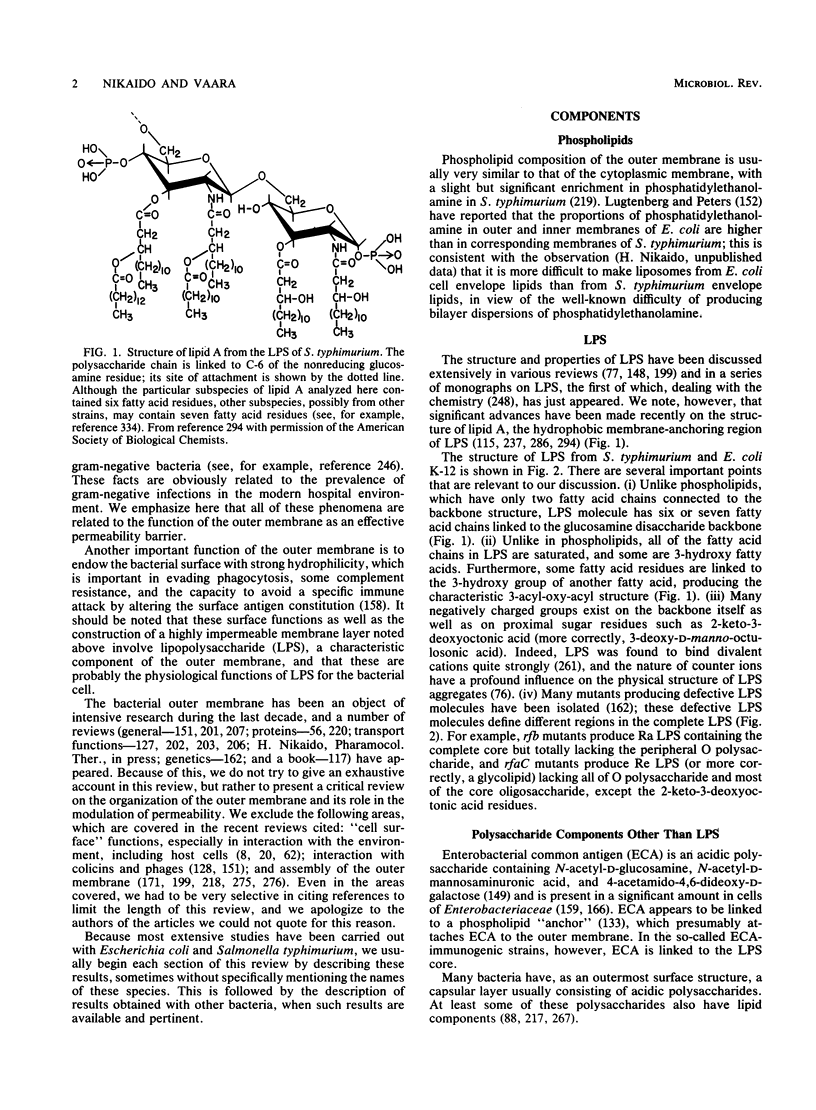
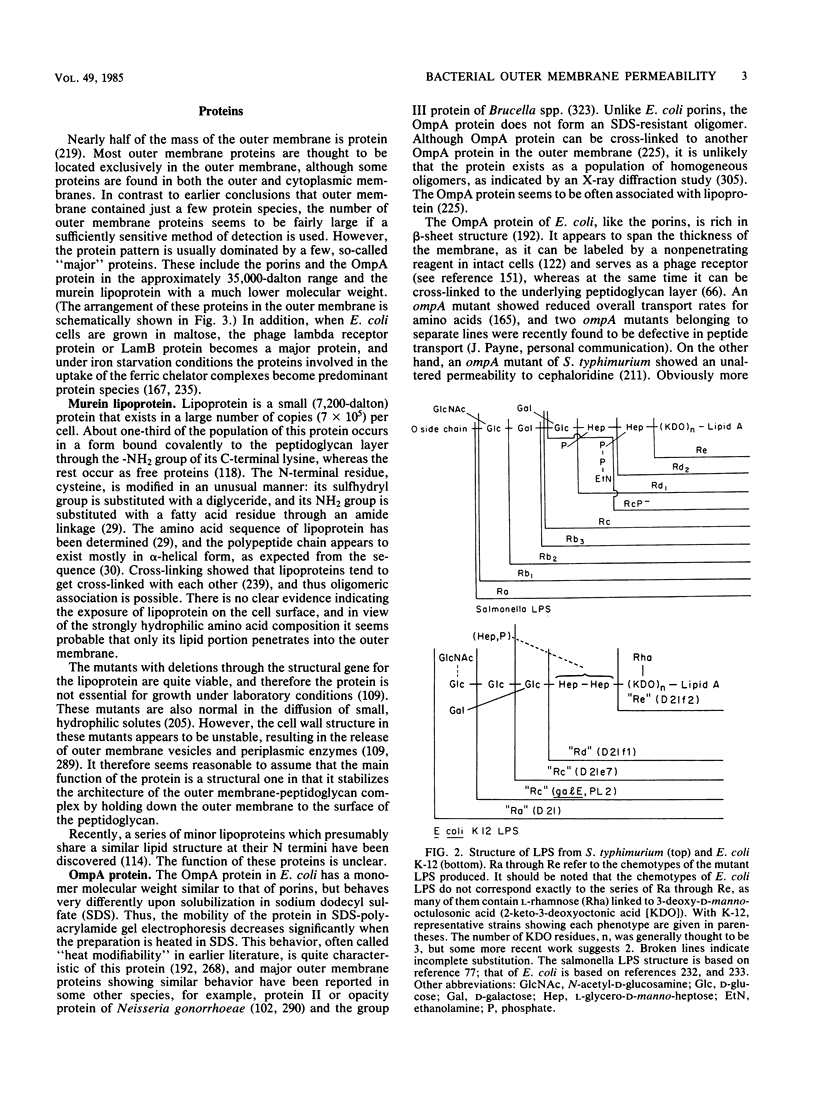
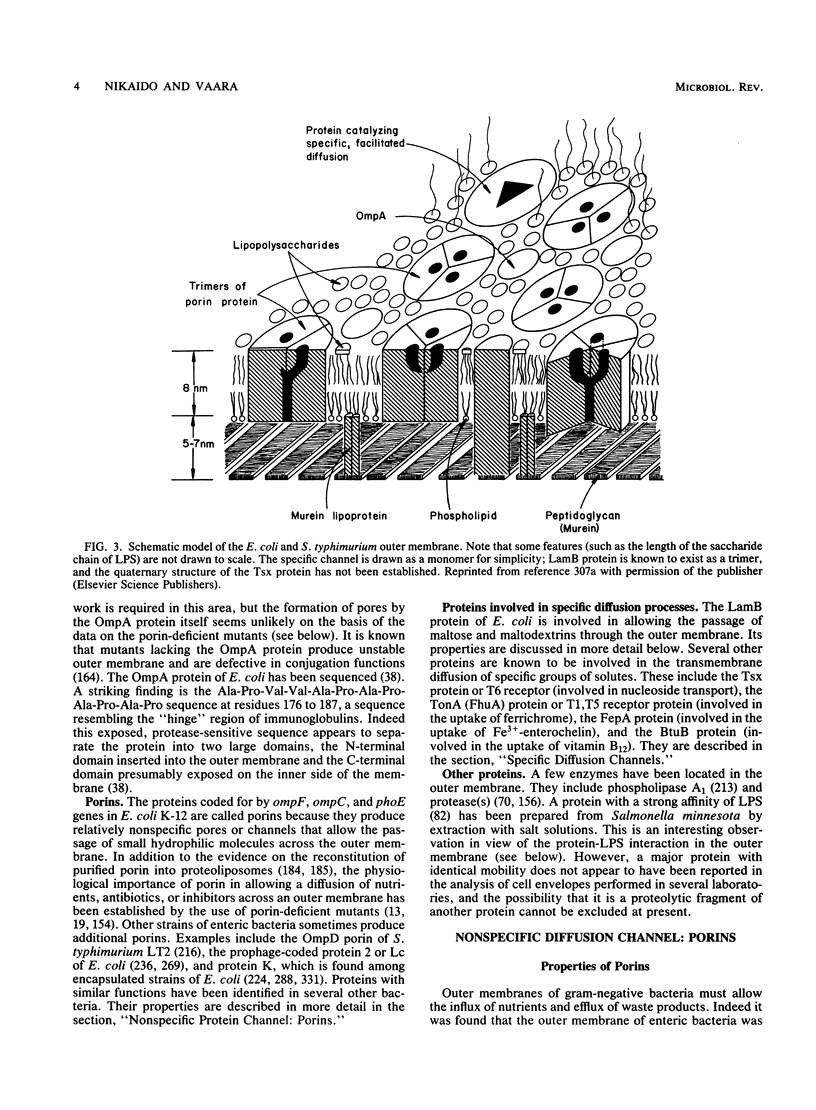
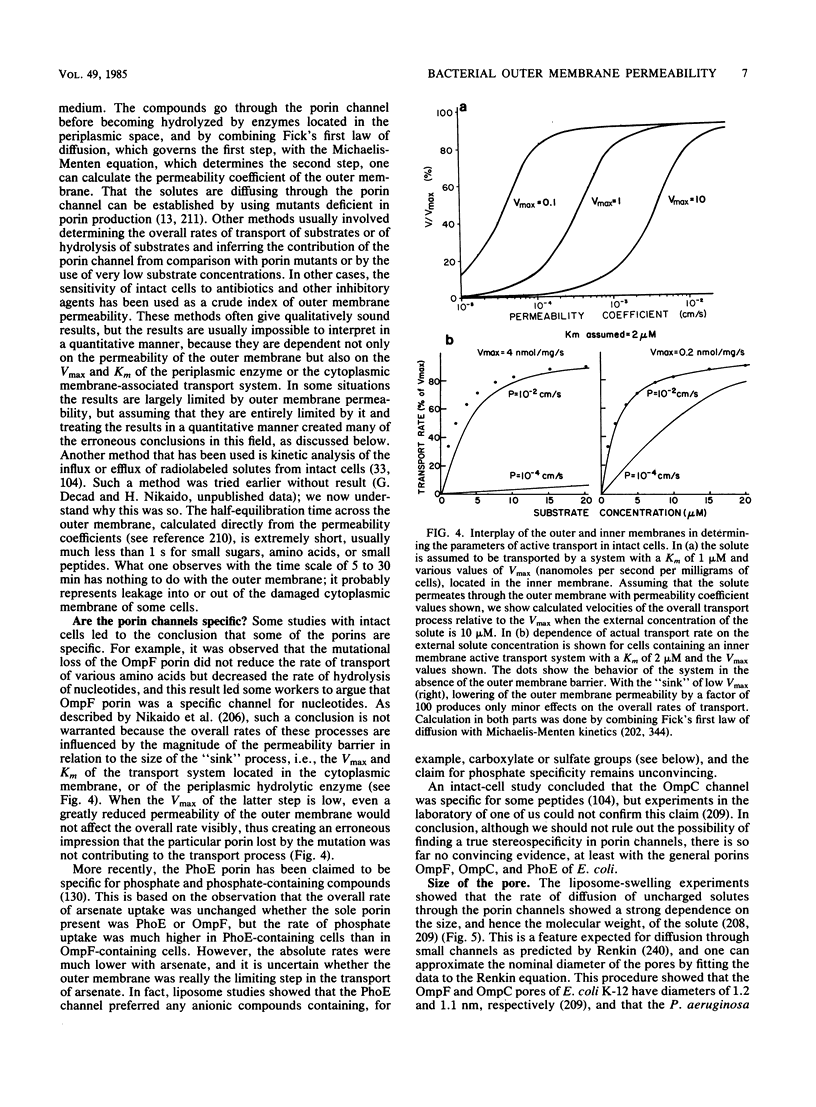
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Nakano M., Inuzuka M., Tomoeda M. Specific role of sex pili in the effective eliminatory action of sodium dodecyl sulfate on sex and drug resistance factors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1114–1124. doi: 10.1128/jb.109.3.1114-1124.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus B. L., Hancock R. E. Outer membrane porin proteins F, P, and D1 of Pseudomonas aeruginosa and PhoE of Escherichia coli: chemical cross-linking to reveal native oligomers. J Bacteriol. 1983 Sep;155(3):1042–1051. doi: 10.1128/jb.155.3.1042-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Boos W. Co-regulation in Escherichia coli of a novel transport system for sn-glycerol-3-phosphate and outer membrane protein Ic (e, E) with alkaline phosphatase and phosphate-binding protein. J Bacteriol. 1980 Jul;143(1):142–150. doi: 10.1128/jb.143.1.142-150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Nikaido H. Physical interaction between the phage lambda receptor protein and the carrier-immobilized maltose-binding protein of Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11385–11388. [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Wandersman C., Schwartz M., Nikaido H. A mutant form of maltose-binding protein of Escherichia coli deficient in its interaction with the bacteriophage lambda receptor protein. J Bacteriol. 1983 Aug;155(2):919–921. doi: 10.1128/jb.155.2.919-921.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R., Haas D., Yagil E. Mutants of Escherichia coli "cryptic" for certain periplasmic enzymes: evidence for an alteration of the outer membrane. J Bacteriol. 1977 Feb;129(2):1034–1044. doi: 10.1128/jb.129.2.1034-1044.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M., Pugsley A., Schnaitman C. Correlation between the expression of an Escherichia coli cell surface protein and the ability of the protein to bind to lipopolysaccharide. J Bacteriol. 1980 Jul;143(1):403–410. doi: 10.1128/jb.143.1.403-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Benz R., Tokunaga H., Nakae T. Properties of chemically modified porin from Escherichia coli in lipid bilayer membranes. Biochim Biophys Acta. 1984 Jan 25;769(2):348–356. doi: 10.1016/0005-2736(84)90316-x. [DOI] [PubMed] [Google Scholar]
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass J. M., Boos W., Hengge R. Reconstitution of maltose transport in malB mutants of Escherichia coli through calcium-induced disruptions of the outer membrane. J Bacteriol. 1981 Apr;146(1):10–17. doi: 10.1128/jb.146.1.10-17.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass J. M., Ehmann U., Bukau B. Reconstitution of maltose transport in Escherichia coli: conditions affecting import of maltose-binding protein into the periplasm of calcium-treated cells. J Bacteriol. 1983 Jul;155(1):97–106. doi: 10.1128/jb.155.1.97-106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Rotering H., Ohms J. P., Hagenmaier H. Conformational studies on murein-lipoprotein from the outer membrane of Escherichia coli. Eur J Biochem. 1976 Nov 15;70(2):601–610. doi: 10.1111/j.1432-1033.1976.tb11051.x. [DOI] [PubMed] [Google Scholar]
- Burnell E., van Alphen L., Verkleij A., de Kruijff B., Lugtenberg B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli. III. The outer membrane. Biochim Biophys Acta. 1980 Apr 24;597(3):518–532. doi: 10.1016/0005-2736(80)90224-2. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Klapper D. G., Blackman E. Y., Sparling P. F. Genetic locus (nmp-1) affecting the principal outer membrane protein of Neisseria gonorrhoeae. J Bacteriol. 1980 Aug;143(2):847–851. doi: 10.1128/jb.143.2.847-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Clement J. M., Hofnung M. Further sequence analysis of the phage lambda receptor site. Possible implications for the organization of the lamB protein in Escherichia coli K12. J Mol Biol. 1984 May 25;175(3):395–401. doi: 10.1016/0022-2836(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Ross H., Sanderson K. E. Leakage of periplasmic enzymes from lipopolysaccharide-defective mutants of Salmonella typhimurium. Can J Microbiol. 1976 Oct;22(10):1549–1560. doi: 10.1139/m76-227. [DOI] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Chen-Schmeisser U., Henning U. Primary structure of major outer-membrane protein I (ompF protein, porin) of Escherichia coli B/r. Biochem J. 1982 Apr 1;203(1):33–43. doi: 10.1042/bj2030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Bangham A. D. Diffusion of small non-electrolytes across liposome membranes. Nature. 1972 Mar 24;236(5343):173–174. doi: 10.1038/236173a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Sonntag I., Henning U. Cloning and expression in Escherichia coli K-12 of the genes for major outer membrane protein OmpA from Shigella dysenteriae, Enterobacter aerogenes, and Serratia marcescens. J Bacteriol. 1982 Jan;149(1):145–150. doi: 10.1128/jb.149.1.145-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr The rfaD gene codes for ADP-L-glycero-D-mannoheptose-6-epimerase. An enzyme required for lipopolysaccharide core biosynthesis. J Biol Chem. 1983 Feb 10;258(3):1985–1990. [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Corwin L. M., Rothman S. W., Kim R., Talevi L. A. Mechanisms and genetics of resistance to sodium lauryl sulfate in strains of Shigella and Escherichia coli. Infect Immun. 1971 Sep;4(3):287–294. doi: 10.1128/iai.4.3.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin R. T., Tonsager S., McGroarty E. J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983 Apr 12;22(8):2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., MacIntyre S., Buckley J. T., Hancock R. E. Purification and reconstitution in lipid bilayer membranes of an outer membrane, pore-forming protein of Aeromonas salmonicida. J Bacteriol. 1983 Dec;156(3):1006–1011. doi: 10.1128/jb.156.3.1006-1011.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Donaldson D. M., Roberts R. R., Larsen H. S., Tew J. G. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun. 1974 Sep;10(3):657–666. doi: 10.1128/iai.10.3.657-666.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorset D. L., Engel A., Häner M., Massalski A., Rosenbusch J. P. Two-dimensional crystal packing of matrix porin. A channel forming protein in Escherichia coli outer membranes. J Mol Biol. 1983 Apr 25;165(4):701–710. doi: 10.1016/s0022-2836(83)80275-7. [DOI] [PubMed] [Google Scholar]
- Dorset D. L., Engel A., Massalski A., Rosenbusch J. P. Three Dimensional Structure of a Membrane Pore: Electron Microscopical Analysis of Escherichia coli Outer Membrane Matrix Porin. Biophys J. 1984 Jan;45(1):128–129. doi: 10.1016/S0006-3495(84)84135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Emmerling G., Henning U., Gulik-Krzywicki T. Order-disorder conformation transition of hydrocarbon chains in lipopolysaccharide from Escherichia coli. Eur J Biochem. 1977 Sep;78(2):503–509. doi: 10.1111/j.1432-1033.1977.tb11763.x. [DOI] [PubMed] [Google Scholar]
- Endermann R., Krämer C., Henning U. Major outer membrane proteins of Escherichia coli K-12: evidence for protein II being a transmembrane protein. FEBS Lett. 1978 Feb 1;86(1):21–24. doi: 10.1016/0014-5793(78)80089-1. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Lee K. S. Directed evolution of the lambda receptor of Escherichia coli through affinity chromatographic selection. J Mol Biol. 1982 Sep 25;160(3):431–444. doi: 10.1016/0022-2836(82)90306-0. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Schwentorat M., Ullrich S., Vilmart J. Lambda receptor in the outer membrane of Escherichia coli as a binding protein for maltodextrins and starch polysaccharides. J Bacteriol. 1980 May;142(2):521–526. doi: 10.1128/jb.142.2.521-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Hollifield W. C., Jr, Neilands J. B. Absence of ferric enterobactin receptor modification activity in mutants of Escherichia coli K-12 lacking protein a. Biochem Biophys Res Commun. 1979 Nov 14;91(1):29–34. doi: 10.1016/0006-291x(79)90578-3. [DOI] [PubMed] [Google Scholar]
- Flammann H. T., Weckesser J. Porin isolated from the cell envelope of Rhodopseudomonas capsulata. J Bacteriol. 1984 Jul;159(1):410–412. doi: 10.1128/jb.159.1.410-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried V. A., Rothfield L. I. Interactions between lipopolysaccharide and phosphatidylethanolamine in molecular monolayers. Biochim Biophys Acta. 1978 Dec 4;514(1):69–82. doi: 10.1016/0005-2736(78)90077-9. [DOI] [PubMed] [Google Scholar]
- Funahara Y., Nikaido H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1463–1465. doi: 10.1128/jb.141.3.1463-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Yamada H., Mizushima S. Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):1071–1080. doi: 10.1128/jb.140.3.1071-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Pluschke G., Overath P., Seelig J. Structure of Escherichia coli membranes. Fatty acyl chain order parameters of inner and outer membranes and derived liposomes. Biochemistry. 1980 Apr 15;19(8):1638–1643. doi: 10.1021/bi00549a018. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J. A., Neuhaus J. M., Pugsley A. P., Rosenbusch J. P. Structural investigations of outer membrane proteins from Escherichia coli. Ann Microbiol (Paris) 1982 Jan;133A(1):37–41. [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Geyer R., Galanos C., Westphal O., Golecki J. R. A lipopolysaccharide-binding cell-surface protein from Salmonella minnesota. Isolation, partial characterization and occurrence in different Enterobacteriaceae. Eur J Biochem. 1979 Jul;98(1):27–38. doi: 10.1111/j.1432-1033.1979.tb13156.x. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Bergmann H., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Different release of lipopolysaccharide from wild type and lipopolysaccharide mutant cells by EDTA treatment. Arch Microbiol. 1980 Jan;124(1):69–71. doi: 10.1007/BF00407030. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular composition of the outer membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch Microbiol. 1980 Sep;127(2):81–86. doi: 10.1007/BF00428010. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981 Dec;8(6):429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Poole K., Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982 May;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Phage T6--colicin K receptor and nucleoside transport in Escherichia coli. FEBS Lett. 1976 Nov;70(1):109–112. doi: 10.1016/0014-5793(76)80737-5. [DOI] [PubMed] [Google Scholar]
- Hardaway K. L., Buller C. S. Effect of ethylenediaminetetraacetate on phospholipids and outer membrane function in Escherichia coli. J Bacteriol. 1979 Jan;137(1):62–68. doi: 10.1128/jb.137.1.62-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. Role of the receptor for bacteriophage lambda in the functioning of the maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Oct;124(1):119–126. doi: 10.1128/jb.124.1.119-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in Escherichia coli cell envelope structure and the sites of fluorescence probe binding caused by carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Biochemistry. 1977 Sep 6;16(18):4109–4117. doi: 10.1021/bi00637a026. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Wilson T. H. Selectivity of the Escherichia coli outer membrane porins ompC and ompF. FEBS Lett. 1981 Jul 6;129(2):253–255. doi: 10.1016/0014-5793(81)80177-9. [DOI] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Henson J. M., Walker J. R. Genetic analysis of acrA and lir mutations of Escherichia coli. J Bacteriol. 1982 Dec;152(3):1301–1302. doi: 10.1128/jb.152.3.1301-1302.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. Periplasmic maltose-binding protein confers specificity on the outer membrane maltose pore of Escherichia coli. J Bacteriol. 1980 Feb;141(2):431–435. doi: 10.1128/jb.141.2.431-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. The tsx protein of Escherichia coli can act as a pore for amino acids. J Bacteriol. 1981 Sep;147(3):1113–1116. doi: 10.1128/jb.147.3.1113-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma R., Yamaguchi A., Sawai T. The effect of lipopolysaccharide on lipid bilayer permeability of beta-lactam antibiotics. FEBS Lett. 1984 May 21;170(2):268–272. doi: 10.1016/0014-5793(84)81326-5. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Neilands J. B. Ferric enterobactin transport system in Escherichia coli K-12. Extraction, assay, and specificity of the outer membrane receptor. Biochemistry. 1978 May 16;17(10):1922–1928. doi: 10.1021/bi00603a019. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Fecycz I. T., Campbell J. N. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J Bacteriol. 1980 Nov;144(2):844–847. doi: 10.1128/jb.144.2.844-847.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976 Jun 15;15(12):2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Takahashi I., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes containing purified porins. Antimicrob Agents Chemother. 1982 Nov;22(5):775–780. doi: 10.1128/aac.22.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteland J., Tommassen J., Lugtenberg B. PhoE protein pore of the outer membrane of Escherichia coli K12 is a particularly efficient channel for organic and inorganic phosphate. Biochim Biophys Acta. 1982 Sep 9;690(2):282–289. doi: 10.1016/0005-2736(82)90332-7. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. M., Neter E., Mayer H. Modification of the lipid moiety of the enterobacterial common antigen by the "Pseudomonas factor". Infect Immun. 1983 May;40(2):696–700. doi: 10.1128/iai.40.2.696-700.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Telesetsky S., Coleman W. G., Jr, Carr D. Tetracyclines of various hydrophobicities as a probe for permeability of Escherichia coli outer membranes. Antimicrob Agents Chemother. 1984 May;25(5):539–544. doi: 10.1128/aac.25.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Bewick M. A. Use of a nonpenetrating substrate analogue to study the molecular mechanism of the outer membrane dicarboxylate transport system in Escherichia coli K12. J Biol Chem. 1981 Jun 10;256(11):5511–5517. [PubMed] [Google Scholar]
- Lo T. C., Sanwal B. D. Isolation of the soluble substrate recognition component of the dicarboxylate transport system of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1600–1602. [PubMed] [Google Scholar]
- Lounatmaa K. Ultrastructure of the outer membrane of Salmonella typhimurium bacteriocin-resistant mutants deficient in the 33K protein. J Bacteriol. 1979 Aug;139(2):646–651. doi: 10.1128/jb.139.2.646-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Bacteriophage lambda receptor protein in Escherichia coli K-12: lowered affinity of some mutant proteins for maltose-binding protein in vitro. J Bacteriol. 1983 Feb;153(2):1056–1059. doi: 10.1128/jb.153.2.1056-1059.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., Peters R. Distribution of lipids in cytoplasmic and outer membranes of Escherichia coli K12. Biochim Biophys Acta. 1976 Jul 20;441(1):38–47. doi: 10.1016/0005-2760(76)90279-4. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Earhart C. F. Gene envY of Escherichia coli K-12 affects thermoregulation of major porin expression. J Bacteriol. 1984 Jan;157(1):262–268. doi: 10.1128/jb.157.1.262-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Neisseria gonorrhoeae cell envelope: permeability to hydrophobic molecules. J Bacteriol. 1981 Feb;145(2):946–952. doi: 10.1128/jb.145.2.946-952.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Zubrzycki L., Coyle M. B., Chila M., Warner P. Genetic analysis of drug resistance in Neisseria gonorrhoeae: production of increased resistance by the combination of two antibiotic resistance loci. J Bacteriol. 1975 Nov;124(2):834–842. doi: 10.1128/jb.124.2.834-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Pugsley A. P., Reeves P. Defective growth functions in mutants of Escherichia coli K12 lacking a major outer membrane protein. J Mol Biol. 1977 Oct 25;116(2):285–300. doi: 10.1016/0022-2836(77)90217-0. [DOI] [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Chemistry and biology of the enterobacterial common antigen (ECA). Curr Top Microbiol Immunol. 1979;85:99–153. doi: 10.1007/978-3-642-67322-1_3. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Effect of iron of the relative abundance of two large polypeptides of the Escherichia coli outer membrane. Biochem Biophys Res Commun. 1976 May 3;70(1):315–322. doi: 10.1016/0006-291x(76)91144-x. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Metal ion dependence of a heat-modifiable protein from the outer membrane of Escherichia coli upon sodium dodecyl sulfate-gel electrophoresis. J Bacteriol. 1977 Oct;132(1):314–320. doi: 10.1128/jb.132.1.314-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Levy S. B. Transport of the lipophilic analog minocycline differs from that of tetracycline in susceptible and resistant Escherichia coli strains. Antimicrob Agents Chemother. 1982 Nov;22(5):791–799. doi: 10.1128/aac.22.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D. L., Steim J. M. Thermotropic transitions in biomembranes. Annu Rev Biophys Bioeng. 1976;5:205–238. doi: 10.1146/annurev.bb.05.060176.001225. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Reeves P. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J Bacteriol. 1982 Jun;150(3):1016–1023. doi: 10.1128/jb.150.3.1016-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulford C. A., Osborn M. J. An intermediate step in translocation of lipopolysaccharide to the outer membrane of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1159–1163. doi: 10.1073/pnas.80.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. Reversal of the bactericidal reaction of serum by magnesium ion. J Bacteriol. 1966 Apr;91(4):1399–1402. doi: 10.1128/jb.91.4.1399-1402.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Schmidt G., Mayer H., Nikaido H., Whang H. Y., Neter E. Enterobacterial common antigen in rfb deletion mutants of Salmonella typhimurium. J Bacteriol. 1976 Sep;127(3):1141–1149. doi: 10.1128/jb.127.3.1141-1149.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Nakae R., Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982 Oct;22(4):554–559. doi: 10.1128/aac.22.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. A porin activity of purified lambda-receptor protein from Escherichia coli in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):774–781. doi: 10.1016/0006-291x(79)91475-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J. Permeability properties of Escherichia coli outer membrane containing, pore-forming proteins: comparison between lambda receptor protein and porin for saccharide permeation. J Bacteriol. 1980 Jun;142(3):735–740. doi: 10.1128/jb.142.3.735-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neuhaus J. M., Schindler H., Rosenbusch J. P. The periplasmic maltose-binding protein modifies the channel-forming characteristics of maltoporin. EMBO J. 1983;2(11):1987–1991. doi: 10.1002/j.1460-2075.1983.tb01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Alteration of susceptibility to EDTA, polymyxin B and gentamicin in Pseudomonas aeruginosa by divalent cation regulation of outer membrane protein H1. J Gen Microbiol. 1983 Feb;129(2):509–517. doi: 10.1099/00221287-129-2-509. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol C. P., Davis J. H., Weeks G., Bloom M. Quantitative study of the fluidity of Escherichia coli membranes using deuterium magnetic resonance. Biochemistry. 1980 Feb 5;19(3):451–457. doi: 10.1021/bi00544a008. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Bavoil P., Hirota Y. Outer membranes of gram-negative bacteria. XV. Transmembrane diffusion rates in lipoprotein-deficient mutants of Escherichia coli. J Bacteriol. 1977 Dec;132(3):1045–1047. doi: 10.1128/jb.132.3.1045-1047.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Luckey M., Rosenberg E. Y. Nonspecific and specific diffusion channels in the outer membrane of Escherichia coli. J Supramol Struct. 1980;13(3):305–313. doi: 10.1002/jss.400130304. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Permeability of the outer membrane of bacteria. Angew Chem Int Ed Engl. 1979 May;18(5):337–350. doi: 10.1002/anie.197903373. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Takeuchi Y., Ohnishi S. I., Nakae T. Outer membrane of Salmonella typhimurium. Electron spin resonance studies. Biochim Biophys Acta. 1977 Feb 14;465(1):152–164. doi: 10.1016/0005-2736(77)90363-7. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Nogami T., Mizushima S. Outer membrane porins are important in maintenance of the surface structure of Escherichia coli cells. J Bacteriol. 1983 Oct;156(1):402–408. doi: 10.1128/jb.156.1.402-408.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Lounatmaa K., Sarvas M., Mäkelä P. H., Nakae T. Bacteriophage-resistant mutants of Salmonella typhimurium deficient in two major outer membrane proteins. J Bacteriol. 1976 Aug;127(2):941–955. doi: 10.1128/jb.127.2.941-955.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Overath P., Brenner M., Gulik-Krzywicki T., Shechter E., Letellier L. Lipid phase transitions in cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1975 May 6;389(2):358–369. doi: 10.1016/0005-2736(75)90328-4. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Van Scharrenburg G., Lugtenberg B. Antigenic relationships between pore proteins of Escherichia coli K12. Eur J Biochem. 1980 Sep;110(1):247–254. doi: 10.1111/j.1432-1033.1980.tb04862.x. [DOI] [PubMed] [Google Scholar]
- Paakkanen J., Gotschlich E. C., Mäkelä P. H. Protein K: a new major outer membrane protein found in encapsulated Escherichia coli. J Bacteriol. 1979 Sep;139(3):835–841. doi: 10.1128/jb.139.3.835-841.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T. Protein interactions in the outer membrane of Escherichia coli. Eur J Biochem. 1979 Feb 1;93(3):495–503. doi: 10.1111/j.1432-1033.1979.tb12848.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Randall L. L. Arrangement of protein I in Escherichia coli outer membrane: cross-linking study. J Bacteriol. 1978 Jan;133(1):279–286. doi: 10.1128/jb.133.1.279-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Westermann P. Arrangement of the maltose-inducible major outer membrane proteins, the bacteriophage lambda receptor in Escherichia coli and the 44 K protein in Salmonella typhimurium. FEBS Lett. 1979 Mar 1;99(1):77–80. doi: 10.1016/0014-5793(79)80253-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Portis A., Pangborn W. Calcium-induced lipid phase transitions and membrane fusion. Ann N Y Acad Sci. 1978;308:50–66. doi: 10.1111/j.1749-6632.1978.tb22013.x. [DOI] [PubMed] [Google Scholar]
- Parton R. Envelope proteins in Salmonella minnesota mutants. J Gen Microbiol. 1975 Jul;89(1):113–123. doi: 10.1099/00221287-89-1-113. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Schmidt G., Jann B., Jann K. The cell-wall lipopolysaccharide of Escherichia coli K-12. Structure and acceptor site for O-antigen and other substituents. Eur J Biochem. 1976 Nov 1;70(1):171–177. doi: 10.1111/j.1432-1033.1976.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K., Boman H. G. Cell-wall lipopolysaccharides of ampicillin-resistant mutants of Escherichia coli K-12. Eur J Biochem. 1976 Jul 1;66(2):369–377. doi: 10.1111/j.1432-1033.1976.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Neilands J. B. Genetic and biochemical characterization of the Escherichia coli K-12 fhuB mutation. J Bacteriol. 1984 Mar;157(3):874–880. doi: 10.1128/jb.157.3.874-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Increased production of the outer membrane receptors for colicins B, D and M by Escherichia coli under iron starvation. Biochem Biophys Res Commun. 1976 Jun 7;70(3):846–853. doi: 10.1016/0006-291x(76)90669-0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Heller D., Fenselau C. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12947–12951. [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Cross-linking of the proteins in the outer membrane of Escherichia coli. Biochim Biophys Acta. 1977 Apr 18;466(2):245–256. doi: 10.1016/0005-2736(77)90222-x. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Curtis N. A. The interplay of beta-lactamases and intrinsic factors in the resistance of gram-negative bacteria to penicillins and cephalosporins. Ann N Y Acad Sci. 1974 May 10;235(0):553–568. doi: 10.1111/j.1749-6632.1974.tb43290.x. [DOI] [PubMed] [Google Scholar]
- Rick P. D., Neumeyer B. A., Young D. A. Effect of altered lipid A synthesis on the synthesis of the OmpA protein in Salmonella typhimurium. J Biol Chem. 1983 Jan 10;258(1):629–635. [PubMed] [Google Scholar]
- Roantree R. J., Kuo T. T., MacPhee D. G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977 Dec;103(2):223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Rosenthal K. S., Storm D. R. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J Antibiot (Tokyo) 1977 Dec;30(12):1087–1092. doi: 10.7164/antibiotics.30.1087. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Leive L. Effect of variations in lipopolysaccharide on the fluidity of the outer membrane of Escherichia coli. J Biol Chem. 1977 Mar 25;252(6):2077–2081. [PubMed] [Google Scholar]
- Rottem S., Markowitz O., Hasin M., Razin S. Outer membrane proteins of smooth and rough strains of Proteus mirabilis. Eur J Biochem. 1979 Jun;97(1):141–146. doi: 10.1111/j.1432-1033.1979.tb13095.x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Ultrastructural study of Salmonella typhimurium treated with membrane-active agents: specific reaction dansylchloride with cell envelope components. J Bacteriol. 1978 Jul;135(1):198–206. doi: 10.1128/jb.135.1.198-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Schmidt G. Möglichkeiten zur Differenzierung von Salmonella-R-Formen mittels Antibiotica und antibakterieller Farbstoffe. Zentralbl Bakteriol Orig. 1970;212(2):505–511. [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Schmidt G., Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 3. Typisierung von S. minnesota-Mutanten mittels chemischer Agenzien. Zentralbl Bakteriol Orig. 1969 Dec;212(1):88–96. [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Schwarz H., Sonntag I., Henning U. Mutational change of membrane architecture. Mutants of Escherichia coli K12 missing major proteins of the outer cell envelope membrane. Biochim Biophys Acta. 1976 Oct 19;448(3):474–491. doi: 10.1016/0005-2736(76)90301-1. [DOI] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidén I., Boman H. G. Escherichia coli mutants with an altered sensitivity to cecropin D. J Bacteriol. 1983 Apr;154(1):170–176. doi: 10.1128/jb.154.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan-Lotter H., Gupta M., Sanderson K. E. The influence of cations on the permeability of the outer membrane of Salmonella typhimurium and other gram-negative bacteria. Can J Microbiol. 1979 Apr;25(4):475–485. doi: 10.1139/m79-070. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Fesik S. W., Armitage I. M. Structure and metal-binding properties of lipopolysaccharides from heptoseless mutants of Escherichia coli studied by 13C and 31P nuclear magnetic resonance. J Biol Chem. 1983 Nov 25;258(22):13466–13477. [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J., Blumenthal R., Walter A., Foulds J. Escherichia coli outer membrane protein K is a porin. J Bacteriol. 1983 Nov;156(2):867–872. doi: 10.1128/jb.156.2.867-872.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Takeuchi Y., Nikaido H. Persistence of segregated phospholipid domains in phospholipid--lipopolysaccharide mixed bilayers: studies with spin-labeled phospholipids. Biochemistry. 1981 Feb 3;20(3):523–529. doi: 10.1021/bi00506a013. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecoma E. S., Wu D. Membrane deenergization by colicin K affects fluorescence of exogenously added but not biosynthetically esterified parinaric acid probes in Escherichia coli. J Bacteriol. 1980 Jun;142(3):931–938. doi: 10.1128/jb.142.3.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Teuber M. Lysozyme-dependent production of spheroplast-like bodies from polymyxin B-treated Salmonella typhimurium. Arch Mikrobiol. 1970;70(2):139–146. doi: 10.1007/BF00412204. [DOI] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Permeability properties of chemically modified porin trimers from Escherichia coli B. J Biol Chem. 1981 Aug 10;256(15):8024–8029. [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Nakae T. The outer membrane permeability of Gram-negative bacteria: determination of permeability rate in reconstituted vesicle membranes. FEBS Lett. 1979 Oct 1;106(1):85–88. doi: 10.1016/0014-5793(79)80700-0. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T., Mitsui T., Nikaido H. X-ray diffraction studies of outer membranes of Salmonella typhimurium. J Biochem. 1979 Jan;85(1):173–182. doi: 10.1093/oxfordjournals.jbchem.a132307. [DOI] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Jensen M., Helander I., Nurminen M., Rietschel E. T., Mäkelä P. H. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 1981 Jun 29;129(1):145–149. doi: 10.1016/0014-5793(81)80777-6. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981 Apr;19(4):578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Sarvas M. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):664–667. doi: 10.1128/jb.139.2.664-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Vaara M., Viljanen P., Vaara T., Mäkelä P. H. An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J Immunol. 1984 May;132(5):2582–2589. [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Van Alphen L., Lugtenberg B., Rietschel E. T., Mombers C. Architecture of the outer membrane of Escherichia coli K12. Phase transitions of the bacteriophage K3 receptor complex. Eur J Biochem. 1979 Nov;101(2):571–579. doi: 10.1111/j.1432-1033.1979.tb19752.x. [DOI] [PubMed] [Google Scholar]
- Verkleij A., van Alphen L., Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli K12. II. Freeze fracture morphology of wild type and mutant strains. Biochim Biophys Acta. 1977 Apr 18;466(2):269–282. doi: 10.1016/0005-2736(77)90224-3. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen P., Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother. 1984 Jun;25(6):701–705. doi: 10.1128/aac.25.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Release of lipopolysaccharide in Escherichia coli resistant to the permeability increase induced by ethylenediaminetetraacetate. J Biol Chem. 1970 Mar 10;245(5):1108–1114. [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Zalman L. S., Nikaido H. Porin from Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jul;159(1):199–205. doi: 10.1128/jb.159.1.199-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Hancock R. E., Costerton J. W. Outer membrane protein K of Escherichia coli: purification and pore-forming properties in lipid bilayer membranes. J Bacteriol. 1983 Nov;156(2):873–879. doi: 10.1128/jb.156.2.873-879.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Konisky J. Increased binding of a hydrophobic, photolabile probe to Escherichia coli inversely correlates to membrane potential but not adenosine 5'-triphosphate levels. J Bacteriol. 1981 Jan;145(1):341–347. doi: 10.1128/jb.145.1.341-347.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Takamatsu N., Yoshikawa M. Preferential inhibitory action of sodium cholate on an Escherichia coli strain carrying a plasmid in an integrated state. J Bacteriol. 1978 Jan;133(1):406–408. doi: 10.1128/jb.133.1.406-408.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M. Screening method of agents against the R factor by the use of an Hfr made by integrative suppression with an R factor. Antimicrob Agents Chemother. 1974 Apr;5(4):362–365. doi: 10.1128/aac.5.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Yamada H., Mizushima S. Role of lipopolysaccharide in the receptor function for bacteriophage TuIb in Escherichia coli. J Bacteriol. 1981 Nov;148(2):712–715. doi: 10.1128/jb.148.2.712-715.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration through the gram-negative cell wall: a co-determinant of the efficacy of beta-lactam antibiotics. Int J Clin Pharmacol Biopharm. 1979 Mar;17(3):131–134. [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Hack A. M., Verhoef C., Havekes L. meoA is the structural gene for outer membrane protein c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):147–155. doi: 10.1007/BF00271665. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Verhoef K. Architecture of the outer membrane of Escherichia coli K12. I. Action of phospholipases A2 and C on wild type strains and outer membrane mutants. Biochim Biophys Acta. 1977 Apr 18;466(2):257–268. doi: 10.1016/0005-2736(77)90223-1. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Leunissen-Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli. III. Protein-lipopolysaccharide complexes in intramembraneous particles. J Bacteriol. 1978 Jun;134(3):1089–1098. doi: 10.1128/jb.134.3.1089-1098.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Nikaido H. Outer membrane of gram-negative bacteria. XVII. Secificity of transport process catalyzed by the lambda-receptor protein in Escherichia coli. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1100–1107. doi: 10.1016/0006-291x(77)90534-4. [DOI] [PubMed] [Google Scholar]


