kang Y, Liebert M, Wu XR, Taylor JA. Current preclinical models for the advancement of translational bladder cancer research. Mol Cancer Ther. 2013;12(2):121–130. doi: 10.1158/1535-7163.MCT-12-0508. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Li X, Liu S, Xu X, Tian X, Simoneau AR, Wu RU, Zi X. Flavokawain A inhibits urinary bladder carcinogenesis in the UPII-SV40T transgenic mouse bladder cancer model. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. 2012 Abstract: 619. [Google Scholar]
- 35.Johnson AM, O'Connell MJ, Messing EM, Reeder JE. Decreased bladder cancer growth in parous mice. Urology. 2008;72(3):470–473. doi: 10.1016/j.urology.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol. 2012;42(7):569–577. doi: 10.1093/jjco/hys072. [DOI] [PubMed] [Google Scholar]
- 37.Takimoto R, Wang W, Dicker DT, Rastinejad F, Lyssikatos J, el-Deiry WS. The mutant p53-conformation modifying drug, CP-31398, can induce apoptosis of human cancer cells and can stabilize wild-type p53 protein. Cancer Biol Ther. 2002;1(1):47–55. doi: 10.4161/cbt.1.1.41. [DOI] [PubMed] [Google Scholar]
- 38.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22(51):8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H, Pressey JG, Elmets CA, Kopelovich L, Athar M. Targeting wild-type and mutant p53 with small molecule CP-31398 blocks the growth of rhabdomyosarcoma by inducing reactive oxygen species-dependent apoptosis. Cancer Res. 2010;70(16):6566–6576. doi: 10.1158/0008-5472.CAN-10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roh JL, Kang SK, Minn I, Califano JA, Sidransky D, Koch WM. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47(1):8–15. doi: 10.1016/j.oraloncology.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorini C, Menegazzi M, Padroni C, Dando I, Dalla Pozza E, Gregorelli A, Costanzo C, Palmieri M, Donadelli M. Autophagy induced by p53-reactivating molecules protects pancreatic cancer cells from apoptosis. Apoptosis. 2012;18(3):337–346. doi: 10.1007/s10495-012-0790-6. [DOI] [PubMed] [Google Scholar]
- 42.Muret J, Hasmim M, Stasik I, Jalil A, Mallavialle A, Nanbakhsh A, Lacroix L, Billot K, Baud V, Thiery J, et al. Attenuation of soft-tissue sarcomas resistance to the cytotoxic action of TNF-α by restoring p53 function. PLoS One. 2012;7(6):e38808. doi: 10.1371/journal.pone.0038808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein JP, Ginsberg DA, Grossfeld GD, Chatterjee SJ, Esrig D, Dickinson MG, Groshen S, Taylor CR, Jones PA, Skinner DG, et al. Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998;90(14):1072–1079. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 44.Hong SH, Choi YS, Cho HJ, Lee JY, Hwang TK, Kim SW. Induction of apoptosis of bladder cancer cells by zinc-citrate compound. Korean J Urol. 2012;53(11):800–806. doi: 10.4111/kju.2012.53.11.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crew JP, O'Brien T, Bradburn M, Fuggle S, Bicknell R, Cranston D, Harris AL. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res. 1997;57:5281–5285. [PubMed] [Google Scholar]
- 46.Yang CC, Chu KC, Yeh WM. The expression of vascular endothelial growth factor in transitional cell carcinoma of urinary bladder is correlated with cancer progression. Urol Oncol. 2004;22:1–6. doi: 10.1016/S1078-1439(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 47.Bernardini S, Fauconnet S, Chabannes E, Henry PC, Adessi G, Bittard H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J Urol. 2001;166:1275–1279. [PubMed] [Google Scholar]
- 48.Inoue K, Slaton JW, Davis DW, Hicklin DJ, McConkey DJ, Karashima T, Radinsky R, Dinney CP. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res. 2000;6:2635–2643. [PubMed] [Google Scholar]
- 49.Zhang L, Yu D, Hu M, Xiong S, Lang A, Ellis LM, Pollock RE. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 2000;60:3655–3661. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.