Abstract
Prenatal exposure to maternal stress, anxiety, and depression can have lasting effects on infant development with consequences for risk of psychopathology. Though the impact of prenatal maternal distress has been well documented, the potential mechanisms through which maternal psychosocial variables shape development have yet to be fully elucidated. Advances in molecular biology have highlighted the role of epigenetic mechanisms in regulating gene activity, neurobiology, and behavior and the potential role of environmentally-induced epigenetic variation in linking early life exposures to long-term biobehavioral outcomes. In this review, we discuss evidence illustrating the association between maternal prenatal distress and both fetal and infant developmental trajectories and the potential role of epigenetic mechanisms in mediating these effects. Postnatal experiences may have a critical moderating influence on prenatal effects, and here we review findings illustrating prenatal-postnatal interplay and the developmental and epigenetic consequences of postnatal mother-infant interactions. The in utero environment is regulated by placental function and there is emerging evidence that the placenta is highly susceptible to maternal distress and a target of epigenetic dysregulation. Integrating studies of prenatal exposures, placental function, and postnatal maternal care with the exploration of epigenetic mechanisms may provide novel insights into the pathophysiology induced by maternal distress.
Keywords: prenatal stress, maternal depression, DNA methylation, development, epigenetic
The developmental origins of disease risk have been established through epidemiological studies in humans and illustrate the profound impact of early life adversity. During prenatal development, the fetus is particularly vulnerable to the effects of a broad range of environmental exposures, with consequences that can persist into infancy, adolescence, and adulthood. In particular, maternal distress during pregnancy, in the form of exposure to chronic or acute stressors, depression, and/or anxiety, can influence both fetal and infant behavioral and physiological outcome measures. For example, antenatal depression and anxiety symptoms predict increased behavioral reactivity and cortisol in response to novelty in infants (Kaplan, Evans & Monk, 2007; O’Connor et al., 2002a), higher resting cortisol throughout the day amongst adolescents (Van den Bergh et al., 2008), and a reduction in gray matter density in the prefrontal cortex (Pruessner et al., 2008). These human studies parallel the findings from decades of animal research indicating that prenatal stress can induce long-term neurobiological and behavioral effects with particular consequences for the hypothalamic-pituitary-adrenal (HPA) response to stress (Weinstock, 2005). The stress-vulnerability that is established in utero in response to exposure to maternal prenatal stress, depression, and anxiety may ultimately lead to increased risk of psychopathology (O’Connor et al., 2002b; Pawlby et al., 2009; Talge, Neal & Glover, 2007).
A critical question within these studies concerns the mechanism through which maternal distress shapes development. An emerging theme within developmental studies is the dynamic interactions between environmental experiences and gene activity achieved through epigenetic mechanisms – molecular modifications to gene activity that do not involve changes to the underlying DNA sequence. Though these mechanisms were once thought to be limited in their plasticity to the very early stages of embryonic development, more recent evidence indicates that epigenetic variation can be induced across the lifespan in response to a broad range of environmental exposures (Champagne, 2010; Jirtle & Skinner, 2007). These epigenetic pathways can give rise to altered gene expression levels in multiple tissues, including the brain, with consequences for the functioning and connectivity of neural circuits, which can confer risk for later life physical and psychiatric disorder. Thus, the exploration of epigenetic mechanisms in the context of studies of prenatal maternal distress may provide insight into the biological pathways linking fetal exposure to maternal distress and both acute and long-term outcomes.
In this review, we highlight evidence for the impact of human maternal distress on fetal and infant outcomes and research exploring the role of epigenetic mechanisms in linking this form of prenatal adversity to neurobiological and behavioral outcomes. Within the study of prenatal effects, a critical consideration is the continued influence of prenatal maternal adversity during the postnatal period mediated through variations in mother-infant interactions. The quality of interactions between mothers and infants during postnatal development can have a profound impact on development and these effects may also involve epigenetic pathways. Thus to compliment the findings from studies of prenatal adversity, here we describe the evidence for shifts in developmental trajectories associated with postnatal maternal care in humans and illustrate evidence from both human and animal studies that these postnatal effects are associated with epigenetic modifications. Finally, during fetal development the placenta serves as a critical interface between the mother and fetus and dynamic changes in gene regulation within this temporary endocrine structure can have significant implications for growth and development. Epigenetic regulation of gene activity within the placenta can alter functioning of this tissue and in this review we will describe the evidence suggesting that prenatal distress may act through the placenta to alter fetal development. Overall, these studies provide growing support for the hypothesis that epigenetic pathways may serve as a biological link between maternal psychosocial states during and after pregnancy and psychological, physiological, and neurobiological changes in infants that may increase risk of later life psychopathology.
Prenatal Consequences of Maternal Stress, Depression & Anxiety in Humans
There is considerable evidence demonstrating that fetal exposure to maternal psychosocial experiences contributes to the determination of children’s neurodevelopmental trajectories (Bale et al., 2010). Data generated largely over the last two decades shows that when pregnant women experience significant stress, anxiety, or depression (each of which are frequently indexed by cortisol levels), their children are at increased risk for biobehavioral characteristics conceptualized as potential precursors to psychopathology (Beydoun & Saftlas, 2008; Charil et al., 2010; Talge et al., 2007). However, the specific findings generated from these studies can vary considerably, dependent on the methodological approach used to explore the link between maternal adversity and developmental outcomes. Despite wide ranging methodological approaches, it is evident that maternal psychosocial variables assessed during pregnancy can predict developmental outcomes and that this “transmission of risk” likely occurs independent of the inheritance specific gene variants. Thus the quality of the fetal environment, shaped by maternal adversity, can lead to divergent developmental pathways.
Epidemiological studies
Nearly 40 years ago, seminal epidemiological research on birth cohorts from the Dutch Hunger Winter of 1944 showed associations between characteristics of pregnant women’s lives and offspring’s long–term cognitive and mental health development (Stein et al., 1972; Susser & Lin, 1992). Based on adult children of women who were pregnant when food intake from the war–time blockade was reduced to 500 – 1000 kcal per day, these studies showed that fetal development during the blockade was associated with a two–fold increased risk for neurodevelopmental disorders (Susser, Hoek & Brown, 1998). Two interpretations regarding the causal mechanisms of these effects have been suggested: (1) deficiency in many micro– and macronutrients, or (2), maternal distress, secondary to famine - each having neurotoxic effects on the developing brain. Several subsequent studies, each based on the Dutch Famine, found similar elevated risk for adult psychopathology in relation to fetal exposure to this maternal experience. Overall, these studies suggest that exposure to this event in the 1st trimester increases the offspring’s risk for future schizophrenia, while exposure in the 2nd or 3rd trimester is associated with elevated risk for affective disorders (Brown et al., 2000; Hoek, Brown & Susser, 1998; Susser et al., 1998; Susser, Brown & Matte, 1999). However, it is important to note that these findings are based on a population’s experience of the Dutch Famine and do not measure individual exposure.
The impact of prenatal exposures, and in particular maternal distress, on an individual level has been explored in several cohort studies. In a Danish cohort of 1.38 million births, traumatic events occurring during pregnancy were documented and used to predict risk of psychopathology amongst offspring (Khashan et al., 2008). In this cohort, death of a relative during the 1st trimester was associated with an increased risk of schizophrenia - though this did not hold for those who had a family history of mental illness (Khashan et al., 2008). There has been some evidence linking prenatal distress to autism (Beversdorf et al., 2005; Kinney et al., 2008), though a recent population based cohort study (1.49 million births) also using individual exposure assessment, found no association between maternal distress due to the death of a relative and risk for autism (Li et al., 2009); a result which could reflect some specificity of prenatal distress exposure and risk for certain disorders. These studies, based on archived health records, point to links between maternal prenatal distress and children’s risk for psychopathology.
Prospective, event–based studies of prenatal distress effects: Natural disasters
Consistent with the Dutch Famine studies, population exposure to events such as wars (Malaspina et al., 2008; Selten et al., 2003) and earthquakes (Glynn et al., 2001) as a proxy for prenatal distress have been associated with negative effects on child outcomes. One series of studies, based on the 1998 ice storm in Quebec, Canada, prospectively followed a cohort of women pregnant during the ice storm up until the children were 8 years of age (Charil et al., 2010; King & Laplante, 2005; Laplante et al., 2004; Laplante et al., 2008). Given the prospective design, this work differentiated ‘objective distress’ (what events the woman experienced related to the storm, i.e., loss of electrical power) from subjective distress (women’s self–reported psychological reaction to the ice storm); the former is randomly distributed and independent of potentially heritable personality traits while the latter is not. Children prenatally exposed to high vs. low levels of objective distress, particularly during the 1st or 2nd trimester, exhibited poorer cognitive, linguistic, and play abilities at 2, 5, and 8 years of age (Charil et al., 2010; King & Laplante, 2005; Laplante et al., 2004; Laplante et al., 2008).
Longitudinal, observational infant and child research
Spanning infancy through adolescence, numerous reports of prospective, observational studies indicate that prenatal distress exposure predicts an increased likelihood of poor emotion regulation evidenced in difficult temperament and symptoms of attention-deficit-hyperactivity disorder (ADHD). Maternal prenatal anxiety and depression across pregnancy, assessed via self–report, and higher cortisol in the 3rd trimester were found associated with fearful behavior in infants at 2 months of age (Davis et al., 2007), and, during the 3rd trimester, greater motor and cry responses to novelty at 4 months of age (Davis et al., 2004). In a prospective study of 179 women, elevated pregnancy–specific anxiety throughout gestation (in contrast to more global state/trait anxiety) predicted more negative infant temperament at 3 months, after controlling for maternal postnatal distress (Sandman et al., 2011). Amongst 4 month old infants, symptoms of maternal depression and anxiety during the 3rd trimester were associated with greater cry responses to novelty. Infants categorized by calm reactions to novel stimuli, were in contrast, 8.4 times more likely than other infants to have a mother free of psychiatric symptoms (Werner et al., 2007).
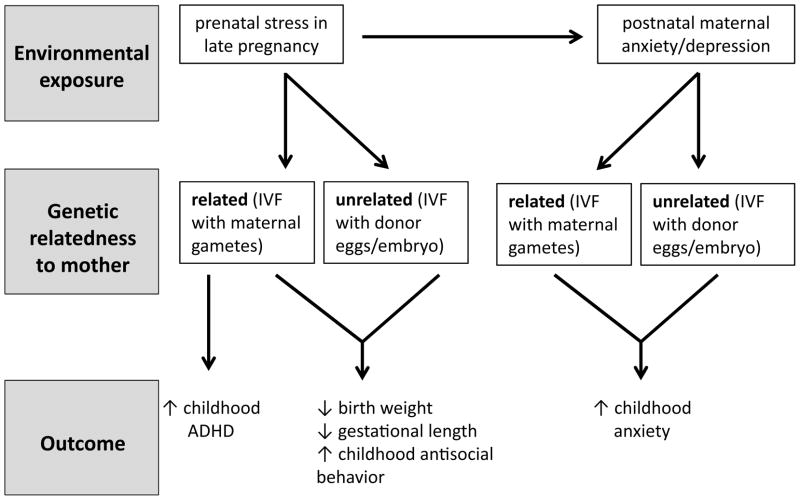
Following children to 2 years of age, higher pregnancy–specific anxiety in the 2nd trimester predicts greater likelihood of increased negative temperament (Blair et al., 2011) and fear behavior (Bergman et al., 2007). Prenatal anxiety exposure in the 3rd trimester has been associated with behavioral/emotional problems amongst 4 year olds (O’Connor et al., 2002b) and ADHD symptoms amongst 6 year olds (O’Connor et al., 2003). For 8 and 9 year old children, elevated prenatal anxiety in the 1st and 2nd trimester accounted for 22% and 15% of the variance in teacher and parent rated ADHD and externalizing symptoms, respectively (Van den Bergh & Marcoen, 2004). Follow up studies of this same cohort to 15 years of age showed that prenatal anxiety predicted poorer performance on a continuous performance task for boys (a validated instrument for detection of ADHD symptoms) (van den Bergh et al., 2006) and greater impulsivity for both boys and girls (Van den Bergh et al., 2005). Finally, depression during pregnancy has been linked to risk for depression in adolescents independent of the impact of mothers’ subsequent depressions throughout the child’s life (Pawlby et al., 2009). What emerges from these studies is a risk profile associated with prenatal exposure to maternal distress: less adaptive emotion regulation, evidenced in particular by greater reactivity to stimuli, which may be an early marker for stress–linked psychopathology, as well as ADHD symptoms. Animal studies of prenatal stress exposure (which largely control for genetic transmission) produce similar results, i.e., offspring who are more distractible and ‘anxious’ (Clarke & Schneider, 1997; Schneider, 1992; Schneider et al., 1999; Takahashi, Baker & Kalin, 1990; Takahashi, Haglin & Kalin, 1992). In an attempt to tease apart genetic from environmental influence on infant/childhood outcomes in humans, a comparison of children conceived through donor eggs (i.e. having maternal genetic dissimilarity) vs. IVF (i.e. having maternal genetic similarity) suggests that the emergence of ADHD was based on shared genetic factors whereas exposure to prenatal distress could increase the risk for antisocial behavior in children (Rice et al., 2009) (see Figure 1).
Figure 1. Prenatal cross-fostering design in the study of the effects of human maternal distress.
Though differentiating the impact of genetic and environmental factors in predicting childhood outcomes associated with maternal distress is challenging in humans, studies examining mothers who have used in vitro fertilization (IVF) may provide a useful experimental design. Rice et al. (2009) examined birth outcomes and childhood psychopathology amongst infants who were genetically related (IVF using mother’s own gametes) or genetically unrelated (IVF with donor eggs/embryo) to their mother. Prenatal stress in late pregnancy was found to predict reduced birth weight and gestational length and a higher incidence of childhood antisocial behavior regardless of genetic relatedness to the mother. Prenatal stress was likewise found to predict increased childhood anxiety regardless of maternal genetic relatedness, though this effect was found to be mediated by prenatal effects on postnatal anxiety/depression. In contrast, increased risk of ADHD was observed only in response to prenatal stress when there was genetic relatedness between mother and infant – suggesting a significant genetic contribution to this outcome.
Importantly, with some exceptions (Bergman et al., 2007; Davis et al., 2004; Pawlby et al., 2009; Van den Bergh & Marcoen, 2004; Van den Bergh et al., 2005; van den Bergh et al., 2006; Werner et al., 2007), these studies rely on maternal reports for the predictive variable (prenatal distress) and dependent variable (child behavior). Although various researchers argue that parents are the best source of description of their children (Rettew & McKee, 2005), others have questioned whether maternal reports of infant behavior are sufficiently unbiased, or instead, reflective of, and thus highly correlated with, maternal distress, which often is maintained from the pregnancy to the postnatal period (DiPietro et al., 1996a; Pesonen et al., 2005; Zeanah et al., 1985). Moreover, although these studies control for maternal postnatal distress proximal to the child’s birth and still identify prenatal effects, the question of whether the prenatal maternal distress is a marker for compromised maternal care throughout development, which itself is the agent of influence, is only rarely considered (see (Bergman et al., 2010; Grant et al., 2009; Kaplan et al., 2007).
Infant and child biological outcomes
The biological impact of prenatal exposure to maternal distress has also been explored and dysregulation of HPA function has been implicated as a primary outcome. At birth, infants exposed to higher levels of maternal plasma cortisol in the 2nd and 3rd trimester had a larger cortisol response to a heal–stick procedure (Davis et al., 2011). In a study of 4–month old infants, exposure to 3rd trimester prenatal depression and anxiety, based on psychiatric interviews, predicted higher cortisol levels upon entering a laboratory setting (Kaplan et al., 2007). Amongst 7 month old infants exposed prenatally to maternal anxiety, heightened cortisol activation was observed even after the experimental procedures were completed (Grant et al., 2009). A high–flattened, day–time cortisol profile (Van den Bergh et al., 2008) and higher cortisol levels upon awakening (O’Connor et al., 2005) have been observed in pre–adolescents and adolescents related to prenatal distress exposure. Neurobiological correlates of exposure to prenatal maternal distress have likewise been identified. In a prospective report, children exposed to maternal prenatal anxiety in the 2nd trimester were found to have reduced gray matter volumes in the prefrontal cortex, premotor cortex, the medial temporal lobe, and other areas involved in learning and memory (Buss et al., 2010). Though these studies of the biological impact of maternal prenatal stress provide promising insights into the pathophysiology of these early life exposures, the issues of the moderating or mediating effects of postnatal factors are typically not explored within these research approaches.
Fetal neurobehavior related to maternal distress
Assessment of fetal neurobehavioral outcomes within the study of prenatal exposures provides an opportunity to examine prenatal effects prior to the potential influence of the postnatal period. During baseline assessments, heightened maternal stress was found to be associated with reduced fetal heart rate variability (DiPietro et al., 1996b) as well as increased fetal movement (DiPietro et al., 2002). Maternal prenatal depression was also associated with increased motor activity (Dieter et al., 2001; Emory & Dieter, 2006). Higher levels of maternal cortisol have been positively associated with greater amplitude in fetal movement, as well as the amount of time spent moving (DiPietro et al., 2009). In contrast, women who endorsed greater trait anxiety had fetuses who spent more time in quiet sleep and showed less gross body movement while in active sleep (Groome et al., 1995). Differences in fetal assessment may account for this seemingly conflicting finding (i.e. ultrasound vs. Doppler actigraphy).
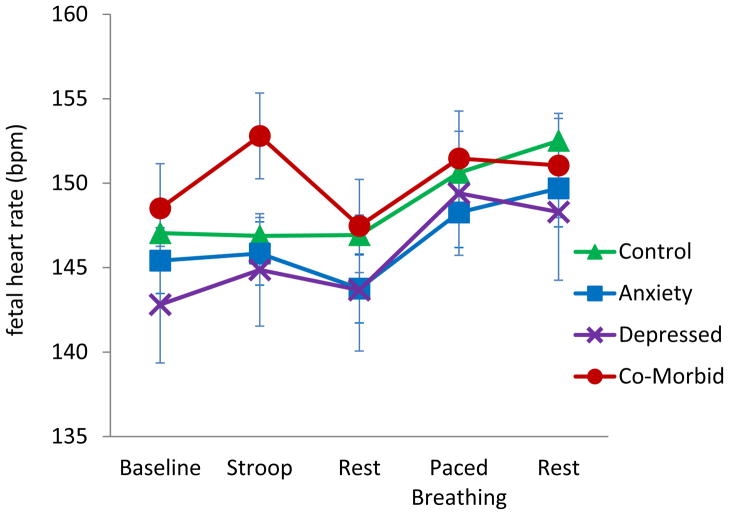
Additional approaches to fetal assessments involve exposing mothers to psychological challenge (i.e. using the Stroop task) or directly stimulating the fetus via a vibroacoustic probe. It has been shown that while fetuses of non–anxious and psychiatrically healthy women do not show a heart rate change during the Stroop task, fetuses of anxious and depressed women have a significant heart rate increase (Monk et al., 2010; Monk et al., 2000; Monk et al., 2004) (see Figure 2). In the most recent results, maternal cortisol levels were marginally positively associated with fetal heart rate activity (Monk et al., 2010). When stimulated directly with a vibroacoustic probe, fetuses of women with elevated levels of corticotropin releasing hormone (CRH) (which, during pregnancy, is predominantly of placental origin, and can be increased by maternal cortisol) failed to respond to a novel stimulus in a habituation/dishabituation paradigm. In addition, higher CRH has been related to higher overall fetal heart rate reactivity to the stimulus (Sandman et al., 1999). Taken together, these findings suggest a similar biobehavioral profile to that of the child exposed to maternal prenatal distress: greater activity and reactivity, and a possible decrement in a rudimentary form of discrimination (i.e. during the habituation studies). Thus these approaches may provide a valuable new strategy for exploring the early origins of maternal adversity-induced risk of psychopathology.
Figure 2. Fetal heart rate during laboratory protocol by women’s diagnostic status.
In a sample of 3rd trimester pregnant women (Monk et al., 2010), fetuses of women with diagnosed depression and anxiety showed greater heart rate reactivity during Stroop compared to those in the other groups. There was a trend for maternal salivary cortisol to be positively associated with fetal heart rate.
Effects of Stress, Depression and Anxiety on Mother-Infant Interactions During the Postpartum Period
Exposure to maternal distress occurring during fetal development is highly correlated with continued exposure during postnatal development; highlighting the importance of considering postnatal factors within the study of prenatal effects. Maternal prenatal depression is strongly associated with risk for postpartum depression (PPD) and a third of pregnant women with depressive symptomatology have been reported to also experience depression during the postpartum period (Beck, 1996; Gotlib et al., 1989; O’Hara & Swain, 1996). Distress during pregnancy is also associated with increased risk for PPD. For example, distress-related risk factors in the prenatal period that predict PPD symptoms include: anxiety (Beck, 1996; O’Hara & Swain, 1996; Sutter-Dallay et al., 2004), recent intimate partner violence (Valentine et al., 2011), martial adjustment (O’Hara & Swain, 1996), overall low social support (O’Hara & Swain, 1996), inadequate support from baby’s father (O’Hara & Swain, 1996), and low socioeconomic status (Goyal, Gay & Lee, 2010). When pregnant women were divided into high and low risk groups based on depression-related risk factors, 25% of those in the high risk group vs. 6% in the low risk group developed postpartum depression, and depressive symptomatology during pregnancy independently predicted PPD (Verkerk et al., 2003). It seems that when a child experiences an altered in utero environment linked to maternal mood, there likely is continuity to the postpartum environment in the form of maternal PPD or depressive symptoms. This continuity has significant implications for the quality of the postnatal environment, particularly the quality of maternal care.
Healthy mother-infant interactions are critical to the development of healthy children and adults. Specifically, the quality of maternal caregiving behavior (MCB) during infancy has been linked to outcomes such as emotion regulation, social adjustment, stress reactivity, cognitive abilities, and risk for psychopathology. Maternal adversity, in the form of psychosocial distress, negatively impacts MCB, and thus results in poor outcomes for children and adolescents. Maternal depression during infancy (or since birth) predicts higher waking cortisol levels during adolescence (Murray et al., 2010), larger amygdala (but not hippocampal) volume and higher cortisol level at 10 years (Lupien et al., 2011), greater childhood internalizing and total behavior problems (Bagner et al., 2010), and greater childhood and adolescent depressive symptoms (Bureau, Easterbrooks & Lyons-Ruth, 2009). Further, adverse outcomes can emerge as early as infancy (Kikkert, Middelburg & Hadders-Algra, 2010). Maternal trait anxiety, but not paternal trait anxiety, has been related to less optimal neurological development of the infant as measured by the Towen Infant Neurological Examination at 10 months of age. Even typical variation in MCB predicts differences in infant emotion regulation (Hane & Fox, 2006). Variation in the quality of MCB, assessed in terms of maternal sensitivity and responsiveness during multiple mother-infant interactions, was found to predict infant stress reactivity indexed by right frontal EEG asymmetry, fear reactivity and positive sociability with a stranger. Relative to high MCB, low MCB was associated with greater right frontal EEG asymmetry and fear reactivity and less positive sociability, and these findings have been extended to stress reactivity outcomes at 2 and 3 years of age (Hane et al., 2010).
Maternal distress and MCB
Assessment of the quality of postnatal mother-infant interactions with a focus on the mother typically measure variables such as maternal sensitivity and responsivity occurring during dyadic interactions such as free play, diaper changing, and feeding. Postnatal maternal depression has been shown to reduce maternal responsivity to infants 6 months of age (Forman et al., 2007) and increase maternal hostility-intrusiveness to infants 18 months of age (Bureau et al., 2009). Mothers with anxiety show hyperarousal during free play with infants 6 months of age (Kaitz et al., 2010), less sensitivity and reduced emotional tone during free play with infants 10–14 months of age (Nicol-Harper, Harvey & Stein, 2007), less maternal sensitivity during free play with infants 9 months of age (Feldman et al., 2009), and exaggerated behavior during free play and teaching with infants 6 months of age (Kaitz et al., 2010). Finally, maternal stress has been associated with less maternal sensitivity to infant cues in a vocal elicitation paradigm (Crnic et al., 1984).
Maternal distress and infant behavior
Maternal distress can also lead to altered behavioral reactions to mothers. One assessment approach is the use of the Still-Face Paradigm, during which mothers are asked to become unresponsive to their infants for a brief period of time, and infant reactivity is measured. In a study that compared responses to the Still-Face Paradigm in 5 month-old infants of depressed vs. non–depressed mothers, infants of depressed mothers were found to engage in a self-soothing strategy whereas infants of non–depressed mothers engaged in an attention strategy (Manian & Bornstein, 2009). This finding suggests that infants use varying emotion regulation strategies as a function of maternal depression, and is consistent with earlier work showing that infants of depressed mothers first attempt to use external regulation, and then engage in self-directed regulatory behavior (Gianino & Tronick, 1985; Tronick & Gianino, 1986). Infants have also been observed to display negative affect when interacting with mothers who have depression or with healthy mothers who simulate depression (Gianino & Tronick, 1985; Tronick & Gianino, 1986). Depressed mood in healthy women reduces infant responsivity and infant positive responses (Zekoski, O’Hara & Wills, 1987); infants look less at depressed mothers and appear distressed (Cohn et al., 1986; Tronick, 1989); and infants of depressed mothers show low social engagement (Feldman et al., 2009). Similarly, maternal anxiety was found to be associated with reduced social engagement amongst infants (Feldman et al., 2009), though less negative affect in the Still Face Paradigm was observed (Kaitz et al., 2010), and maternal stress was found to be associated with less infant responsivity (Crnic et al., 1984). Infant attachment, as measured by the Strange Situation Task, also varies significantly in association with maternal distress. Infants of depressed mothers are more likely to show insecure attachment (Martins & Gaffan, 2000; McMahon et al., 2006; Teti et al., 1995), and a disorganized attachment style has been linked to the chronicity of maternal depression (Teti et al., 1995). Maternal trauma has likewise been found to predict insecure infant attachment (Lyons-Ruth & Block, 1996).
Maternal distress and mother–child interactions
The coordination of behavior between mother and infant may also be sensitive to the effects of maternal distress. For example, dyadic gaze rhythm can be measured to assess the level of predictability and expectancy between a mother and infant when they look at and away from one another’s faces. In a study that compared mother-infant dyads with either low or high distressed mothers, maternal distress related to less efficient gaze rhythm, suggesting that establishing predictable patterns of eye gaze may be more difficult for dyads of infants and high distress mothers (Beebe et al., 2008). Predictability of behavioral responses can also be assessed through the observed contingency of behavior between dyads (interactive contingency) or within an individual (self-contingency). Anxious mothers have been found to exhibit heightened (vigilant) interactive and self-contingencies in some modalities and lowered (withdrawn) interactive and self-contingencies in others, while infants of anxious mothers exhibited lowered self-contingency (Beebe et al., 2011). Anxious mothers were found to engage in heightened visual monitoring, but lowered emotional coordination with their infants. Taken together, these studies suggest that maternal adversity may lead to aberrant forms of social coordination/communication between mothers and infants.
Potential underlying mechanisms
It has been suggested that the primary parenting demands during infancy are 1) to establish a secure attachment with the infant and 2) to foster development of emotion regulation in the infant (Goodman & Gotlib, 1999). In the case of distressed mothers, it appears likely that there is reduced attainment of these goals. Infants of distressed mothers exhibit insecure attachment styles (Martins & Gaffan, 2000; McMahon et al., 2006; Teti et al., 1995) and increased fear reactivity (Feldman et al., 2009) whereas in healthy participants, the quality of MCB has been linked to reduced fear reactivity (Hane & Fox, 2006). It may also be the case that exposure to maternal depression-associated negative cognition, affect and behavior impacts the infant through social learning or modeling (Goodman & Gotlib, 1999). Indeed, infants of depressed mothers tend to show more negative affect, flatter affect and less positive responsivity (Dawson et al., 1992; Gianino & Tronick, 1985; Tronick, 1989; Zekoski et al., 1987). Infants of depressed mothers may also experience inadequate arousal and stimulation (Field, 1998). Depressed mothers have been found to be less responsive to verbal utterances (Bettes, 1989) and to provide less tactile stimulation (gentle touching, stroking; (Field, 2002)). Tactile experience during the postnatal period has been demonstrated to influence pain sensitivity, affect, and growth in neonates (Field, 2010) and the reciprocal tactile stimulation between mother and infant may contribute to increased maternal responsiveness and infant attachment (Bystrova et al., 2009; Field, 2010). Thus, mood/stress-induced reductions in this form of MCB may serve as a critical mechanism for shifting psychological and physical development in infants.
Epigenetic Perspectives on the Impact of Prenatal Adversity
Studies of the impact of prenatal maternal distress suggest that this form of early life adversity can lead to neurobiological, behavioral, and psychological consequences for infants. Moreover, this distress can also lead to altered mother-infant interactions during the postpartum period which in themselves have been demonstrated to shift developmental trajectories. Thus, it is evident that development is a dynamic process during which shifts in the experience of the fetus and infant can have profound and long-term consequences. In the case of maternal distress, the psychosocial characteristics of the mother can induce these effects – raising the question of how these effects are achieved. An evolving approach that has been applied to address this question involves exploration of the biological mechanisms through which environmental exposures come to shape the activity of genes within the developing organism. This epigenetic perspective has been a significant breakthrough in linking the psychological and physiological experiences of an individual to mechanistic pathways within cells that either enhance or reduce gene expression with consequences for multiple biological and behavioral outcomes.
Epigenetic mechanisms and gene regulation
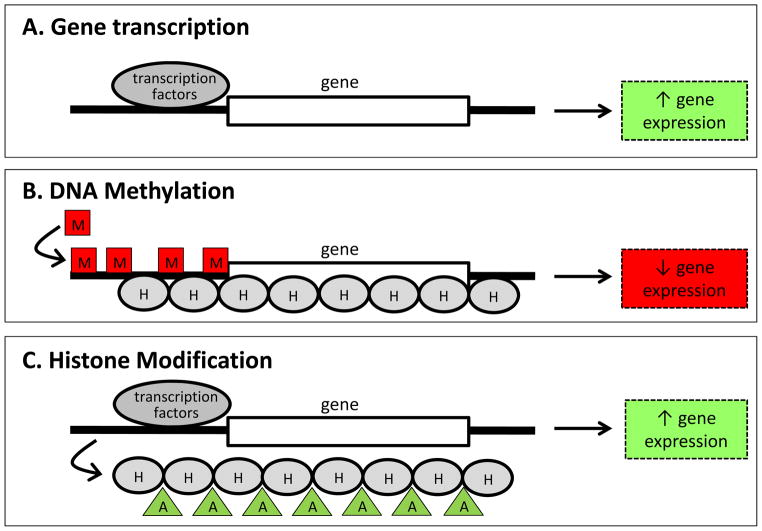
The term “epigenetic” is attributed to the developmental biologist Conrad Waddington, who in the 1940’s and 50’s posited that interactions between genes and their products lead to the diverging characteristics of cells in an organism (Jablonka & Lamb, 2002). This epigenesis of cellular “phenotypes” emphasized the dynamic process of development and the stable maintenance of characteristics once they had emerged. Advances in molecular biology have led to the identification of specific molecular mechanisms through which this process is achieved and thus more modern applications of the term “epigenetic” is in reference to those mechanisms. Gene regulation is a complex process and is dependent on a cascade of protein-protein, protein-DNA, and enzymatic interactions which ultimately determine the accessibility of DNA to the process of gene transcription (Turner, 2001). DNA methylation and post-translational histone modifications (Peterson & Laniel, 2004; Razin, 1998; Umlauf, Goto & Feil, 2004) are two mechanisms through which this accessibility can be altered (see Figure 3) and have been explored within the context of environmentally induced changes in gene activity that may be relevant for our understanding of the pathways through which prenatal adversity can lead to altered infant and childhood outcomes.
Figure 3. Schematic illustrating epigenetic regulation of gene expression.
A. Gene expression (production of mRNA from DNA) is dependent on the binding of transcription factors to the region upstream of the coding region of the gene. B. DNA methylation involves the addition of methyl chemical groups (M) to the DNA. This modification reduces accessibility of the DNA to transcription factors, increases the interactions between histone proteins (H) and DNA and generally leads to decreased gene expression. C. Modification of histone proteins, such as addition of acetyl chemicals (A), can reduce the interactions between histones and DNA, increase the access of DNA to transcription factors and lead to increased gene expression.
DNA methylation & histone modifications
Within DNA, cytosine nucleotides can undergo the process of methylation – the addition of a methyl chemical group to the cytosine. This modification is not a mutation and is potentially reversible. When cytosines become methylated there is generally less accessibility to the DNA (though the location of the methylated cytosine within the gene sequence will be an important predictor of this accessibility) and consequently DNA methylation is thought to be a process leading to gene silencing (Razin, 1998). Methylated cytosines can also attract methyl-binding proteins which cluster around the DNA and attract enzymes which can shift the state of the histone proteins around which the DNA is wrapped – a process which can further reduce access to the DNA (Fan & Hutnick, 2005). The addition of methyl groups to DNA is accomplished via a class of enzymes – DNA methyltransferases (e.g. DNMT1 and DNMT3a/b) (Turek-Plewa & Jagodzinski, 2005). Disruption to these enzymes, using targeted gene deletion techniques, results in genome-wide hypomethylation, impairments in growth, and embryonic or postnatal lethality (Li, Bestor & Jaenisch, 1992; Okano et al., 1999) – highlighting the critical role of DNA methylation in development. In particular, DNA methylation is a process that maintains cellular differentiation. As cells become more specialized, taking on the characteristics of a specific cell type (i.e. muscle cells, neurons, skin cells), DNA methylation patterns must be reliably copied during the process of cell division or the unique characteristics of the cell will be lost when the cell divides. Thus, DNA methylation patterns are heritable during cell division and also potentially very stable, generating cellular diversity amongst genetically identical cells (Jones & Taylor, 1980).
Modification to histone proteins is another critical epigenetic process which can both increase and decrease accessibility to DNA. In order to compact DNA within the cell nucleus, DNA is wrapped around a core of histone proteins (Turner, 2001). The “tails” of these histone proteins can undergo multiple post-translational modifications which alter the dynamic interactions between these proteins and the DNA. For example, histone acetylation is a process whereby an acetyl chemical is added to the histone protein tail and is typically associated with increased gene expression due to a looser interaction between the histones and the DNA (Peterson & Laniel, 2004; Umlauf et al., 2004). Histone methylation can result in either enhanced or reduced gene expression, dependent on the location within the histone tail of the methyl group (Barski et al., 2007; Koch et al., 2007). There are many chemicals which can be added or removed from the histone tails resulting in histone phosphorylation, methylation, and ubiquitination and multiple other chemical modifications. Collectively, these modifications result in a “histone code” – a very dynamic and complex strategy for reducing or enhancing gene expression (Jenuwein & Allis, 2001). As is the case for DNA methylation, there are specific enzymes that facilitate these epigenetic modifications (Legube & Trouche, 2003) and disruption to these enzymes can result in significant impairments in development. For example, targeted deletion of a histone acetyltransferase gene can be lethal and induce deficits in neural tube closing (Xu et al., 2000; Yao et al., 1998) and mutation of the histone methyltransferase EHMT1 has been found to be associated with severe developmental delay in humans (Kleefstra et al., 2006).
Epigenetic Effects of Prenatal Adversity
Though epigenetic modifications (particularly DNA methylation) were once thought to be limited in plasticity to early embryonic development, it is becoming evident that both DNA methylation and histone modifications are dynamic and changeable across the lifespan (Champagne, 2010). Given this plasticity, epigenetic variation may be induced by experiences occurring during prenatal and postnatal development and thus be a mechanistic link between maternal adversity and infant outcomes. In humans, the epidemiological studies involving the Dutch Hunger Winter birth cohort have explored whether DNA methylation is altered as a function of this early life exposure. Analysis of whole blood samples from exposed vs. non-exposed sibling pairs has indicated that periconceptual exposure to conditions of famine/stress are associated with differential methylation of several genes amongst adults (Heijmans et al., 2008; Tobi et al., 2009). In particular, decreased DNA methylation was found within the insulin-like growth factor II (IGF2) gene amongst exposed compared to non-exposed siblings (Heijmans et al., 2008). IGF2 plays a significant role in growth and development (Smith, Garfield & Ward, 2006) and is likely a key factor in regulating many of the outcomes associated with prenatal adversity. Within this cohort, several other genes, including INSIGF, IL10, LEP, ABCA1, GNASAS and MEG3 were found to have altered DNA methylation levels (Tobi et al., 2009), suggesting widespread epigenetic effects of this early life exposure. These effects appear to be timing- specific, with differential DNA methylation most evident when exposure occurred during the periconceptual period rather than in late gestation.
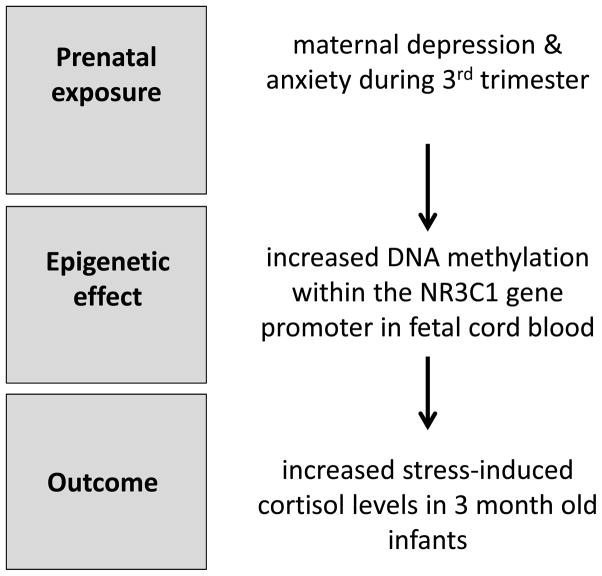
Though sibling studies within the Dutch Hunger Winter cohort did not find significant effects on genes associated primarily with the HPA axis, such as CRH and the glucocorticoid receptor (NR3C1), studies of prenatal maternal stress and depression have provided evidence for epigenetic dysregulation within HPA pathways. Analysis of cord blood samples from infants born to mothers with elevated ratings of depression (using the Hamilton Depression Scale) during the 3rd trimester of pregnancy has indicated elevated levels of DNA methylation within the NR3C1 gene (Oberlander et al., 2008). These epigenetic effects were observed in fetal but not maternal blood samples. At 3 months of age, infant HPA reactivity was assessed using a habituation information-processing task during which salivary cortisol levels were measured. Levels of NR3C1 DNA methylation in fetal cord blood were found to predict infant cortisol response to stress, suggesting a functional consequence of this epigenetic variation (see Figure 4). The persistence of these prenatal effects on NR3C1 DNA methylation beyond infancy has also been demonstrated. Children and adolescents (age 10–19 years) born to women who had experienced stress in the form of intimate partner violence during pregnancy were found to have elevated NR3C1 DNA methylation levels in whole blood samples (Radtke et al., 2011). As was observed in the case of prenatal maternal depression, these epigenetic effects were present in offspring blood samples, but not evident in maternal blood samples. Methylation within the SLC6A4 gene, which encodes the serotonin transporter, has been found to be reduced in cord blood samples in response to increased maternal depressed mood during the 2nd trimester, whereas no effects on BDNF (brain-derived neurotrophic factor) were observed (Devlin et al., 2010).
Figure 4. Impact of prenatal maternal depression/anxiety on DNA methylation and infant stress response.
Oberlander et al. (2008) found that maternal depression and anxiety assessed during pregnancy (3rd trimester) was significantly correlated with the degree of DNA methylation within the glucocorticoid receptor gene (NR3C1) measured in fetal cord blood. At 3 months of age, this epigenetic effect was found to predict increased infant cortisol response to stress.
Though there is increasing exploration of epigenetic effects in human cohort studies, interpretation of the meaning of these epigenetic changes in blood is unclear. However, using animal models, it has been possible to demonstrate that under controlled laboratory conditions exposure to prenatal stress can induce epigenetic variation within brain regions that are involved in the behavioral characteristics associated with this form of prenatal adversity. In mice, chronic variable stress exposure during the first gestational week (1st trimester) results in increased corticosterone response to stress and increased depressive like behavior (Mueller & Bale, 2008). In adult male offspring, prenatal stress is associated with decreased DNA methylation of the CRH gene and increased methylation of the NR3C1 gene in hypothalamic tissue of adult male offspring. Moreover, there is altered expression of these genes (increased CRH expression and decreased GR expression) in response to prenatal stress which likely induce increased HPA reactivity in offspring. Thus animal studies can be used to provide support for the validity of target genes identified in human studies using peripheral blood samples and explore the mechanistic pathways through which prenatal adversity alters brain and behavior.
Epigenetic Effects During Postnatal Development
The epigenetic impact of postnatal mother-infant interactions has also been explored in both humans and animals and, as has been previously described, may be an important consideration in studies of prenatal adversity. Deprivation of parental care, such as that observed in institutionalized infants, has been found to have broad epigenetic consequences. Amongst institution reared (since birth) children aged 7–10 years, analysis of blood samples indicates increased DNA methylation throughout the genome when compared to age-matched children reared by their biological parents (Naumova et al., 2011). Amongst the differentially methylated genes are those implicated in brain development, including genes within vasopressinergic, serotonergic, glutamatergic, and GABAergic pathways. The epigenetic effects of childhood abuse have likewise been observed in human brain tissue (McGowan et al., 2009). Analysis of gene expression and DNA methylation levels in postmortem hippocampal tissue from adults with or without a documented history of childhood abuse has indicated decreased expression of the NR3C1 gene and increased DNA methylation within the regulatory region of the NR3C1 gene associated with a history of childhood abuse. Decreased levels of glucocorticoid receptors within the hippocampus can lead to heightened HPA response to stress and, in particular, a decreased capacity to down-regulate the stress response. As such, the epigenetic effects on NR3C1 induced by postnatal adversity may account for the increased risk of psychopathology and poorer emotional regulation amongst individuals with a history of abuse.
Variation in maternal care in rodents
Though there is certainly emerging data from human cohorts indicating and impact of postnatal experiences on epigenetic pathways, animal studies have provided a significant basis for this exploration. In particular, studies in laboratory rodents illustrate the neurobiological and behavioral consequences of variations in maternal care within the normal range and the role of epigenetic mechanisms in the long-term consequences of maternal care on offspring outcomes (Meaney, 2001; Meaney & Szyf, 2005). Rodents provide tactile stimulation to offspring through licking/grooming of pups (LG) and this form of maternal care has been found to influence HPA reactivity, cognition, and reproductive behavior of offspring (Caldji et al., 1998; Cameron et al., 2008a; Champagne et al., 2008; Champagne et al., 2003a; Liu et al., 2000; Liu et al., 1997). Gene expression within the hippocampus and hypothalamus has been found to be altered in adult offspring that have experienced low vs. high levels of LG during the postnatal period and may account for the behavioral and physiological effects of LG. Males that have experienced low vs. high LG are observed to have reduced levels of hippocampal glucocorticoid receptors within the hippocampus which likely accounts for the increased HPA reactivity of these offspring (Liu et al., 1997). Analysis of DNA methylation within the NR3C1 gene indicates that the experience of low levels of LG is associated with increased DNA methylation at several sites within this gene (Weaver et al., 2004). These adult patterns of DNA methylation are not observed prenatally or at the time of birth but instead emerge during the first postnatal week. Histone acetylation (H3K9) is also reduced within the NR3C1 gene in low LG compared to high LG offspring. Interestingly, these early life effects on NR3C1 can be modified in adulthood through pharmacological manipulations which increase histone acetylation or increase the potential for DNA methylation – suggesting a high degree of plasticity within these epigenetic pathways (Weaver et al., 2004; Weaver et al., 2005). Maternal LG also leads to altered levels of the DNA methyltransferase DNMT1 in the hippocampus, such that male offspring that receive low levels of LG have elevated levels of DNMT1 mRNA (Zhang et al., 2010). In addition to hippocampal glucocorticoid receptors, maternal LG has been found to alter expression of the enzyme glutamic acid decarboxylase (GAD1) gene and analysis of DNA methylation within the GAD1 gene indicates that among offspring that experience low levels of postnatal LG there is heighted DNA methylation and reduced histone acetylation of this gene that can be observed to persist into adulthood (Zhang et al., 2010). Genome wide analysis of gene expression profiles in the hippocampus of male offspring that have received low vs. high levels of maternal care indicate that over 900 genes are differentially expressed in the hippocampus as a function of maternal LG, and that overall there is increased gene expression amongst adult offspring of high LG compared to low LG dams (Weaver, Meaney & Szyf, 2006). These findings suggest widespread epigenetic consequences of maternal care.
The effects of maternal LG on female offspring have primarily focused on reproductive outcomes such as sexual and maternal behavior (Cameron et al., 2008a; Cameron, Fish & Meaney, 2008b; Champagne et al., 2003a); though it should be noted that females display similar stress responsivity profiles to their male siblings as a function of maternal LG (i.e. low LG associated with behavioral inhibition to novelty) (Champagne & Meaney, 2007). Amongst female offspring that received low levels of LG during the postnatal period there is decreased mRNA and protein levels of estrogen receptor alpha (ERα) in the MPOA, which may account for the reduced estrogen sensitivity and maternal care evident in these offspring (Champagne et al., 2001; Champagne et al., 2003b). Analysis of levels of DNA methylation within the ERα gene (ESR1) in MPOA tissue indicates that offspring that experience low LG have elevated ESR1 DNA methylation in adulthood (Champagne et al., 2006). This persistent epigenetic effect may account for the transmission of maternal LG behavior across generations (Champagne, 2008) and illustrates the profound effect of early rearing experiences on the neurobiological circuits involved in reproductive behavior. Experience-driven epigenetic effects on ESR1 may also lead to sexual dimorphism. Within the MPOA there are sex differences in ESR1 DNA methylation such that males have higher levels of ESR1 DNA methylation in this brain region compared to females (Kurian, Olesen & Auger, 2010). However, if females are provided with additional tactile stimulation during postnatal days 5–7, there are increases in ESR1 DNA methylation, such that females become indistinguishable from males. Previous studies have indicated that male pups receive elevated levels of maternal LG compared to females (Moore, 1984; Moore & Morelli, 1979) and thus it may be the case that differential levels of LG can trigger epigenetic pathways which enhance sex differences in the brain. Moreover, frequency of LG may be an important consideration in studies of prenatal adversity as this form of maternal care has been found to be reduced among females that experienced stress during gestation (Champagne & Meaney, 2006).
Epigenetic effects of neglect and abuse in animal models
Maternal separation studies in laboratory primates and rodents have been used as a strategy to model early maternal deprivation/neglect and have demonstrated the impact of this form of postnatal adversity on neurobiological and behavioral outcomes (Lehmann et al., 2002; Spinelli et al., 2009; Suomi et al., 1975; Suomi, Harlow & Kimball, 1971). Rhesus macaques that are reared under conditions of maternal separation and possess the risk allele of the SLC6A4 gene (i.e. the short version which has been associated with increased anxiety-like behavior) were found to have increased DNA methylation of SLC6A4 gene in blood samples (Kinnally et al., 2010). These findings highlight the interaction between genetic variation and epigenetic effects in response to early life adversity that are likely an important consideration in human studies. In mice, maternal separation occurring daily during the postnatal period leads to increased stress sensitivity and an up-regulation in the expression of hypothalamic vasopressin (AVP) that can be observed in adulthood (Murgatroyd et al., 2009). DNA methylation levels within key regulatory regions of the AVP gene have been found to vary as a function of exposure to postnatal maternal separation, such that maternally separated offspring have decreased hypothalamic AVP DNA methylation compared to normally reared offspring. This separation induced hypomethylation of AVP was also associated with reduced levels of binding of MeCP2 (a methyl binding protein) to the AVP gene (Murgatroyd et al., 2009).
Maternal abuse of offspring can also be modeled in laboratory rodents and has illustrated the role of epigenetic modifications in maintaining the effects of abuse across the lifespan. In laboratory rats, aggressive behavior toward pups (such as stepping on pups, aggressive grooming, and transporting of pups by a limb) can be induced by reducing the nesting materials provided to lactating dams during the postnatal period (Roth & Sullivan, 2005). This manipulation also reduces the frequency of nurturing behaviors such as maternal LG. When pups are exposed to caregivers (non-biological mothers) that are provided with limited nesting materials and placed in an unfamiliar environment, there is increased exposure of pups to aggressive encounters (Roth et al., 2009). Within the prefrontal cortex of abuse-exposed offspring, there is a down-regulation of the expression of BDNF. Analysis of DNA methylation within the regulatory regions of the BDNF gene indicates that amongst non-abused offspring there are very low levels of DNA methylation whereas abused offspring have elevated levels of this repressive epigenetic modification (Roth et al., 2009). These epigenetic effects of abuse are also observed in the offspring of abused vs. non-abused females, possibly suggesting an epigenetic basis to the multigenerational transmission of abuse and its consequences for neurobiological and behavioral functioning.
Gene Regulation in the Placenta and Fetal Development
The mechanistic pathways linking prenatal adversity to infant development are likely to be very complex, varying dependent on the timing of exposure, the type of exposure, and underlying genetic and environmental risk factors. However, functioning of the placenta will likely be of critical importance within these diverse pathways. This temporary endocrine structure regulates the transfer of nutrients to the developing fetus, buffers the fetus from toxins and maternal glucocorticoids, and by altering maternal hormone levels can also influence maternal mood and the priming of the maternal brain with consequences for the quality of postnatal mother-infant interactions (Cottrell & Seckl, 2009; Desforges & Sibley, 2010; Levy, 1981; Mann & Bridges, 2001) (see Figure 5). Thus gene regulation within the placenta can have functional consequences for both fetal and infant development. Gene transcription within the placenta varies during the course of gestation and it is likely that epigenetic mechanisms, such as variation in the levels of placental DNA methyltransferase levels, account for the temporal shifts in placental gene expression profiles (Novakovic et al., 2010). Altered gene expression within the placenta has also been associated with adverse birth outcomes, such as intrauterine growth retardation (Angiolini et al., 2006; Lee et al., 2010; McCarthy et al., 2007; McMinn et al., 2006; Sitras et al., 2009). Many of the genes identified as differentially expressed in these studies are imprinted genes (McMinn et al., 2006; Tycko, 2006) – genes that are subject to epigenetic silencing mechanisms to achieve parent-of-origin expression patterns – and there is emerging evidence for altered DNA methylation levels genome wide and within specific target genes that may account for this altered transcriptional profile (Lambertini et al., 2011; Nelissen et al., 2011; Tabano et al., 2010).
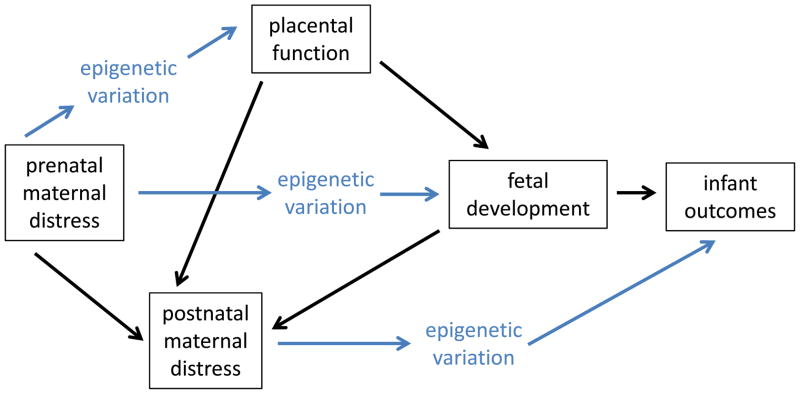
Figure 5. Pathways linking prenatal maternal distress to infant outcomes.
There are both direct and indirect pathways through which epigenetic modifications may shape infant outcomes associated with prenatal maternal distress. Within these pathways, postnatal maternal distress and placental function are critical factors.
Interestingly, there is significant overlap in the genes identified as differentially methylated in the placenta in studies of fetal growth retardation and those genes identified as differentially methylated in blood samples from famine exposed individuals in the Dutch Famine cohort (e.g. IGF2, GNAS, LEP, MEG3) (Heijmans et al., 2008; McMinn et al., 2006; Tobi et al., 2009). Thus, it is likely the case that maternal distress can lead to altered gene expression and epigenetic profiles in placental tissues. In mice, chronic variable stress during the 1st trimester is associated with altered placental gene expression, including altered mRNA levels of the DNA methyltransferase DNMT1 (Mueller & Bale, 2008). One gene target of particular importance in linking maternal distress to fetal outcomes (particularly the HPA reactivity that is often observed) is 11β–HSD2 encoding the enzyme 11β-hydroxysteroid dehydrogenase 2. 11β–HSD2 is highly expressed in the placenta and functions to inactivate glucocorticoids (Cottrell & Seckl, 2009). Thus, the degree of expression of 11β–HSD2 in the placenta is predicted to influence the exposure of the fetus to circulating maternal stress hormones. In humans, heightened maternal anxiety during pregnancy has been found to be negatively correlated with placental 11β–HSD2 mRNA levels (O’Donnell et al., 2011) and reduced placental 11β–HSD2 mRNA levels have also been found associated with intrauterine growth retardation and pre-term birth (Borzsonyi et al., 2012; Dy et al., 2008). In rats, chronic restraint stress during gestational days 11–20 was found to decrease placental 11β–HSD2 mRNA levels of this gene (Mairesse et al., 2007) and increased DNA methylation within the 11β–HSD2 gene may account for this down-regulation of gene expression. Thus, it is likely that the placenta is a central target of maternal distress effects and a key mechanistic link between maternal distress and infant outcomes.
Future Directions
Though both the acute and long-term consequences of prenatal exposure to maternal distress are evident in humans, the biological mechanisms through which these effects are achieved have yet to be fully elucidated. Exploration of epigenetic factors within the study of maternal distress may provide a promising strategy for determining the pathways through which development is altered as a function of prenatal adversity and there is increasing evidence in humans for the epigenetic consequences of the early life environment. From this emerging literature, it is clear that there are critical methodological issues and empirical questions that must be addressed in order to advance our understanding of the mechanisms of prenatal effects. First, the moderating and mediating influence of postnatal experiences following prenatal adversity must be carefully considered, as prenatal adversity is highly predictive of postnatal adversity and postnatal experiences, particularly those involving variation in mother-infant interactions, can have significant epigenetic, neurobiological, and behavioral consequences. The investigation of prenatal-postnatal interplay in shaping development may provide insights into the pathways through which fetal exposures lead to childhood and adolescent outcomes and also identify potential interventions through which the long-term consequences of fetal adversity can be attenuated. Second, the validity and interpretation of human peripheral tissue samples in the study of epigenetic effects must be carefully considered. Unlike genetic markers, epigenetic variation is likely to occur within and across tissue types. Though fetal exposures will very likely have system-wide consequences for epigenetic modifications and gene expression, the nature of this consequence may differ in brain compared to blood cells, thus limiting the capacity to explore the pathophysiology of psychological and behavioral outcomes linked to fetal adversity based exclusively on peripheral tissue samples. To address this issue, animal models in which both brain and blood epigenetic profiles can be compared and responsiveness to environmental exposures assessed, could provide an essential methodological tool. Finally, from both a neuroendocrine and epigenetic perspective, the placenta is a critical interface between maternal adversity and fetal/infant outcomes (see Figure 5). Continued investigation of the impact of prenatal adversity on placental function and gene regulation may contribute significantly to our understanding of the routes through which maternal psychosocial characteristics induce biological effects in the developing fetus. Future studies addressing these issues and incorporating an epigenetic perspective in the study of prenatal maternal distress have the potential to illustrate the complex interplay between genes and environments in shaping infant outcomes and to determine the mechanisms, both genetic and epigenetic, through which the transmission of risk of psychopathology from mothers to infants is achieved.
Acknowledgments
This research was supported by Grants DP2OD001674 from the Office of the Director National Institutes of Health (FAC) and 1R01MH092580-01A1 from the National Institutes of Mental Health (CM & FAC).
References
- Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98–102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Pettit JW, Lewinsohn PM, Seeley JR. Effect of maternal depression on child behavior: a sensitive period? Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(7):699–707. doi: 10.1016/j.jaac.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biological Psychiatry. 2010;68(4):314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nursing Research. 1996;45(5):297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beebe B, Badalamenti A, Jaffe J, Feldstein S, Marquette L, Helbraun E, Demetri-Friedman D, Flaster C, Goodman P, Kaminer T, Kaufman-Balamuth L, Putterman J, Stepakoff S, Ellman L. Distressed mothers and their infants use a less efficient timing mechanism in creating expectancies of each other’s looking patterns. Journal of Psycholinguist Ressearch. 2008;37(5):293–307. doi: 10.1007/s10936-008-9078-y. [DOI] [PubMed] [Google Scholar]
- Beebe B, Steele M, Jaffe J, Buck K, Chen H, Cohen P, Kaitz M, Markese S, Andrews H, Margolis A, Feldstein S. Maternal anxiety symptoms and mother-infant self- and interactive contingency. Infant Mental Health Journal. 2011;32(2):174–206. doi: 10.1002/imhj.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biological Psychiatry. 2010;67(11):1026–32. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(11):1454–63. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Bettes BA. Maternal depression and motherese: Temporal and intonational features. Child Development. 1989;59:1089–96. doi: 10.1111/j.1467-8624.1988.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. Journal of Autism and Devopmental Disorders. 2005;35(4):471–8. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatric and Perinatal Epidemiology. 2008;22(5):438–66. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14(6):644–51. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzsonyi B, Demendi C, Pajor A, Rigo J, Jr, Marosi K, Agota A, Nagy ZB, Joo JG. Gene expression patterns of the 11beta-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. European Journal of Obstetrics & Gynecology and Reprodroductive Biology. 2012;161(1):12–17. doi: 10.1016/j.ejogrb.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. American Journal of Psychiatry. 2000;157(2):190–5. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Bureau JF, Easterbrooks MA, Lyons-Ruth K. Maternal depressive symptoms in infancy: unique contribution to children’s depressive symptoms in childhood and adolescence? Development and Psychopathology. 2009;21(2):519–37. doi: 10.1017/S0954579409000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35(1):141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrova K, Ivanova V, Edhborg M, Matthiesen AS, Ransjo-Arvidson AB, Mukhamedrakhimov R, Uvnas-Moberg K, Widstrom AM. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009;36(2):97–109. doi: 10.1111/j.1523-536X.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Acadedemy of Sciences U S A. 1998;95(9):5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008a;3(5):e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Hormones & Behavior. 2008b;54(1):178–84. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience. 2008;28(23):6037–45. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences U S A. 2001;98(22):12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology. 2008;29(3):386–97. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental Psychobiology. 2010;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology & Behavior. 2003a;79(3):359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behavioral Neuroscience. 2007;121(6):1353–63. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003b;144(11):4720–4. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Research Reviews. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Effects of prenatal stress on behavior in adolescent rhesus monkeys. Annals of the New York Academy of Sciences. 1997;807:490–1. doi: 10.1111/j.1749-6632.1997.tb51947.x. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Matias R, Tronick EZ, Connell D, Lyons-Ruth K. Face-to-face interactions of depressed mothers and their infants. New Directions for Child Development. 1986;(34):31–45. doi: 10.1002/cd.23219863405. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in Behavioral Neuroscience. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnic KA, Greenberg MT, Robinson NM, Ragozin AS. Maternal stress and social support: effects on the mother-infant relationship from birth to eighteen months. American Journal of Orthopsychiatry. 1984;54(2):224–35. doi: 10.1111/j.1939-0025.1984.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(6):737–46. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52(2):119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PE, Snidman N, Wadhwa P, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–31. [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Hill D, Spieker S. Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child Development. 1992;63:725–37. [PubMed] [Google Scholar]
- Desforges M, Sibley CP. Placental nutrient supply and fetal growth. International Journal of Developmental Biology. 2010;54(2–3):377–90. doi: 10.1387/ijdb.082765md. [DOI] [PubMed] [Google Scholar]
- Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS ONE. 2010;5(8):e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter JNI, Field T, Hernandez-Reif M, Jones NA, Lecanuet JP, Salman FA, Mercedes R. Maternal depression and increased fetal activity. Journal of Obstetrics & Gynaecology. 2001;21(5):468–73. doi: 10.1080/01443610120072009. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Bornstein MH, Costigan KA, Pressman EK, Hahn CS, Painter K, Smith BA, Yi LJ. What does fetal movement predict about behavior during the first two years of life? Developmental Psychobiology. 2002;40(4):358–71. doi: 10.1002/dev.10025. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TRB. Fetal antecedents of infant temperament. Child Development. 1996a;67:2568–83. [PubMed] [Google Scholar]
- DiPietro JA, Hodgson KA, Costigan SC, Johnson TRB. Developmental of fetal movement – fetal heart rate coupling from 20 weeks through term. Early Human Development. 1996b;44:139–51. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Devopmental Psychobiology. 2009;51(6):505–12. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. Placental 11beta-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta. 2008;29(2):193–200. doi: 10.1016/j.placenta.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Emory EK, Dieter JN. Maternal depression and psychotropic medication effects on the human fetus. Annals of the New York Academy of Sciences. 2006;1094:287–91. doi: 10.1196/annals.1376.036. [DOI] [PubMed] [Google Scholar]
- Fan G, Hutnick L. Methyl-CpG binding proteins in the nervous system. Cell Research. 2005;15(4):255–61. doi: 10.1038/sj.cr.7290294. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):919–27. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Field T. Maternal depression effects on infants and early interventions. Preventive Medicine. 1998;27(2):200–3. doi: 10.1006/pmed.1998.0293. [DOI] [PubMed] [Google Scholar]
- Field T. Early interactions between infants and their postpartum depressed mothers. Infant Behavior and Development. 2002;25(1):25–9. [Google Scholar]
- Field T. Touch for socioemotional and physical well-being: A review. Developmental Review. 2010;30:367–83. [Google Scholar]
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Development and Psychopathology. 2007;19(2):585–602. doi: 10.1017/S0954579407070289. [DOI] [PubMed] [Google Scholar]
- Gianino A, Tronick E. The mutual regulation model: The infant’s self and interactive regulation and coping and defensive capacities. In: Field T, McCabe P, Schneiderman N, editors. Stress and coping. Hillsdale, NJ: Erlbaum; 1985. pp. 47–68. [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics & Gynecology. 2001;184(4):637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–90. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. Journal of Consulting and Clinical Psychology. 1989;57(2):269–74. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee KA. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Womens Health Issues. 2010;20(2):96–104. doi: 10.1016/j.whi.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Devopmental Psychobiology. 2009 doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Swiber MJ, Bentz LS, Holland SB, Atterbury JL. Maternal anxiety during pregnancy: Effect on fetal behavior at 38 to 40 weeks of gestation. Developmental and Behavioral Pediatrics. 1995;16(6):391–6. [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological Science. 2006;17(6):550–6. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane AA, Henderson HA, Reeb-Sutherland BC, Fox NA. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow-up study. Devopmental Psychobiology. 2010;52(6):558–67. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences U S A. 2008;105(44):17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW, Brown AS, Susser E. The Dutch famine and schizophrenia spectrum disorders. Social Psychiatry and Psychiatric Epidemiology. 1998;33(8):373–9. doi: 10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ. The changing concept of epigenetics. Annals of the New York Academy of Sciences. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews in Genetics. 2007;8(4):253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kaitz M, Maytal HR, Devor N, Bergman L, Mankuta D. Maternal anxiety, mother-infant interactions, and infants’ response to challenge. Infant Behavior & Development. 2010;33(2):136–48. doi: 10.1016/j.infbeh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development. 2007;84(4):249–56. doi: 10.1016/j.earlhumdev.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry. 2008;65(2):146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kikkert HK, Middelburg KJ, Hadders-Algra M. Maternal anxiety is related to infant neurological condition, paternal anxiety is not. Early Human Development. 2010;86(3):171–7. doi: 10.1016/j.earlhumdev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. 2005;8(1):35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes, Brain and Behavior. 2010;9:575–82. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. Journal of Autism and Developmental Disorders. 2008;38(3):481–8. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, Genevieve D, Cormier-Daire V, van Esch H, Fryns JP, Hamel BC, Sistermans EA, de Vries BB, van Bokhoven H. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. American Journal of Human Genetics. 2006;79(2):370–7. doi: 10.1086/505693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CM, Andrews RM, Flicek P, Dillon SC, Karaoz U, Clelland GK, Wilcox S, Beare DM, Fowler JC, Couttet P, James KD, Lefebvre GC, Bruce AW, Dovey OM, Ellis PD, Dhami P, Langford CF, Weng Z, Birney E, Carter NP, Vetrie D, Dunham I. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Research. 2007;17(6):691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151(5):2297–305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini L, Lee TL, Chan WY, Lee MJ, Diplas A, Wetmur J, Chen J. Differential methylation of imprinted genes in growth-restricted placentas. Reproductive Sciences. 2011;18(11):1111–7. doi: 10.1177/1933719111404611. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Ressarch. 2004;56(3):400–10. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(9):1063–72. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Lee MH, Jeon YJ, Lee SM, Park MH, Jung SC, Kim YJ. Placental gene expression is related to glucose metabolism and fetal cord blood levels of insulin and insulin-like growth factors in intrauterine growth restriction. Early Human Development. 2010;86(1):45–50. doi: 10.1016/j.earlhumdev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Reports. 2003;4(10):944–7. doi: 10.1038/sj.embor.embor941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Jongen-Relo AL, Stohr T, Pothuizen HH, Feldon J. Comparison of maternal separation and early handling in terms of their neurobehavioral effects in aged rats. Neurobiology of Aging. 2002;23(3):457–66. doi: 10.1016/s0197-4580(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Levy G. Pharmacokinetics of fetal and neonatal exposure to drugs. Obstetrics & Gynecology. 1981;58(5 Suppl):9S–16S. [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009;123(4):1102–7. doi: 10.1542/peds.2008-1734. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Seguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences U S A. 2011;108(34):14324–9. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Block D. The disturbed caregiving system: Relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health Journal. 1996;17(3):257–275. [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. American Journal of Physiology Endocrinology & Metabolism. 2007;292(6):E1526–33. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manian N, Bornstein MH. Dynamics of emotion regulation in infants of clinically depressed and nondepressed mothers. Journal of Child Psychology and Psychiatry. 2009;50(11):1410–8. doi: 10.1111/j.1469-7610.2009.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Lactogenic hormone regulation of maternal behavior. Progress in Brain Research. 2001;133:251–62. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- Martins C, Gaffan EA. Effects of early maternal depression on patterns of infant-mother attachment: a meta-analytic investigation. Journal of Child Psychology and Psychiatry. 2000;41(6):737–46. [PubMed] [Google Scholar]
- McCarthy C, Cotter FE, McElwaine S, Twomey A, Mooney EE, Ryan F, Vaughan J. Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. American Journal of Obstetrics & Gynecology. 2007;196(1):70 e1–6. doi: 10.1016/j.ajog.2006.08.027. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CA, Barnett B, Kowalenko NM, Tennant CC. Maternal attachment state of mind moderates the impact of postnatal depression on infant attachment. Journal of Child Psychology and Psychiatry. 2006;47(7):660–9. doi: 10.1111/j.1469-7610.2005.01547.x. [DOI] [PubMed] [Google Scholar]
- McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, Smith AC, Weksberg R, Thaker HM, Tycko B. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27(6–7):540–9. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Reviews in Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28(9):456–63. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Bagiella E, Duong JK, Chen IS, Leotti L, Altincatal A. Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Developmental Psychobiology. 2010;53(3):221–233. doi: 10.1002/dev.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Sloan RP, Myers MM, Trien L, Hurtado A. Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Developmental Psychobiology. 2000;36:67–77. [PubMed] [Google Scholar]
- Monk C, Myers MM, Sloan RP, Werner L, Jeon J, Tager F, Fifer WP. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):283–90. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Developmental Psychobiology. 1984;17(4):347–56. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. Journal of Comparative Physiological Psychology. 1979;93(4):677–84. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008;28(36):9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience. 2009;12(12):1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murray L, Halligan SL, Goodyer I, Herbert J. Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: a preliminary study. Journal of Affective Disorders. 2010;122(3):218–23. doi: 10.1016/j.jad.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Development and Psychopathology. 2011:1–13. doi: 10.1017/S0954579411000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Human Reproduction Update. 2011;17(3):397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- Nicol-Harper R, Harvey AG, Stein A. Interactions between mothers and infants: impact of maternal anxiety. Infant Behavior and Development. 2007;30(1):161–7. doi: 10.1016/j.infbeh.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic B, Wong NC, Sibson M, Ng HK, Morley R, Manuelpillai U, Down T, Rakyan VK, Beck S, Hiendleder S, Roberts CT, Craig JM, Saffery R. DNA methylation-mediated down-regulation of DNA methyltransferase-1 (DNMT1) is coincident with, but not essential for, global hypomethylation in human placenta. Journal of Biological Chemistry. 2010;285(13):9583–93. doi: 10.1074/jbc.M109.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Glover V The ALSPAC Study Team. Antenatal anxiety predicts child behavior/emotional problems independently of postnatal depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2002a;41(12):1470–7. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioral/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. British Journal of Psychiatry. 2002b;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. Journal of Child Psychology and Psychiatry. 2003;44(7):1025–36. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression - a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O’Keane V. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. Journal of Affective Disorders. 2009;113(3):236–43. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Strandberg TE, Jarvenpaa AL. Continuity of maternal stress from the pre- to the postnatal period: Associations with infant’s positive, negative, and overall temperamental reactivity. Infant Behavior & Development. 2005;28:36–47. [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Current Biology. 2004;14(14):R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63(2):234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO Journal. 1998;17(17):4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew DC, McKee L. Temperament and its role in developmental psychopathology. Harvard Review of Psychiatry. 2005;13(1):14–27. doi: 10.1080/10673220590923146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychological Medicine. 2009:1–11. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65(9):760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–31. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to Prenatal Psychobiological Stress Exerts Programming Influences on the Mother and Her Fetus. Neuroendocrinology. 2011 doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34:163–73. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Schneider ML. The effect of mild stress during pregnancy on birthweight and neuromotor maturation in rhesus monkey infants (Macaca mulatta) Infant Behavior and Development. 1992;15:389–403. [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Development. 1999;70:263–74. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E, Nahon D, Levav I, Aleman A, Kahn RS. No relationship between risk of schizophrenia and prenatal exposure to stress during the Six- Day War or Yom Kippur War in Israel. Schizophrenia Research. 2003;63(1–2):131–5. doi: 10.1016/s0920-9964(02)00375-4. [DOI] [PubMed] [Google Scholar]
- Sitras V, Paulssen R, Leirvik J, Vartun A, Acharya G. Placental gene expression profile in intrauterine growth restriction due to placental insufficiency. Reproductive Sciences. 2009;16(7):701–11. doi: 10.1177/1933719109334256. [DOI] [PubMed] [Google Scholar]
- Smith FM, Garfield AS, Ward A. Regulation of growth and metabolism by imprinted genes. Cytogenetic Genome Research. 2006;113(1–4):279–91. doi: 10.1159/000090843. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–65. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178(62):708–13. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. Journal of Abnormal Psychology. 1975;84(5):576–8. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF, Kimball SD. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psychological Reports. 1971;29(3):1171–7. doi: 10.2466/pr0.1971.29.3f.1171. [DOI] [PubMed] [Google Scholar]
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. American Journal of Epidemiology. 1998;147(3):213–6. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- Susser EB, Brown A, Matte TD. Prenatal factors and adult mental and physical health. Canadian Journal of Psychiatry. 1999;44:326–34. doi: 10.1177/070674379904400402. [DOI] [PubMed] [Google Scholar]
- Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Archives of General Psychiatry. 1992;49(12):983–8. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. European Psychiatry. 2004;19(8):459–63. doi: 10.1016/j.eurpsy.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Tabano S, Colapietro P, Cetin I, Grati FR, Zanutto S, Mando C, Antonazzo P, Pileri P, Rossella F, Larizza L, Sirchia SM, Miozzo M. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010;5(4):313–24. doi: 10.4161/epi.5.4.11637. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Baker EW, Kalin NH. Ontogeny of behavioral and hormonal responses to stress in prenatallay stressed male rat pups. Physiology & Behavior. 1990;47:357–64. doi: 10.1016/0031-9384(90)90154-v. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Haglin C, Kalin NH. Prenatal stress potentiates stress-induced behavior and reduces the propensity to play in juvenile rats. Physiology & Behavior. 1992;51:319–23. doi: 10.1016/0031-9384(92)90147-t. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry. 2007;48(3–4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti DM, Felfand DM, Messinger DS, Isabella R. Maternal depression and the quality of early attachment: An examination of intants, preschoolers, and their mothers. Developmental Psychobiology. 1995;31:364–76. [Google Scholar]
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics. 2009;18(21):4046–53. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, Gianino A. The transmission of maternal disturbance to the infant. In: Tronick E, Field T, editors. Maternal depression and infant disturbance. San Francisco: Jossey-Bass; 1986. pp. 5–12. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Emotions and emotinal communication in infants. American Psychologist. 1989;44:112–9. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Turek-Plewa J, Jagodzinski PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cellular and Molecular Biology Letters. 2005;10(4):631–47. [PubMed] [Google Scholar]
- Turner B. Chromatin and Gene Regulation. Oxford: Blackwell Science Ltd; 2001. [Google Scholar]
- Tycko B. Imprinted genes in placental growth and obstetric disorders. Cytogenetic and Genome Research. 2006;113(1–4):271–8. doi: 10.1159/000090842. [DOI] [PubMed] [Google Scholar]
- Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods in Molecular Biology. 2004;287:99–120. doi: 10.1385/1-59259-828-5:099. [DOI] [PubMed] [Google Scholar]
- Valentine JM, Rodriguez MA, Lapeyrouse LM, Zhang M. Recent intimate partner violence as a prenatal predictor of maternal depression in the first year postpartum among Latinas. Archives of Women’s Mental Health. 2011;14(2):135–43. doi: 10.1007/s00737-010-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems and anxiety in 8–9 year olds. Child Development. 2004;75(4):1085–97. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neuroscience and Biobehavioral Reviews. 2005;29(2):259–69. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- van den Bergh BR, Mennes M, Stevens V, van der Meere J, Borger N, Stiers P, Marcoen A, Lagae L. ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatric Research. 2006;59(1):78–82. doi: 10.1203/01.pdr.0000191143.75673.52. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Verkerk GJ, Pop VJ, Van Son MJ, Van Heck GL. Prediction of depression in the postpartum period: a longitudinal follow-up study in high-risk and low-risk women. Journal of Affective Disorders. 2003;77(2):159–66. doi: 10.1016/s0165-0327(02)00146-5. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. Journal of Neuroscience. 2005;25(47):11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences U S A. 2006;103(9):3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity. 2005;19(4):296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Werner A, Myers MM, Fifer WP, Cheng B, Fang Y, Allen R, Monk C. Prenatal predictors of infant temperament. Developmental Psychobiology. 2007;49:474–84. doi: 10.1002/dev.20232. [DOI] [PubMed] [Google Scholar]
- Xu W, Edmondson DG, Evrard YA, Wakamiya M, Behringer RR, Roth SY. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nature Genetics. 2000;26(2):229–32. doi: 10.1038/79973. [DOI] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93(3):361–72. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Keener MA, Stewart L, Anders TF. Prenatal perception of infant personality: a preliminary investigation. Journal of the American Academy of Child Psychiatry. 1985;24(2):204–10. doi: 10.1016/s0002-7138(09)60449-0. [DOI] [PubMed] [Google Scholar]
- Zekoski EM, O’Hara MW, Wills KE. The effects of maternal mood on mother-infant interaction. Journal of Abnormal Child Psychology. 1987;15(3):361–78. doi: 10.1007/BF00916455. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. Journal of Neuroscience. 2010;30(39):13130–7. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]