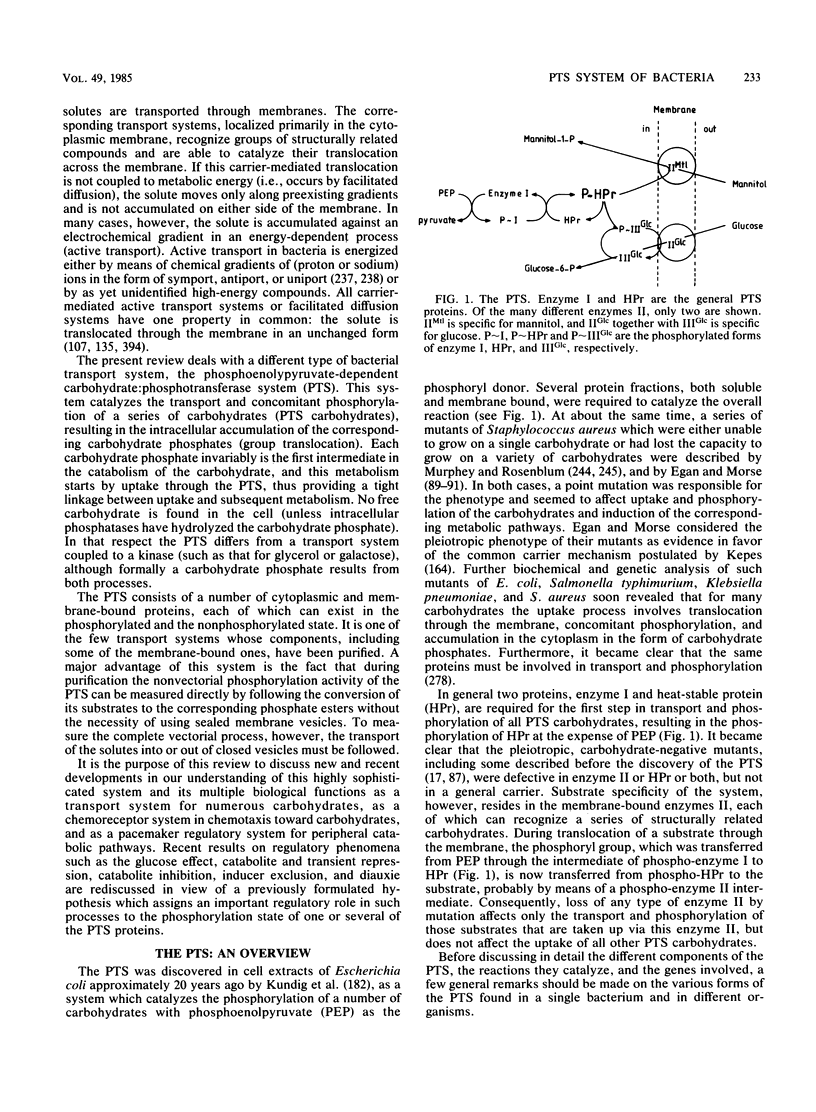
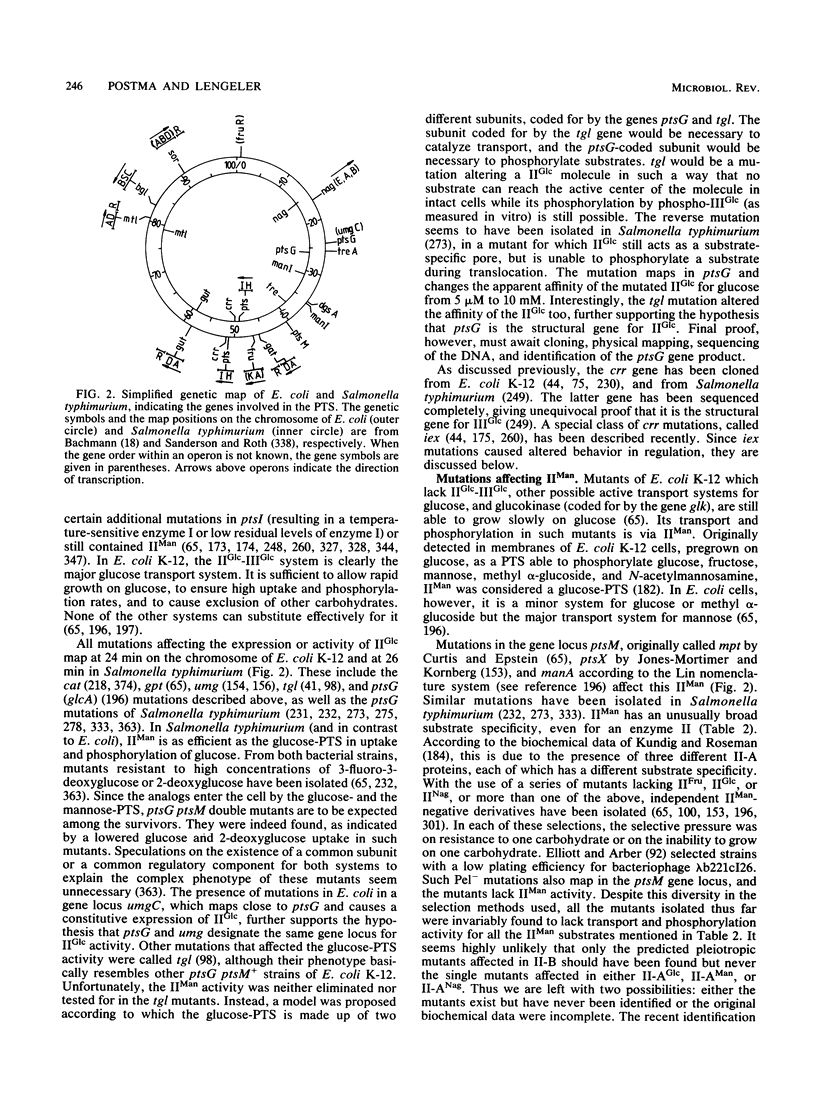
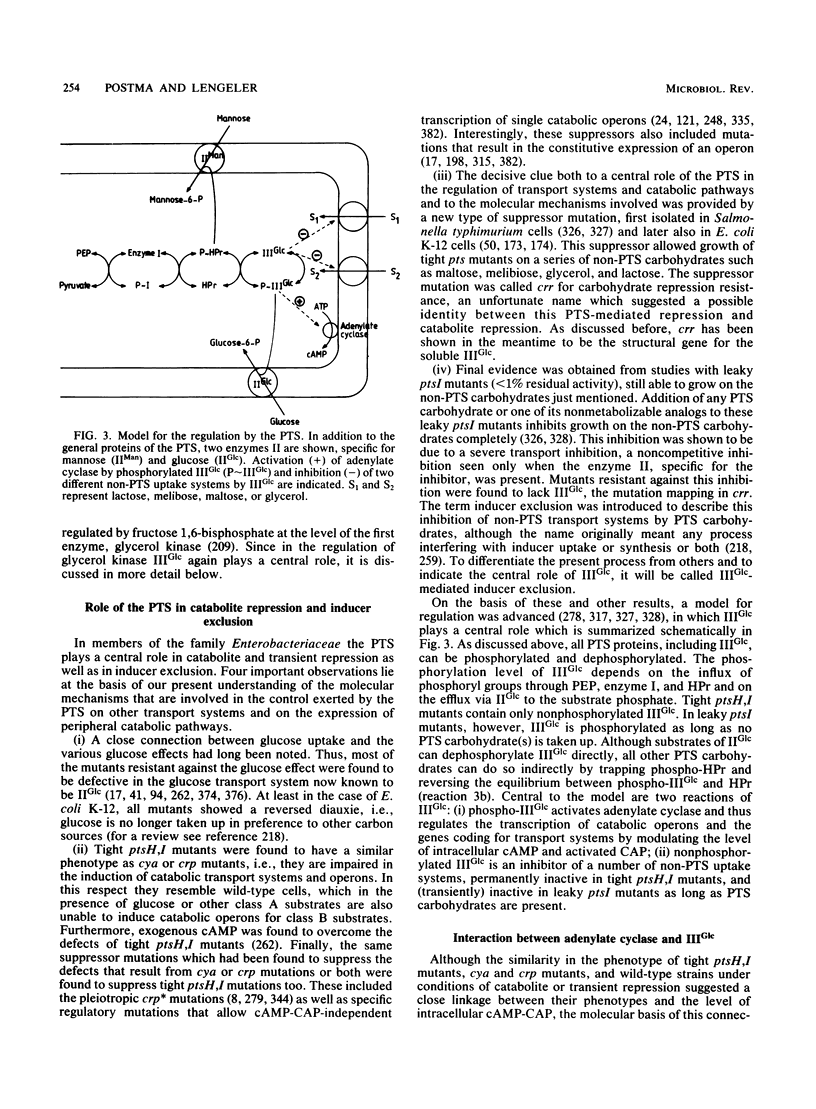
Full text
PDF

































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASENSIO C., AVIGAD G., HORECKER B. L. PREFERENTIAL GALACTOSE UTILIZATION IN A MUTANT STRAIN OF E. COLI. Arch Biochem Biophys. 1963 Dec;103:299–309. doi: 10.1016/0003-9861(63)90419-3. [DOI] [PubMed] [Google Scholar]
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Scraba D. The nature of the gal3 mutation of Escherichia coli. Mol Gen Genet. 1975;136(3):233–242. doi: 10.1007/BF00334018. [DOI] [PubMed] [Google Scholar]
- Aiba H., Mori K., Tanaka M., Ooi T., Roy A., Danchin A. The complete nucleotide sequence of the adenylate cyclase gene of Escherichia coli. Nucleic Acids Res. 1984 Dec 21;12(24):9427–9440. doi: 10.1093/nar/12.24.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaeddinoglu N. G., Charles H. P. Transfer of a gene for sucrose utilization into Escherichia coli K12, and consequent failure of expression of genes for D-serine utilization. J Gen Microbiol. 1979 Jan;110(1):47–59. doi: 10.1099/00221287-110-1-47. [DOI] [PubMed] [Google Scholar]
- Alexander J. K. Suppression of defects in cyclic adenosine 3',5'-monophosphate metabolism in Escherichia coli. J Bacteriol. 1980 Oct;144(1):205–209. doi: 10.1128/jb.144.1.205-209.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. K., Tyler B. Genetic analysis of succinate utilization in enzyme I mutants of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Bacteriol. 1975 Oct;124(1):252–261. doi: 10.1128/jb.124.1.252-261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert C. A., Frank R., Stüber K., Deutscher J., Hengstenberg W. Phosphoenolpyruvate-dependent protein kinase enzyme I of Streptococcus faecalis: purification and properties of the enzyme and characterization of its active center. Biochemistry. 1985 Feb 12;24(4):959–964. doi: 10.1021/bi00325a023. [DOI] [PubMed] [Google Scholar]
- Amaral D., Kornberg H. L. Regulation of fructose uptake by glucose in Escherichia coli. J Gen Microbiol. 1975 Sep;90(1):157–168. doi: 10.1099/00221287-90-1-157. [DOI] [PubMed] [Google Scholar]
- Ammer J., Brennenstuhl M., Schindler P., Höltje J. V., Zähner H. Phosphorylation of streptozotocin during uptake via the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. Antimicrob Agents Chemother. 1979 Dec;16(6):801–807. doi: 10.1128/aac.16.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B., Weigel N., Kundig W., Roseman S. Sugar transport. 3. Purification and properties of a phosphocarrier protein (HPr) of the phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):7023–7033. [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews K. J., Lin E. C. Selective advantages of various bacterial carbohydrate transport mechanisms. Fed Proc. 1976 Aug;35(10):2185–2189. [PubMed] [Google Scholar]
- Arai T. Effect of arsenate on chemotactic behavior of Escherichia coli. J Bacteriol. 1981 Feb;145(2):803–807. doi: 10.1128/jb.145.2.803-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr Regulation of adenylate cyclase synthesis in Escherichia coli: studies with cya-lac operon and protein fusion strains. J Bacteriol. 1982 Sep;151(3):1346–1357. doi: 10.1128/jb.151.3.1346-1357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley G. S., Hansen D. E., Jacobson G. R., Knowles J. R. Stereochemical course of the reactions catalyzed by the bacterial phosphoenolpyruvate:glucose phosphotransferase system. Biochemistry. 1982 Oct 26;21(22):5552–5556. doi: 10.1021/bi00265a026. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation and characterization of membrane vesicles from mutant and wild type Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14565–14575. [PubMed] [Google Scholar]
- Berkowitz D. D-Mannitol utilization in Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):232–240. doi: 10.1128/jb.105.1.232-240.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Kurtz M., Lin E. C., Richey D. P. Promoter-like mutant with increased expression of the glycerol kinase operon of Escherichia coli. J Bacteriol. 1971 Jun;106(3):724–731. doi: 10.1128/jb.106.3.724-731.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M., Lin E. C. Glycerol-specific revertants of a phosphoenolpyruvate phosphotransferase mutant: suppression by the desensitization of glycerol kinase to feedback inhibition. J Bacteriol. 1971 Jan;105(1):113–120. doi: 10.1128/jb.105.1.113-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M., Zwaig N., Lin E. C. Suppression of a pleiotropic mutant affecting glycerol dissimilation. Biochem Biophys Res Commun. 1970 Jan 23;38(2):272–278. doi: 10.1016/0006-291x(70)90708-4. [DOI] [PubMed] [Google Scholar]
- Bernsmann P., Alpert C. A., Muss P., Deutscher J., Hengstenberg W. The bacterial PEP-dependent phosphotransferase system mechanism of gluconate phosphorylation in Streptococcus faecalis. FEBS Lett. 1982 Feb 8;138(1):101–103. doi: 10.1016/0014-5793(82)80404-3. [DOI] [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Bhumiratana A., Anderson R. L., Costilow R. N. Trehalose metabolism by Bacillus popilliae. J Bacteriol. 1974 Aug;119(2):484–493. doi: 10.1128/jb.119.2.484-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Genetic evidence for the physiological significance of the D-tagatose 6-phosphate pathway of lactose and D-galactose degradation in staphylococcus aureus. J Bacteriol. 1974 Sep;119(3):698–704. doi: 10.1128/jb.119.3.698-704.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Wenger W. C., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. II. Isomerization of D-galactose 6-phosphate to D-tagatose 6-phosphate by a specific D-galactose-6-phosphate isomerase. J Biol Chem. 1980 Sep 25;255(18):8740–8744. [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Adenylate cyclase is required for chemotaxis to phosphotransferase system sugars by Escherichia coli. J Bacteriol. 1983 Mar;153(3):1187–1195. doi: 10.1128/jb.153.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Involvement of cyclic GMP in intracellular signaling in the chemotactic response of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3879–3883. doi: 10.1073/pnas.77.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenberg M., Yanofsky C. Regulatory region of the Klebsiella aerogenes tryptophan operon. J Bacteriol. 1982 Oct;152(1):49–56. doi: 10.1128/jb.152.1.49-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakova T. N., Dobrynina O. Y., Gershanovitch V. N. Isolation and investigation of the Escherichia coli mutant with the deletion in the ptsH gene. FEBS Lett. 1979 Nov 1;107(1):169–172. doi: 10.1016/0014-5793(79)80488-3. [DOI] [PubMed] [Google Scholar]
- Bolshakova T. N., Gabrielyan T. R., Bourd G. I., Gershanovitch V. N. Involvement of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system in regulation of transcription of catabolic genes. Eur J Biochem. 1978 Sep 1;89(2):483–490. doi: 10.1111/j.1432-1033.1978.tb12552.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boronat A., Jones-Mortimer M. C., Kornberg H. L. A specialized transducing phage, lambda psrlA, for the sorbitol phosphotransferase of Escherichia coli K12. J Gen Microbiol. 1982 Mar;128(3):605–611. doi: 10.1099/00221287-128-3-605. [DOI] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic AMP phosphodiesterase in Salmonella typhimurium: characteristics and physiological function. J Bacteriol. 1984 Nov;160(2):826–830. doi: 10.1128/jb.160.2.826-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Bourd G. I., Erlagaeva R. S., Bolshakova T. N., Gershanovitch V. N. Glucose catabolite repression in Escherichia coli K12 mutants defective in methyl-alpha-d-glucoside transport. Eur J Biochem. 1975 May 6;53(2):419–427. doi: 10.1111/j.1432-1033.1975.tb04082.x. [DOI] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton P., Boronat A., Hartley D. A., Jones-Mortimer M. C., Kornberg H. L., Parra F. Phosphotransferase-mediated regulation of carbohydrate utilization in Escherichia coli K12: location of the gsr (tgs) and iex (crr) genes by specialized transduction. J Gen Microbiol. 1983 Feb;129(2):349–356. doi: 10.1099/00221287-129-2-349. [DOI] [PubMed] [Google Scholar]
- Britton P., Lee L. G., Murfitt D., Boronat A., Jones-Mortimer M. C., Kornberg H. L. Location and direction of transcription of the ptsH and ptsI genes on the Escherichia coli K12 genome. J Gen Microbiol. 1984 Apr;130(4):861–868. doi: 10.1099/00221287-130-4-861. [DOI] [PubMed] [Google Scholar]
- Britton P., Murfitt D., Parra F., Jones-Mortimer M. C., Kornberg H. L. Phosphotransferase-mediated regulation of carbohydrate utilisation in Escherichia coli K12: identification of the products of genes on the specialised transducing phages lambda iex (crr) and lambda gsr (tgs). EMBO J. 1982;1(8):907–911. doi: 10.1002/j.1460-2075.1982.tb01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M., Elferink M. G., Robillard G. T. Phosphoenolpyruvate-dependent fructose phosphotransferase system of Rhodopseudomonas sphaeroides: purification and physicochemical and immunochemical characterization of a membrane-associated enzyme I. Biochemistry. 1982 Jan 5;21(1):82–88. doi: 10.1021/bi00530a015. [DOI] [PubMed] [Google Scholar]
- CLARK D. J., MARR A. G. STUDIES ON THE REPRESSION OF BETA-GALACTOSIDASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 23;92:85–94. doi: 10.1016/0926-6569(64)90272-x. [DOI] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Analysis of the differentiation and of the heterogeneity within a population of Escherichia coli undergoing induced beta-galactosidase synthesis. J Bacteriol. 1959 Nov;78:613–623. doi: 10.1128/jb.78.5.613-623.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959 Nov;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L., Feucht B. U., Morse M. L., Saier M. H., Jr Regulation of carbohydrate permeases and adenylate cyclase in Escherichia coli. Studies with mutant strains in which enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system is thermolabile. J Biol Chem. 1976 Sep 25;251(18):5522–5527. [PubMed] [Google Scholar]
- Chalumeau H., Delobbe A., Gay P. Biochemical and genetic study of D-glucitol transport and catabolism in Bacillus subtilis. J Bacteriol. 1978 Jun;134(3):920–928. doi: 10.1128/jb.134.3.920-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B., Holms W. H. Control of the sequential utilization of glucose and fructose by Escherichia coli. J Gen Microbiol. 1976 Aug;96(2):191–201. doi: 10.1099/00221287-95-2-191. [DOI] [PubMed] [Google Scholar]
- Cordaro C. Genetics of the bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Genet. 1976;10:341–359. doi: 10.1146/annurev.ge.10.120176.002013. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Anderson R. P., Grogan E. W., Jr, Wenzel D. J., Engler M., Roseman S. Promoter-like mutation affecting HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):245–252. doi: 10.1128/jb.120.1.245-252.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Melton T., Stratis J. P., Atagün M., Gladding C., Hartman P. E., Roseman S. Fosfomycin resistance: selection method for internal and extended deletions of the phosphoenolpyruvate:sugar phosphotransferase genes of Salmonella typhimurium. J Bacteriol. 1976 Dec;128(3):785–793. doi: 10.1128/jb.128.3.785-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R., Morse H. G., Morse M. L. Carbohydrate transport and cyclic 3',5' adenosine monophosphate (cAMP) levels in a temperature sensitive phosphotransferase mutant of Escherichia coli. Mol Gen Genet. 1974 Mar 6;129(1):1–10. doi: 10.1007/BF00269261. [DOI] [PubMed] [Google Scholar]
- Danchin A., Dondon L., Daniel J. Metabolic alterations mediated by 2-ketobutyrate in Escherichia coli K12. Mol Gen Genet. 1984;193(3):473–478. doi: 10.1007/BF00382086. [DOI] [PubMed] [Google Scholar]
- Danchin A., Guiso N., Roy A., Ullmann A. Identification of the Escherichia coli cya gene product as authentic adenylate cyclase. J Mol Biol. 1984 May 25;175(3):403–408. doi: 10.1016/0022-2836(84)90356-5. [DOI] [PubMed] [Google Scholar]
- Daniel J. Enzyme III stimulation of cyclic AMP synthesis in an Escherichia coli crp mutant. J Bacteriol. 1984 Mar;157(3):940–941. doi: 10.1128/jb.157.3.940-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Joseph E., Danchin A. Role of 2-ketobutyrate as an alarmone in E. coli K12: inhibition of adenylate cyclase activity mediated by the phosphoenolpyruvate: glycose phosphotransferase transport system. Mol Gen Genet. 1984;193(3):467–472. doi: 10.1007/BF00382085. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Huttner E., Danchin A. Analysis of the ptsH-ptsI-crr region in Escherichia coli K-12: evidence for the existence of a single transcriptional unit. Gene. 1984 Dec;32(1-2):31–40. doi: 10.1016/0378-1119(84)90029-5. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Roy A., Danchin A. Analysis of the ptsH-ptsI-crr region in Escherichia coli K-12: nucleotide sequence of the ptsH gene. Gene. 1985;35(1-2):199–207. doi: 10.1016/0378-1119(85)90172-6. [DOI] [PubMed] [Google Scholar]
- Defez R., De Felice M. The metabolism of beta-glucosides in Escherichia coli K12. Ann Microbiol (Paris) 1982 May-Jun;133(3):347–350. [PubMed] [Google Scholar]
- Delidakis C. E., Jones-Mortimer M. C., Kornberg H. L. A mutant inducible for galactitol utilization in Escherichia coli K12. J Gen Microbiol. 1982 Mar;128(3):601–604. doi: 10.1099/00221287-128-3-601. [DOI] [PubMed] [Google Scholar]
- Delobbe A., Chalumeau H., Claverie J. M., Gay P. Phosphorylation of intracellular fructose in Bacillus subtilis mediated by phosphoenolpyruvate-1-fructose phosphotransferase. Eur J Biochem. 1976 Jul 15;66(3):485–491. doi: 10.1111/j.1432-1033.1976.tb10573.x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W., Jr The hexose phosphate transport system of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1976;44:237–259. doi: 10.1002/9780470122891.ch7. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Schmidt M. R., Saier M. H., Jr Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):239–244. doi: 10.1002/jcb.1982.240180211. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983 Feb;153(2):861–866. doi: 10.1128/jb.153.2.861-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrogosz W. J., Hall G. W., Sherba D. K., Silva D. O., Harman J. G., Melton T. Regulatory interactions among the cya, crp and pts gene products in Salmonella typhimurium. Mol Gen Genet. 1983;192(3):477–486. doi: 10.1007/BF00392194. [DOI] [PubMed] [Google Scholar]
- Dooijewaard G., Roossien F. F., Robillard G. T. Escherichia coli phosphoenolpyruvate dependent phosphotransferase system. Copurification of HPr and alpha 1-6 glucan. Biochemistry. 1979 Jul 10;18(14):2990–2996. doi: 10.1021/bi00581a013. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörschug M., Frank R., Kalbitzer H. R., Hengstenberg W., Deutscher J. Phosphoenolpyruvate-dependent phosphorylation site in enzyme IIIglc of the Escherichia coli phosphotransferase system. Eur J Biochem. 1984 Oct 1;144(1):113–119. doi: 10.1111/j.1432-1033.1984.tb08438.x. [DOI] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):172–183. doi: 10.1016/0926-6585(65)90101-9. [DOI] [PubMed] [Google Scholar]
- Elliott J., Arber W. E. coli K-12 pel mutants, which block phage lambda DNA injection, coincide with ptsM, which determines a component of a sugar transport system. Mol Gen Genet. 1978 Apr 25;161(1):1–8. doi: 10.1007/BF00266608. [DOI] [PubMed] [Google Scholar]
- Elvin C. M., Kornberg H. L. A mutant beta-D-glucoside transport system of Escherichia coli resistant to catabolite inhibition. FEBS Lett. 1982 Oct 18;147(2):137–142. doi: 10.1016/0014-5793(82)81027-2. [DOI] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlagaeva R. S., Bolshakova T. N., Shulgina M. V., Bourd G. I., Gershanovitch V. N. Glucose effect in tgl mutant of Escherichia col K12 defective in methyl-alpha-D-glucoside transport. Eur J Biochem. 1977 Jan 3;72(1):127–135. doi: 10.1111/j.1432-1033.1977.tb11232.x. [DOI] [PubMed] [Google Scholar]
- Erni B., Trachsel H., Postma P. W., Rosenbusch J. P. Bacterial phosphotransferase system. Solubilization and purification of the glucose-specific enzyme II from membranes of Salmonella typhimurium. J Biol Chem. 1982 Nov 25;257(22):13726–13730. [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The role of phosphotransferase-mediated syntheses of fructose 1-phosphate and fructose 6-phosphate in the growth of Escherichia coli on fructose. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):105–119. doi: 10.1098/rspb.1974.0065. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The utilization of fructose by Escherichia coli. Properties of a mutant defective in fructose 1-phosphate kinase activity. Biochem J. 1973 Feb;132(2):341–347. doi: 10.1042/bj1320341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Wilson G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Mar;59(3):988–995. doi: 10.1073/pnas.59.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Fraenkel D. G. The phosphoenolpyruvate-initiated pathway of fructose metabolism in Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6458–6463. [PubMed] [Google Scholar]
- Friedman S. A., Hays J. B. Initial characterization of hexose and hexitol phosphoenolpyruvate-dependent phosphotransferases of Staphylococcus aureus. J Bacteriol. 1977 Jun;130(3):991–999. doi: 10.1128/jb.130.3.991-999.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. A new assay of the phosphotransferase system in Escherichia coli. Biochem Biophys Res Commun. 1969 Feb 21;34(4):382–387. doi: 10.1016/0006-291x(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Galloway R. J., Taylor B. L. Histidine starvation and adenosine 5'-triphosphate depletion in chemotaxis of Salmonella typhimurium. J Bacteriol. 1980 Dec;144(3):1068–1075. doi: 10.1128/jb.144.3.1068-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Mayrand D., Vadeboncoeur C. Isolation of a novel protein involved in the transport of fructose by an inducible phosphoenolpyruvate fructose phosphotransferase system in Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):755–763. doi: 10.1128/jb.160.2.755-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Cordier P., Marquet M., Delobbe A. Carbohydrate metabolism and transport in Bacillus subtilis. A study of ctr mutations. Mol Gen Genet. 1973 Mar 19;121(4):355–368. doi: 10.1007/BF00433234. [DOI] [PubMed] [Google Scholar]
- Gee D. L., Baumann P., Baumann L. Enzymes of D-fructose catabolism in species of Beneckea and Photobacterium. Arch Microbiol. 1975 Apr 7;103(2):205–207. doi: 10.1007/BF00436351. [DOI] [PubMed] [Google Scholar]
- Gershanovitch V. N., Ilyina T. S., Rusina O. Y., Yourovitskaya N. V., Bolshakova T. N. Repression of inducible enzyme synthesis in a mutant of Escherichia coli K 12 deleted for the ptsH gene. Mol Gen Genet. 1977 Jun 8;153(2):185–190. doi: 10.1007/BF00264734. [DOI] [PubMed] [Google Scholar]
- Glesyna M. L., Bolshakova T. N., Gershanovitch V. N. Effect of ptsI and ptsH mutations on initiation of transcription of the Escherichia coli lactose operon. Mol Gen Genet. 1983;190(3):417–420. doi: 10.1007/BF00331070. [DOI] [PubMed] [Google Scholar]
- Goldenbaum P. E., Hall G. A. Transport of cyclic adenosine 3',5'-monophosphate across Escherichia coli vesicle membranes. J Bacteriol. 1979 Nov;140(2):459–467. doi: 10.1128/jb.140.2.459-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F. C., Waygood E. B., Saier M. H., Jr Bacterial phosphotransferase system: regulation of mannitol enzyme II activity by sulfhydryl oxidation. Biochemistry. 1985 Jan 1;24(1):47–51. doi: 10.1021/bi00322a008. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5799–5801. doi: 10.1073/pnas.77.10.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Isolation and characterization of an Escherichia coli mutant affected in the regulation of adenylate cyclase. J Bacteriol. 1981 Dec;148(3):753–761. doi: 10.1128/jb.148.3.753-761.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiso N., Blazy B. Regulatory aspects of the cyclic AMP receptor protein in Escherichia coli K-12. Biochem Biophys Res Commun. 1980 May 14;94(1):278–283. doi: 10.1016/s0006-291x(80)80217-8. [DOI] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Different sidedness of functionally homologous essential thiols in two membrane-bound phosphotransferase enzymes of Escherichia coli detected by permeant and nonpermeant thiol reagents. J Biol Chem. 1980 Jun 10;255(11):5075–5081. [PubMed] [Google Scholar]
- Haguenauer R., Kepes A. NaF inhibition of phosphorylation and dephosphorylation involved in -methyl-D glucoside transport in E. coli K 12. A pH dependant phenomenon sensitive to uncoupling agents. Biochimie. 1972;54(4):505–512. doi: 10.1016/s0300-9084(72)80235-9. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Imai K., Romano C. P. Genetics of the lac-PTS system of Klebsiella. Genet Res. 1982 Jun;39(3):287–302. doi: 10.1017/s0016672300020966. [DOI] [PubMed] [Google Scholar]
- Hanson T. E., Anderson R. L. Phosphoenolpyruvate-dependent formation of D-fructose 1-phosphate by a four-component phosphotransferase system. Proc Natl Acad Sci U S A. 1968 Sep;61(1):269–276. doi: 10.1073/pnas.61.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman J. G., Dobrogosz W. J. Mechanism of CRP-mediated cya suppression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):191–199. doi: 10.1128/jb.153.1.191-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Gazdar C., Prasad C., Peterkofsky A., Curtis S. J., Epstein W. Involvement of the glucose enzymes II of the sugar phosphotransferase system in the regulation of adenylate cyclase by glucose in Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2462–2468. [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Hays J. B., Simoni R. D., Roseman S. Sugar transport. V. A trimeric lactose-specific phosphocarrier protein of the Staphylococcus aureus phosphotransferase system. J Biol Chem. 1973 Feb 10;248(3):941–956. [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Sensory transduction in bacterial chemotaxis. Int Rev Cytol. 1983;81:33–70. doi: 10.1016/s0074-7696(08)62334-7. [DOI] [PubMed] [Google Scholar]
- Hengge R., Boos W. Maltose and lactose transport in Escherichia coli. Examples of two different types of concentrative transport systems. Biochim Biophys Acta. 1983 Aug 11;737(3-4):443–478. doi: 10.1016/0304-4157(83)90009-6. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W. Enzymology of carbohydrate transport in bacteria. Curr Top Microbiol Immunol. 1977;77:97–126. doi: 10.1007/978-3-642-66740-4_4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Asensio M., Del Campo F. F. Enhancement of alpha-methylglucoside efflux by respiration in respiratory mutants of Escherichia coli K-12. Arch Biochem Biophys. 1980 Apr 1;200(2):309–318. doi: 10.1016/0003-9861(80)90360-4. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolution of ribosomal proteins in Enterobacteriaceae. J Bacteriol. 1978 Mar;133(3):1089–1095. doi: 10.1128/jb.133.3.1089-1095.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdig H., Hengstenberg W. The bacterial phosphoenolpyruvate dependent phosphotransferase system (PTS): solubilisation and kinetic parameters of the glucose-specific membrane bound enzyme II component of Streptococcus faecalis. FEBS Lett. 1980 May 19;114(1):103–106. doi: 10.1016/0014-5793(80)80869-6. [DOI] [PubMed] [Google Scholar]
- Jablonski E. G., Brand L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Preparation of a fluorescein derivative of the glucose-specific phosphocarrier protein IIIGlc and its binding to the phosphocarrier protein HPr. J Biol Chem. 1983 Aug 25;258(16):9690–9699. [PubMed] [Google Scholar]
- Jacobson G. R., Kelly D. M., Finlay D. R. The intramembrane topography of the mannitol-specific enzyme II of the Escherichia coli phosphotransferase system. J Biol Chem. 1983 Mar 10;258(5):2955–2959. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. I. Properties of the purified permease. J Biol Chem. 1983 Sep 10;258(17):10748–10756. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Saier M. H., Jr Purification of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1979 Jan 25;254(2):249–252. [PubMed] [Google Scholar]
- Jacobson G. R., Tanney L. E., Kelly D. M., Palman K. B., Corn S. B. Substrate and phospholipid specificity of the purified mannitol permease of Escherichia coli. J Cell Biochem. 1983;23(1-4):231–240. doi: 10.1002/jcb.240230120. [DOI] [PubMed] [Google Scholar]
- Jaffor Ullah A. H., Cirillo V. P. Mycoplasma phosphoenolpyruvate-dependent sugar phosphotransferase system: purification and characterization of enzyme I. J Bacteriol. 1977 Sep;131(3):988–996. doi: 10.1128/jb.131.3.988-996.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R. Z., Lin E. C. An inducible phosphoenolpyruvate: dihydroxyacetone phosphotransferase system in Escherichia coli. J Gen Microbiol. 1984 Jan;130(1):83–88. doi: 10.1099/00221287-130-1-83. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Amino-sugar transport systems of Escherichia coli K12. J Gen Microbiol. 1980 Apr;117(2):369–376. doi: 10.1099/00221287-117-2-369. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Genetic control of inducer exclusion by Escherichia coli. FEBS Lett. 1974 Nov 1;48(1):93–95. doi: 10.1016/0014-5793(74)81070-7. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Genetical analysis of fructose utilization by Escherichia coli. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):121–131. doi: 10.1098/rspb.1974.0066. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Uptake of fructose by the sorbitol phosphotransferase of Escherichia coli K12. J Gen Microbiol. 1976 Oct;96(2):383–391. doi: 10.1099/00221287-96-2-383. [DOI] [PubMed] [Google Scholar]
- Joseph E., Bernsley C., Guiso N., Ullmann A. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol Gen Genet. 1982;185(2):262–268. doi: 10.1007/BF00330796. [DOI] [PubMed] [Google Scholar]
- KEPES A. [Kinetic studies on galactoside permease of Escherichia coli]. Biochim Biophys Acta. 1960 May 6;40:70–84. doi: 10.1016/0006-3002(60)91316-0. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Regulation of sugar transport in isolated bacterial membrane preparations from Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):724–731. doi: 10.1073/pnas.63.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- Kelker N. E., Anderson R. L. Evidence for vectorial phosphorylation of D-fructose by intact cells of Aerobacter aerogenes. J Bacteriol. 1972 Dec;112(3):1441–1443. doi: 10.1128/jb.112.3.1441-1443.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker N. E., Anderson R. L. Sorbitol metabolism in Aerobacter aerogenes. J Bacteriol. 1971 Jan;105(1):160–164. doi: 10.1128/jb.105.1.160-164.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker N. E., Simkins R. A., Anderson R. L. Pathway of L-sorbose metabolism in Aerobacter aerogenes. J Biol Chem. 1972 Mar 10;247(5):1479–1483. [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Kolb A., Spassky A., Chapon C., Blazy B., Buc H. On the different binding affinities of CRP at the lac, gal and malT promoter regions. Nucleic Acids Res. 1983 Nov 25;11(22):7833–7852. doi: 10.1093/nar/11.22.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Robillard G. T. Physical mechanism for regulation of proton solute symport in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5480–5484. doi: 10.1073/pnas.79.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop A. H., Hartley M., Bourgeois S. Analysis of the cya locus of Escherichia coli. Gene. 1984 May;28(2):133–146. doi: 10.1016/0378-1119(84)90250-6. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Britton P., Jones-Mortimer M. C., Lee L. G. The role of the phosphoenolpyruvate-phosphotransferase system in inducer exclusion. Biochem Soc Trans. 1984 Apr;12(2):157–159. doi: 10.1042/bst0120157. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Fine control of sugar uptake by Escherichia coli. Symp Soc Exp Biol. 1973;27:175–193. [PubMed] [Google Scholar]
- Kornberg H. L., Riordan C. Uptake of galactose into Escherichia coli by facilitated diffusion. J Gen Microbiol. 1976 May;94(1):75–89. doi: 10.1099/00221287-94-1-75. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Watts P. D. tgs and crr: Genes involved in catabolite inhibition and inducer exclusion in Escherichia coli. FEBS Lett. 1979 Aug 15;104(2):313–316. doi: 10.1016/0014-5793(79)80841-8. [DOI] [PubMed] [Google Scholar]
- Kornberg H., Watts P. D., Brown K. Mechanisms of 'inducer exclusion' by glucose. FEBS Lett. 1980 Aug 25;117 (Suppl):K28–K36. doi: 10.1016/0014-5793(80)80567-9. [DOI] [PubMed] [Google Scholar]
- Korte T., Hengstenberg W. Purification and characterization of the inducible lactose-specific membrane-bound component of the staphylococcal phosphenolpyruvate-dependent phosphotransferase system. Eur J Biochem. 1971 Nov 11;23(2):295–302. doi: 10.1111/j.1432-1033.1971.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Iuchi S., Fujisawa A., Tanaka S. Separation of four components of the phosphoenolpyruvate: glucose phosphotransferase system in Vibrio parahaemolyticus. Microbiol Immunol. 1979;23(3):131–146. doi: 10.1111/j.1348-0421.1979.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Harrington W. F., Roseman S. Sugar transport by the bacterial phosphotransferase system. Studies on the molecular weight and association of enzyme I. J Biol Chem. 1982 Dec 10;257(23):14470–14476. [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lee C. A., Jacobson G. R., Saier M. H., Jr Plasmid-directed synthesis of enzymes required for D-mannitol transport and utilization in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7336–7340. doi: 10.1073/pnas.78.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. J Biol Chem. 1983 Sep 10;258(17):10761–10767. [PubMed] [Google Scholar]
- Lee L. G., Britton P., Parra F., Boronat A., Kornberg H. Expression of the ptsH+ gene of Escherichia coli cloned on plasmid pBR322. A convenient means for obtaining the histidine-containing carrier protein HPr. FEBS Lett. 1982 Nov 29;149(2):288–292. doi: 10.1016/0014-5793(82)81119-8. [DOI] [PubMed] [Google Scholar]
- Leib T. K., Gerlt J. A. Evidence for a small catalytic domain in the adenylate cyclase from Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12982–12987. [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Analysis of mutations affecting the dissmilation of galactitol (dulcitol) in Escherichia coli K 12. Mol Gen Genet. 1977 Mar 28;152(1):83–91. doi: 10.1007/BF00264944. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Analysis of the physiological effects of the antibiotic streptozotocin on Escherichia coli K 12 and other sensitive bacteria. Arch Microbiol. 1980 Dec;128(2):196–203. doi: 10.1007/BF00406158. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Characterisation of mutants of Escherichia coli K12, selected by resistance to streptozotocin. Mol Gen Genet. 1980;179(1):49–54. doi: 10.1007/BF00268445. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Steinberger H. Analysis of regulatory mechanisms controlling the activity of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978 Nov 16;167(1):75–82. doi: 10.1007/BF00270323. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Steinberger H. Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978 Aug 17;164(2):163–169. doi: 10.1007/BF00267381. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Untersuchungen zum Glukose-Effekt bei der Synthese der Galaktose-Enzyme von Escherichia coli. Z Vererbungsl. 1966;98(3):203–229. [PubMed] [Google Scholar]
- Leonard J. E., Saier M. H., Jr Genetic dissection of catalytic activities of the Salmonella typhimurium mannitol enzyme II. J Bacteriol. 1981 Feb;145(2):1106–1109. doi: 10.1128/jb.145.2.1106-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. II. Reconstitution of vectorial transphosphorylation in phospholipid vesicles. J Biol Chem. 1983 Sep 10;258(17):10757–10760. [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Link C. D., Reiner A. M. Genotypic exclusion: a novel relationship between the ribitol-arabitol and galactitol genes of E. coli. Mol Gen Genet. 1983;189(2):337–339. doi: 10.1007/BF00337827. [DOI] [PubMed] [Google Scholar]
- Link C. D., Reiner A. M. Inverted repeats surround the ribitol-arabitol genes of E. coli C. Nature. 1982 Jul 1;298(5869):94–96. doi: 10.1038/298094a0. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. Different cyclic AMP requirements for induction of the arabinose and lactose operons of Escherichia coli. J Mol Biol. 1973 Sep 5;79(1):149–162. doi: 10.1016/0022-2836(73)90276-3. [DOI] [PubMed] [Google Scholar]
- Liu K. D., Roseman S. Kinetic characterization and regulation of phosphoenolpyruvate-dependent methyl alpha-D-glucopyranoside transport by Salmonella typhimurium membrane vesicles. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7142–7145. doi: 10.1073/pnas.80.23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Z. Purification and characterization of the IIIXtl phospho-carrier protein of the phosphoenolpyruvate-dependent xylitol:phosphotransferase found in Lactobacillus casei C183. J Bacteriol. 1983 Nov;156(2):611–619. doi: 10.1128/jb.156.2.611-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MURPHEY W. H., ROSENBLUM E. D. GENETIC RECOMBINATION BY TRANSDUCTION BETWEEN MANNITOL-NEGATIVE MUTANTS OF STAPHYLOCOCCUS AUREUS. Proc Soc Exp Biol Med. 1964 Jun;116:544–548. doi: 10.3181/00379727-116-29302. [DOI] [PubMed] [Google Scholar]
- MURPHEY W. H., ROSENBLUM E. D. MANNITOL CATABOLISM BY STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 Aug;107:292–297. doi: 10.1016/0003-9861(64)90332-7. [DOI] [PubMed] [Google Scholar]
- MacDonald C., Riley M. Cloning chromosomal lac genes of Klebsiella pneumoniae. Gene. 1983 Oct;24(2-3):341–345. doi: 10.1016/0378-1119(83)90096-3. [DOI] [PubMed] [Google Scholar]
- Macchia V., Caputo G., Mandato E., Rocino A., Adhya S., Pastan I. Guanylate cyclase activity in Escherichia coli mutants defective in adenylate cyclase. J Bacteriol. 1981 Sep;147(3):931–934. doi: 10.1128/jb.147.3.931-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerfeld I. H., Miller D., Spitz E., Rickenberg H. V. Regulation of the synthesis of adenylate cyclase in Escherichia coli by the cAMP -- cAMP receptor protein complex. Mol Gen Genet. 1981;181(4):470–475. doi: 10.1007/BF00428738. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Ambudkar S. V., Thomas J., Schiller L. Phosphate/hexose 6-phosphate antiport in Streptococcus lactis. J Bacteriol. 1984 Apr;158(1):238–245. doi: 10.1128/jb.158.1.238-245.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell J., Shimamoto G. T., Bissett D. L., Anderson R. L. Pathway of galactitol catabolism in Klebsiella pneumoniae. Biochem Biophys Res Commun. 1976 Jul 12;71(1):221–227. doi: 10.1016/0006-291x(76)90271-0. [DOI] [PubMed] [Google Scholar]
- Marquet M., Creignou M. C., Dedonder R. The phosphoenolpyruvate : methyl-alpha-D-glucoside phosphotransferase system in Bacillus subtilis Marburg 168 : purification and identification of the phosphocarrier protein (HPr). Biochimie. 1976;58(4):435–441. doi: 10.1016/s0300-9084(76)80254-4. [DOI] [PubMed] [Google Scholar]
- Marquet M., Creignou M. C., Dedonder R. The phosphoenolpyruvate : methyl-alpha-d-glucoside phosphotransferase system in Bacillus subtilis Marburg : kinetic studies of enzyme ii and evidence for a phosphoryl enzyme ii intermediate. Biochimie. 1978;60(11-12):1283–1287. doi: 10.1016/s0300-9084(79)80445-9. [DOI] [PubMed] [Google Scholar]
- Mattoo R. L., Khandelwal R. L., Waygood E. B. Isoelectrophoretic separation and the detection of soluble proteins containing acid-labile phosphate: use of the phosphoenolpyruvate:sugar phosphotransferase system as a model system for N1-P-histidine- and N3-P-histidine-containing proteins. Anal Biochem. 1984 May 15;139(1):1–16. doi: 10.1016/0003-2697(84)90383-x. [DOI] [PubMed] [Google Scholar]
- Mattoo R. L., Waygood E. B. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can J Biochem Cell Biol. 1983 Jan;61(1):29–37. doi: 10.1139/o83-005. [DOI] [PubMed] [Google Scholar]
- McEntee K. Genetic analysis of the Escherichia coli K-12 srl region. J Bacteriol. 1977 Dec;132(3):904–911. doi: 10.1128/jb.132.3.904-911.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Meadow N. D., Rosenberg J. M., Pinkert H. M., Roseman S. Sugar transport by the bacterial phosphotransferase system. Evidence that crr is the structural gene for the Salmonella typhimurium glucose-specific phosphocarrier protein IIIGlc. J Biol Chem. 1982 Dec 10;257(23):14538–14542. [PubMed] [Google Scholar]
- Meadow N. D., Saffen D. W., Dottin R. P., Roseman S. Molecular cloning of the crr gene and evidence that it is the structural gene for IIIGlc, a phosphocarrier protein of the bacterial phosphotransferase system. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2528–2532. doi: 10.1073/pnas.79.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton T., Hartman P. E., Stratis J. P., Lee T. L., Davis A. T. Chemotaxis of Salmonella typhimurium to amino acids and some sugars. J Bacteriol. 1978 Feb;133(2):708–716. doi: 10.1128/jb.133.2.708-716.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton T., Kundig W., Hartman P. E., Meadow N. 3-Deoxy-3-fluoro-D-glucose-resistant Salmonella typhimurium mutants defective in the phosphoenolpyruvate:glycose phosphotransferase system. J Bacteriol. 1976 Dec;128(3):794–800. doi: 10.1128/jb.128.3.794-800.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura C. S., Eisenberg L. B., Jacobson G. R. Resolution of the phosphotransferase enzymes of Streptococcus mutans: purification and preliminary characterization of a heat-stable phosphocarrier protein. Infect Immun. 1984 Jun;44(3):708–715. doi: 10.1128/iai.44.3.708-715.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misset O., Blaauw M., Postma P. W., Robillard G. T. Bacterial phosphoenolpyruvate-dependent phosphotransferase system. Mechanism of the transmembrane sugar translocation and phosphorylation. Biochemistry. 1983 Dec 20;22(26):6163–6170. doi: 10.1021/bi00295a019. [DOI] [PubMed] [Google Scholar]
- Misset O., Brouwer M., Robillard G. T. Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Evidence that the dimer is the active form of enzyme I. Biochemistry. 1980 Mar 4;19(5):883–890. doi: 10.1021/bi00546a009. [DOI] [PubMed] [Google Scholar]
- Misset O., Robillard G. T. Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: mechanism of phosphoryl-group transfer from phosphoenolpyruvate to HPr. Biochemistry. 1982 Jun 22;21(13):3136–3142. doi: 10.1021/bi00256a016. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Misko T. P., Roseman S. Sugar transport by the bacterial phosphotransferase system. Regulation of other transport systems (lactose and melibiose). J Biol Chem. 1982 Dec 10;257(23):14553–14564. [PubMed] [Google Scholar]
- Morse H. G., Penberthy W. K., Morse M. L. Biochemical characterization of the ctr mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):690–694. doi: 10.1128/jb.108.2.690-694.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugharbil U., Cirillo V. P. Mycoplasma phosphoenolpyruvate-dependent sugar phosphotransferase system: glucose-negative mutant and regulation of intracellular cyclic AMP. J Bacteriol. 1978 Jan;133(1):203–209. doi: 10.1128/jb.133.1.203-209.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Lengeler J., Postma P. W. Role of IIIGlc of the phosphoenolpyruvate-glucose phosphotransferase system in inducer exclusion in Escherichia coli. J Bacteriol. 1984 Oct;160(1):360–364. doi: 10.1128/jb.160.1.360-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Postma P. W. Interactions in vivo between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and the glycerol and maltose uptake systems of Salmonella typhimurium. Eur J Biochem. 1984 Feb 15;139(1):29–34. doi: 10.1111/j.1432-1033.1984.tb07971.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Schuitema A. R., Benne R., van der Ploeg L. H., Plijter J. S., Aan F., Postma P. W. Molecular cloning, sequencing, and expression of the crr gene: the structural gene for IIIGlc of the bacterial PEP:glucose phosphotransferase system. EMBO J. 1984 Jul;3(7):1587–1593. doi: 10.1002/j.1460-2075.1984.tb02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M., Wright J. K. Chemical modification of the lactose carrier of Escherichia coli by plumbagin, phenylarsinoxide or diethylpyrocarbonate affects the binding of galactoside. Eur J Biochem. 1983 Dec 15;137(3):615–621. doi: 10.1111/j.1432-1033.1983.tb07870.x. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Gay P., Dedonder R. Identification of the structural gene of the PEP-phosphotransferase enzyme I in Bacillus subtilis Marburg. Mol Gen Genet. 1975;136(4):337–349. doi: 10.1007/BF00341718. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Blumenberg M., Yanofsky C. Comparison of the nucleoside sequence of trpA and sequences immediately beyond the trp operon of Klebsiella aerogenes. Salmonella typhimurium and Escherichia coli. Nucleic Acids Res. 1981 Apr 10;9(7):1743–1755. doi: 10.1093/nar/9.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Ueyama K., Niiya S., Kanazawa H., Futai M., Tsuchiya T. Role of inducer exclusion in preferential utilization of glucose over melibiose in diauxic growth of Escherichia coli. J Bacteriol. 1981 Jun;146(3):1030–1037. doi: 10.1128/jb.146.3.1030-1037.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K. Phenomenon of transient repression in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1201–1209. doi: 10.1128/jb.91.3.1201-1209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra F., Jones-Mortimer M. C., Kornberg H. L. Phosphotransferase-mediated regulation of carbohydrate utilization in Escherichia coli K12: the nature of the iex (crr) and gsr (tgs) mutations. J Gen Microbiol. 1983 Feb;129(2):337–348. doi: 10.1099/00221287-129-2-337. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Perret J., Gay P. Kinetic study of a phosphoryl exchange reaction between fructose and fructose 1-phosphate catalyzed by the membrane-bound enzyme II of the phosphoenolpyruvate-fructose 1-phosphotransferase system of Bacillus subtilis. Eur J Biochem. 1979 Dec;102(1):237–246. doi: 10.1111/j.1432-1033.1979.tb06285.x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A. Escherichia coli adenylate cyclase as a sensor of sugar transport function. Adv Cyclic Nucleotide Res. 1981;14:215–228. [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2324–2328. doi: 10.1073/pnas.71.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Measurements of rates of adenosine 3':5'-cyclic monophosphate synthesis in intact Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2149–2152. doi: 10.1073/pnas.70.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Cordaro J. C., Roseman S. Sugar transport. A pleiotropic membrane mutant of Salmonella typhimurium. J Biol Chem. 1977 Nov 10;252(21):7862–7876. [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Involvement of the phosphotransferase system in galactose transport in Salmonella typhimurium. FEBS Lett. 1976 Jan 1;61(1):49–53. doi: 10.1016/0014-5793(76)80169-x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Neyssel O. M., van Ree R. Glucose transport in Salmonella typhimurium and Escherichia coli. Eur J Biochem. 1982 Mar;123(1):113–119. doi: 10.1111/j.1432-1033.1982.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Regulation of sugar transport in Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):261–267. [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Schuitema A., Kwa C. Regulation of methyl beta-galactoside permease activity in pts and crr mutants of Salmonella typhimurium. Mol Gen Genet. 1981;181(4):448–453. doi: 10.1007/BF00428734. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D. A., Roseman S. The primary structure of Salmonella typhimurium HPr, a phosphocarrier protein of the phosphoenolpyruvate:glycose phosphotransferase system. A correction. J Biol Chem. 1984 Dec 25;259(24):15212–15214. [PubMed] [Google Scholar]
- Prasad I., Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974 Nov;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Hengstenberg W., Saier M. H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984 Oct;160(1):333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Panos C., Saier M. H., Jr Mechanism of inducer expulsion in Streptococcus pyogenes: a two-step process activated by ATP. J Bacteriol. 1983 Oct;156(1):354–361. doi: 10.1128/jb.156.1.354-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Stuiver I., Saier M. H., Jr Regulation of glycerol uptake by the phosphoenolpyruvate-sugar phosphotransferase system in Bacillus subtilis. J Bacteriol. 1984 Jul;159(1):243–250. doi: 10.1128/jb.159.1.243-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Involvement of lactose enzyme II of the phosphotransferase system in rapid expulsion of free galactosides from Streptococcus pyogenes. J Bacteriol. 1983 Oct;156(1):236–242. doi: 10.1128/jb.156.1.236-242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Kinetic analyses of the sugar phosphate:sugar transphosphorylation reaction catalyzed by the glucose enzyme II complex of the bacterial phosphotransferase system. J Biol Chem. 1978 Nov 10;253(21):7595–7597. [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Substrate specificity and kinetic characterization of sugar uptake and phosphorylation, catalyzed by the mannose enzyme II of the phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1980 Sep 25;255(18):8585–8591. [PubMed] [Google Scholar]
- Reynolds A. E., Felton J., Wright A. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature. 1981 Oct 22;293(5834):625–629. doi: 10.1038/293625a0. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. A hypothesis for the role of dithiol-disulfide interchange in solute transport and energy-transducing processes. Eur J Biochem. 1982 Oct;127(3):597–604. doi: 10.1111/j.1432-1033.1982.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. Physical mechanism for regulation of phosphoenolpyruvate-dependent glucose transport activity in Escherichia coli. Biochemistry. 1981 Aug 18;20(17):5025–5032. doi: 10.1021/bi00520a032. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Lageveen R. G. Non-vectorial phosphorylation by the bacterial PEP-dependent phosphotransferase system is an artifact of spheroplast and membrane vesicle preparation procedures. FEBS Lett. 1982 Oct 18;147(2):143–148. doi: 10.1016/0014-5793(82)81028-4. [DOI] [PubMed] [Google Scholar]
- Robillard G. T. The enzymology of the bacterial phosphoenolpyruvate-dependent sugar transport systems. Mol Cell Biochem. 1982 Jul 7;46(1):3–24. doi: 10.1007/BF00215577. [DOI] [PubMed] [Google Scholar]
- Roehl R. A., Phibbs P. V., Jr Characterization and genetic mapping of fructose phosphotransferase mutations in Pseudomonas aeruginosa. J Bacteriol. 1982 Mar;149(3):897–905. doi: 10.1128/jb.149.3.897-905.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Vinopal R. T. Genetic locus, distant from ptsM, affecting enzyme IIA/IIB function in Escherichia coli K-12. J Bacteriol. 1980 Apr;142(1):120–130. doi: 10.1128/jb.142.1.120-130.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossien F. F., Blaauw M., Robillard G. T. Kinetics and subunit interaction of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry. 1984 Oct 9;23(21):4934–4939. doi: 10.1021/bi00316a017. [DOI] [PubMed] [Google Scholar]
- Roossien F. F., Robillard G. T. Mannitol-specific carrier protein from the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system can be extracted as a dimer from the membrane. Biochemistry. 1984 Nov 20;23(24):5682–5685. doi: 10.1021/bi00319a003. [DOI] [PubMed] [Google Scholar]
- Roossien F. F., Robillard G. T. Vicinal dithiol-disulfide distribution in the Escherichia coli mannitol specific carrier enzyme IImtl. Biochemistry. 1984 Jan 17;23(2):211–215. doi: 10.1021/bi00297a006. [DOI] [PubMed] [Google Scholar]
- Rose S. P., Fox C. F. The -glucoside system of Escherichia coli. II. Kinetic evidence for a phosphoryl-enzyme II intermediate. Biochem Biophys Res Commun. 1971 Oct 15;45(2):376–380. doi: 10.1016/0006-291x(71)90829-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Pearce S. M., Hardy C. M., Jacomb P. A. Rapid turnover of mannitol-1-phosphate in Escherichia coli. J Bacteriol. 1984 Apr;158(1):63–68. doi: 10.1128/jb.158.1.63-68.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A., Joseph E., Ullmann A. Two functional domains in adenylate cyclase of Escherichia coli. J Mol Biol. 1983 Mar 25;165(1):197–202. doi: 10.1016/s0022-2836(83)80251-4. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Bromberg F. G., Roseman S. Characterization of constitutive galactose permease mutants in Salmonella typhimurium. J Bacteriol. 1973 Jan;113(1):512–514. doi: 10.1128/jb.113.1.512-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Catalytic activities associated with the enzymes II of the bacterial phosphotransferase system. J Supramol Struct. 1980;14(3):281–294. doi: 10.1002/jss.400140303. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Moczydlowski E. G. Sugar phosphate:sugar transphosphorylation coupled to exchange group translocation catalyzed by the enzyme II complexes of the phosphoenolpyruvate:sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):8908–8916. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Hofstadter L. J. Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):883–892. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., McCaman M. T. Regulation of intracellular adenosine cyclic 3':5'-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J Biol Chem. 1975 Oct 10;250(19):7593–7601. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Roseman S. Phosphoenolpyruvate-dependent fructose phosphorylation in photosynthetic bacteria. J Biol Chem. 1971 Dec 25;246(24):7819–7821. [PubMed] [Google Scholar]
- Saier M. H., Jr, Keeler D. K., Feucht B. U. Physiological desensitization of carbohydrate permeases and adenylate cyclase to regulation by the phosphoenolpyruvate:sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Involvement of adenosine cyclic 3',5'-phosphate and inducer. J Biol Chem. 1982 Mar 10;257(5):2509–2517. [PubMed] [Google Scholar]
- Saier M. H., Jr, Newman M. J., Rephaeli A. W. Properties of a phosphoenolpyruvate: mannitol phosphotransferase system in Spirochaeta aurantia. J Biol Chem. 1977 Dec 25;252(24):8890–8898. [PubMed] [Google Scholar]
- Saier M. H., Jr, Novotny M. J., Comeau-Fuhrman D., Osumi T., Desai J. D. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1983 Sep;155(3):1351–1357. doi: 10.1128/jb.155.3.1351-1357.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Lin P. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J Biol Chem. 1980 Sep 25;255(18):8579–8584. [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R. Vectorial and nonvectorial transphosphorylation catalyzed by enzymes II of the bacterial phosphotransferase system. J Bacteriol. 1981 Jan;145(1):391–397. doi: 10.1128/jb.145.1.391-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D. Regulation of carbohydrate uptake in gram-positive bacteria. J Biol Chem. 1976 Feb 10;251(3):893–894. [PubMed] [Google Scholar]
- Saier M. H., Jr, Simoni R. D., Roseman S. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J Biol Chem. 1970 Nov 10;245(21):5870–5873. [PubMed] [Google Scholar]
- Saier M. H., Jr, Staley J. T. Phosphoenolpyruvate:sugar phosphotransferase system in Ancalomicrobium adetum. J Bacteriol. 1977 Aug;131(2):716–718. doi: 10.1128/jb.131.2.716-718.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Straud H., Massman L. S., Judice J. J., Newman M. J., Feucht B. U. Permease-specific mutations in Salmonella typhimurium and Escherichia coli that release the glycerol, maltose, melibiose, and lactose transport systems from regulation by the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1978 Mar;133(3):1358–1367. doi: 10.1128/jb.133.3.1358-1367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Young W. S., 3rd, Roseman S. Utilization and transport of hexoses by mutant strains of Salmonella typhimurium lacking enzyme I of the phosphoenolpyruvate-dependent phosphotransferase system. J Biol Chem. 1971 Sep 25;246(18):5838–5840. [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Sanders R., McGeoch D. A mutant transcription factor that is activated by 3':5'-cyclic guanosine monophosphate. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1017–1021. doi: 10.1073/pnas.70.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M. V., Tenn L. G., Desai A., Chin A. M., Grenier F. C., Saier M. H., Jr Genetic evidence for glucitol-specific enzyme III, an essential phosphocarrier protein of the Salmonella typhimurium glucitol phosphotransferase system. J Bacteriol. 1984 Mar;157(3):953–955. doi: 10.1128/jb.157.3.953-955.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Mutation in the crp gene of Salmonella typhimurium which interferes with inducer exclusion. J Bacteriol. 1980 Feb;141(2):751–757. doi: 10.1128/jb.141.2.751-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Schrecker O., Hengstenberg W. The staphylococcal phosphoenolpyruvate-dependent phosphotransferase system. Purification and characterisation of the galactoside-specific membrane-component enzyme II. Eur J Biochem. 1981 Jan;113(2):289–294. doi: 10.1111/j.1432-1033.1981.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Molecular cloning of the uhp region and evidence for a positive activator for expression of the hexose phosphate transport system of Escherichia coli. J Bacteriol. 1983 Sep;155(3):1062–1070. doi: 10.1128/jb.155.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Takebe Y., Kaziro Y. A possible involvement of cya gene in the synthesis of cyclic guanosine 3':5'-monophosphate in E. coli. Cell. 1977 Oct;12(2):521–528. doi: 10.1016/0092-8674(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Galloway R. J., Niwano M., Chinnock R. E., Taylor B. L. Requirement of ATP in bacterial chemotaxis. J Biol Chem. 1982 Jul 25;257(14):7969–7975. [PubMed] [Google Scholar]
- Simoni R. D., Hays J. B., Nakazawa T., Roseman S. Sugar transport. VI. Phosphoryl transfer in the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):957–965. [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z. Transmissible substrate-utilizing ability in enterobacteria. J Gen Microbiol. 1975 Mar;87(1):129–140. doi: 10.1099/00221287-87-1-129. [DOI] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Miyal K., Lin E. C. Membrane translocation of mannitol in Escherichia coli without phosphorylation. J Bacteriol. 1973 May;114(2):723–728. doi: 10.1128/jb.114.2.723-728.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger G. A., Lengeler J. W. L-Sorbose metabolism in Klebsiella pneumoniae and Sor+ derivatives of Escherichia coli K-12 and chemotaxis toward sorbose. J Bacteriol. 1984 Jan;157(1):39–45. doi: 10.1128/jb.157.1.39-45.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Maderis A. M., Koshland D. E., Jr Bacterial chemotaxis in the absence of receptor carboxylmethylation. Cell. 1981 Nov;27(1 Pt 2):37–44. doi: 10.1016/0092-8674(81)90358-5. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Waygood E. B., Meadow N. D., Postma P. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982 Dec 10;257(23):14543–14552. [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Conservation and DNA sequence arrangement of the DNA polymerase I gene region from Klebsiella aerogenes, Klebsiella pneumoniae and Escherichia coli. J Mol Biol. 1980 Aug 25;141(4):343–368. doi: 10.1016/0022-2836(80)90251-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lin E. C. Two classes of pleiotropic mutants of Aerobacter aerogenes lacking components of a phosphoenolpyruvate-dependent phosphotransferase system. Proc Natl Acad Sci U S A. 1967 Apr;57(4):913–919. doi: 10.1073/pnas.57.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular hexose-6-phosphate:phosphohydrolase from Streptococcus lactis: purification, properties, and function. J Bacteriol. 1983 Oct;156(1):70–80. doi: 10.1128/jb.156.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Intracellular phosphorylation of glucose analogs via the phosphoenolpyruvate: mannose-phosphotransferase system in Streptococcus lactis. J Bacteriol. 1985 Apr;162(1):224–234. doi: 10.1128/jb.162.1.224-234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Uptake and metabolism of sucrose by Streptococcus lactis. J Bacteriol. 1981 Aug;147(2):543–551. doi: 10.1128/jb.147.2.543-551.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J Bacteriol. 1970 May;102(2):411–422. doi: 10.1128/jb.102.2.411-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A. H., Cirillo V. P. Mycoplasma phosphoenolpyruvate-dependent sugar phosphotransferase system: purification and characterization of the phosphocarrier protein. J Bacteriol. 1976 Sep;127(3):1298–1306. doi: 10.1128/jb.127.3.1298-1306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshin A. G., Belkina N. A., Burd G. I. Mekhanizm katabolitnoi repressii u bakterii E. coli: vzaimodeistvie mezhdu transportnymi belkami i adenilattsiklazoi. Biokhimiia. 1983 Oct;48(10):1624–1633. [PubMed] [Google Scholar]
- Wagner E. F., Fabricant J. D., Schweiger M. A novel ATP-driven glucose transport system in Escherichia coli. Eur J Biochem. 1979 Dec;102(1):231–236. doi: 10.1111/j.1432-1033.1979.tb06284.x. [DOI] [PubMed] [Google Scholar]
- Walsh C. T., Kaback H. R. Vinylglycolic acid. An inactivator of the phosphoenolpyruvate-phosphate transferase system in Escherichia coli. J Biol Chem. 1973 Aug 10;248(15):5456–5462. [PubMed] [Google Scholar]
- Walter R. W., Jr, Anderson R. L. Evidence that the inducible phosphoenolpyruvate:D-fructose 1-phosphotransferase system of Aerobacter aerogenes does not require "HPr". Biochem Biophys Res Commun. 1973 May 1;52(1):93–97. doi: 10.1016/0006-291x(73)90958-3. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Clegg D. O., Koshland D. E., Jr Molecular cloning and amplification of the adenylate cyclase gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4684–4688. doi: 10.1073/pnas.78.8.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate Accumulation and Metabolism in Escherichia coli: Characteristics of the Reversions of ctr Mutations. J Bacteriol. 1970 Dec;104(3):1318–1324. doi: 10.1128/jb.104.3.1318-1324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli: the close linkage and chromosomal location of ctr mutations. J Bacteriol. 1969 May;98(2):605–610. doi: 10.1128/jb.98.2.605-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli. I. Description of pleiotropic mutants. J Mol Biol. 1968 Feb 28;32(1):59–66. doi: 10.1016/0022-2836(68)90145-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Mattoo R. L., Peri K. G. Phosphoproteins and the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium and Escherichia coli: evidence for IIImannose, IIIfructose, IIIglucitol, and the phosphorylation of enzyme IImannitol and enzyme IIN-acetylglucosamine. J Cell Biochem. 1984;25(3):139–159. doi: 10.1002/jcb.240250304. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]
- Waygood E. B. Resolution of the phosphoenolpyruvate: fructose phosphotransferase system of Escherichia coli into two components: enzyme IIfructose and fructose-induced HPr-like protein (FPr). Can J Biochem. 1980 Oct;58(10):1144–1146. doi: 10.1139/o80-153. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Steeves T. Enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. Purification to homogeneity and some properties. Can J Biochem. 1980 Jan;58(1):40–48. doi: 10.1139/o80-006. [DOI] [PubMed] [Google Scholar]
- Weigel N., Kukuruzinska M. A., Nakazawa A., Waygood E. B., Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14477–14491. [PubMed] [Google Scholar]
- Weigel N., Powers D. A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Primary structure and active site of a general phosphocarrier protein (HPr) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14499–14509. [PubMed] [Google Scholar]
- Weigel N., Waygood E. B., Kukuruzinska M. A., Nakazawa A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of enzyme I from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14461–14469. [PubMed] [Google Scholar]
- White R. J. The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-D-glucosamine by Escherichia coli. Biochem J. 1970 Jun;118(1):89–92. doi: 10.1042/bj1180089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. K., Uhlin B. E., Amini K. S., Clark A. J. Physical mapping of the srl recA region of Escherichia coli: analysis of Tn10 generated insertions and deletions. Mol Gen Genet. 1981;183(3):497–504. doi: 10.1007/BF00268771. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Compartmentation in the induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1970 Feb;101(2):470–475. doi: 10.1128/jb.101.2.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Efflux and the steady state in alpha-methylglucoside transport in Escherichia coli. J Bacteriol. 1971 May;106(2):362–368. doi: 10.1128/jb.106.2.362-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]
- Woodward M. J., Charles H. P. Genes for l-sorbose utilization in Escherichia coli. J Gen Microbiol. 1982 Sep;128(9):1969–1980. doi: 10.1099/00221287-128-9-1969. [DOI] [PubMed] [Google Scholar]
- Woodward M. J., Charles H. P. Polymorphism in Escherichia coli: rtl atl and gat regions behave as chromosomal alternatives. J Gen Microbiol. 1983 Jan;129(1):75–84. doi: 10.1099/00221287-129-1-75. [DOI] [PubMed] [Google Scholar]
- Yang J. K., Bloom R. W., Epstein W. Catabolite and transient repression in Escherichia coli do not require enzyme I of the phosphotransferase system. J Bacteriol. 1979 Apr;138(1):275–279. doi: 10.1128/jb.138.1.275-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. K., Epstein W. Purification and characterization of adenylate cyclase from Escherichia coli K12. J Biol Chem. 1983 Mar 25;258(6):3750–3758. [PubMed] [Google Scholar]


