Abstract
Maternal obesity is associated to increased fetal risk of obesity and other metabolic diseases. Human amniotic mesenchymal stem cells (hA-MSCs) have not been characterized in obese women. The aim of this study was to isolate and compare hA-MSC immunophenotypes from obese (Ob-) and normal weight control (Co-) women, to identify alterations possibly predisposing the fetus to obesity. We enrolled 16 Ob- and 7 Co-women at delivery (mean/SEM prepregnancy body mass index: 40.3/1.8 and 22.4/1.0 kg/m2, respectively), and 32 not pregnant women. hA-MSCs were phenotyped by flow cytometry; several maternal and newborn clinical and biochemical parameters were also measured. The expression of membrane antigen CD13 was higher on Ob-hA-MSCs than on Co-hA-MSCs (P=0.005). Also, serum levels of CD13 at delivery were higher in Ob- versus Co-pregnant women and correlated with CD13 antigen expression on Ob-hA-MSCs (r2=0.84, P<0.0001). Adipogenesis induction experiments revealed that Ob-hA-MSCs had a higher adipogenic potential than Co-hA-MSCs as witnessed by higher peroxisome proliferator-activated receptor gamma and aP2 mRNA levels (P=0.05 and P=0.05, respectively), at postinduction day 14 associated with increased CD13 mRNA levels from baseline to day 4 postinduction (P<0.05). Adipogenesis was similar in the two sets of hA-MSCs after CD13 silencing, whereas it was increased in Co-hA-MSCs after CD13 overexpression. CD13 expression was high also in Ob-h-MSCs from umbilical cords or visceral adipose tissue of not pregnant women. In conclusion, antigen CD13, by influencing the adipogenic potential of hA-MSCs, could be an in utero risk factor for obesity. Our data strengthen the hypothesis that high levels of serum and MSC CD13 are obesity markers.
Introduction
The increase in the incidence of obesity in pregnant women in the last two decades has paralleled that observed in the general population [1–3]. Although maternal fat stores increase in all pregnant women, irrespective of prepregnancy weight [4], the storage capacity of subcutaneous adipose tissue is impaired, and fat predominantly accumulates in the visceral adipose tissue (VAT) in obeses [5]. VAT is an important risk factor for metabolic imbalance in human subjects, also during pregnancy [6–8]. In fact, maternal obesity is related to offspring obesity [9], and there is an increased risk of adverse outcomes for both mother and child [10–13]. Moreover, the risk of childhood obesity was quadrupled if the mother was obese before pregnancy [14], which suggests that the in utero environment is obesogenic. In mammals, the placenta is the main interface between the fetus and the mother; it regulates intrauterine development and modulates adaptive responses to suboptimal in utero conditions [15,16].
Placenta is also an important source of stem/progenitor cells [17–19]. In particular, human amniotic mesenchymal stem cells (hA-MSCs) have been shown to differentiate into cell types of mesenchymal origin such as chondrocytes, adipocytes, and osteocytes [20–22]. The phenotype of hA-MSCs from normal pregnant women has been characterized and found to differ in terms of cytokine expression from that of pregnant women affected by pre-eclampsia [23]. Thus far, little is known about hA-MSCs from obese women.
The aim of this study was to characterize hA-MSCs from term placenta of obese (Ob-) women and to test their adipogenic potential with respect to that of normal weight control (Co-) women. We also measured several maternal and newborn clinical and biochemical parameters, and looked for correlations between these parameters with the hA-MSC immunophenotype. We found that the Ob-hA-MSC immunophenotype was characterized by increased expression levels of the CD13 surface antigen that correlated with maternal CD13 serum levels. Adipogenesis was higher in Ob-hA-MSCs than in Co-hA-MSCs, and returned to the control value after CD13 silencing. On the other hand, CD13 overexpression increased the adipogenic potential of Co-hA-MSCs. Our findings suggest that CD13 could contribute to obesity programming in the fetus and indicate that maternal serum CD13 is an obesity risk marker.
Materials and Methods
Patients and controls
Sixteen Ob- (age range: 26–39 years) and seven Co-pregnant women, (age range: 26–38 years), prepregnancy body mass index (BMI) (mean/SEM) 40.3/1.8 and 22.4/1.0 kg/m2, respectively, and thirty-two not pregnant women (16 obese and 16 normal weight, BMI>30 kg/m2 and <25 kg/m2, respectively), were recruited at the Dipartimento di Neuroscienze e Scienze Riproduttive ed Odontostomatologiche, University of Naples “Federico II.” The clinical, personal, and family history of the 23 women was recorded during a medical interview conducted by an expert upon hospitalization. Data relative to each pregnancy follow-up and delivery were also recorded. The general characteristics of the newborn and clinical data (birth weight, length, head circumference, Apgar score) were recorded at birth.
Sample collection
Two fasting peripheral blood samples were collected in the morning from not pregnant women and from Ob- and Co-pregnant women, immediately before delivery. One sample was used for DNA extraction, whereas the other was centrifuged at 2,500 rpm for 15 min and serum was stored at −80°C until further processing. At delivery, placentas were collected by the C-section from each enrolled woman and immediately processed. Bioptic samples of VAT were also collected from not pregnant obese and control women during obstetric surgery (ovarian cysts). All patients and controls gave their informed consent to the study and both parents gave consent for their newborns. The study was performed according to the Helsinki II Declaration and was approved by the Ethics Committee of our Faculty.
Biochemical evaluations
The main serum biochemical parameters were evaluated by routine assays. Leptin and adiponectin were measured in maternal serum with Luminex xMAP Technology on a BioRad Multiplex Suspension Array System (Bio-Rad), according to the manufacturer's instructions. The ratio leptin/adiponectin (L/A) was also calculated.
Aminopeptidase N/CD13 ELISA assay
Aminopeptidase N (APN)/CD13 serum levels were measured by ELISA (Life Science). Briefly, the microtiter plate was precoated with a specific anti-CD13 antibody. Standards or samples were then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for CD13. Next, avidin conjugated to horseradish peroxidase was added to each microplate well and incubated for 15 min at room temperature. A TMB substrate solution (3,3′,5,5′-tetramethylbenzidine) was then added to each well. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution and the color change was measured spectrophotometrically at a wavelength of 450 nm. The amount of CD13 in each sample was determined by comparing the absorbance of the sample to a standard curve.
Cell isolation from placenta tissue
Placentas were collected and immediately processed, according to Parolini et al. [24]. After removal of the maternal decidua, the amnion was manually separated from the chorion and extensively washed 5 times in 40 mL of phosphate-buffered saline (PBS) containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 μg/mL amphotericin B (all from Sigma-Aldrich), after which, it was mechanically minced into small pieces [24]. Amnion fragments were digested overnight at 4°C in the ACCUMAX® reagent (Innovative Cell Technology), a combination of DNase, protease, and collagenolytic enzymes [25], containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 μg/mL amphotericin B. The next day, digestion enzymes were inactivated with a complete culture medium constituted by the low-glucose DMEM (Sigma-Aldrich) supplemented with 10% of heat-inactivated bovine serum (FBS), 1% of nonessential amino acids, and 2% of ultraglutamine (all from Lonza). After centrifugation at 300 g for 10 min, cell pellets and digested tissue fragments were seeded in a cell culture dish (BD Falcon) in the complete culture medium and incubated at 37°C in 5% CO2. One week later, digested tissue pieces were removed from the dish and discarded, and isolated cells formed distinct fibroblast colony-forming units. When the colonies reached 70% confluence, they were washed with PBS and detached with trypsin/EDTA (Sigma-Aldrich), counted, and reseeded in the complete medium for expansion at a concentration of about 5,000/cm2 [24].
Cell preparation
hA-MSCs were expanded for several passages. The absence of mycoplasma contamination was assessed as described previously [26]. The population doubling level was calculated for each subcultivation with the following equation: population doubling=[log10 (NH) – log10 (NI)]/log10 (2), where NI is the cell inoculum number and NH is the cell harvest number [27]. The increase in population doubling was added to the population doubling levels of the previous passages to yield the cumulative population doubling level. When 70%–80% confluent cultures reached about 4 population doublings, they were detached with trypsin/EDTA, resuspended in PBS with 10% FBS, and processed for flow cytometry, DNA, and RNA extraction. Cellular viability was assessed by both Trypan blue dye exclusion and the analysis of light scatter proprieties in flow cytometry, and it was never lower than 90%.
Using the above cell isolation and preparation procedures, h-MSCs were also isolated from the umbilical cord (hUC-MSCs) of one obese and one control pregnant woman.
Isolation of hVAT-MSCs
Briefly, VAT bioptic samples were washed with PBS containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 μg/mL amphotericin B (all from Sigma-Aldrich), minced into small pieces, and digested with 1.5 mg/mL collagenase type I (GIBCO) at 37°C. The digestion enzymes were inactivated with FBS. After centrifugation at 1500 g for 5 min, cell pellets and digested tissue fragments were washed and seeded in a cell culture dish (BD Falcon) in the complete culture medium and incubated at 37°C in 5% CO2. When the colonies reached 60%–70% confluence, they were washed with PBS and detached with trypsin/EDTA (Sigma-Aldrich), counted, and reseeded in the complete medium for expansion at a concentration of about 5,000/cm2 [28].
DNA typing
The fetal origin of both amnion and hA-MSCs was verified by DNA typing. Genomic DNA was extracted from the mother's peripheral blood, from amnion samples, and from hA-MSCs using the Nucleon BACC2 extraction kit (Illustra DNA Extraction Kit BACC2; GE Healthcare). The DNA concentration was evaluated using the NanoDrop® ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies). Genomic DNA (1 ng) was amplified in a final volume of 25 μL using the AmpFlSTR® Identifiler™ PCR Amplification Kit (Applied Biosystems). The AmpFlSTR Identifiler PCR Amplification Kit is a short tandem repeat (STR) multiplex assay that amplifies 15 repeat loci and the Amelogenin gender determining marker in a single polymerase chain reaction (PCR) amplification using a primer set labeled with four fluorescent molecules. The amplification was performed with the GeneAmp PCR System 9700 (Applied Biosystems) instrument. PCR products were then analyzed by capillary electrophoresis on the ABI Prism 3130 Genetic Analyzer (Applied Biosystems) together with an allelic ladder, which contained all the most common alleles for the analyzed loci that were present in Caucasian populations and both a negative- and a positive-quality control sample. Typically, 1μL of each sample was diluted in 18.7μL of deionized formamide; each sample was supplemented with 0.3 μL of an internal size standard (LIZ 500 Applied Biosystems) labeled with an additional fluorophore. The samples were denatured at 95°C for 4 min, and then placed in the auto sampler tray (maximum of 96 samples) on the ABI Prism 3130 for automatic injection in the capillaries. The data were analyzed by Gene Mapper Software (Applied Biosystems).
Immunophenotyping of h-MSCs by flow cytometry
We analyzed the expression of 38 hematopoietic, mesenchymal, endothelial, epithelial, and no-lineage membrane antigens on the surface of hA-MSCs, hUC-MSCs, and hVAT-MSCs by four-color flow cytometry (Table 1). The antibody cocktails contained in each tube are detailed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd). All monoclonal antibodies (MoAbs) were from Becton Dickinson (San Jose) except anti-CD338-APC, which was from R&D (Minneapolis), anti-CD-133-PE and anti-CD271-APC MoAbs, which were from Milenyi Biotec (Bergisch Gladbach). For all antibody staining experiments, at least 1×105 hA-MSCs isolated from each placenta sample were incubated at 4°C for 20 min with the appropriate amount of MoAbs, washed twice with PBS, and finally analyzed with an unmodified Becton-Dickinson FACSCanto II flow cytometer (Becton-Dickinson), that was set up according to published guidelines [29]. For each sample, the respective control was prepared to determine the level of background cellular autofluorescence without antibody staining.
Table 1.
Surface Immunophenotypic Profile Investigated in Human Amniotic Mesenchymal Stem Cells by Flow Cytometry
| Fluorochrome | CD antigen | Other names | Molecular weight (kDa) | Cell expression | Function |
|---|---|---|---|---|---|
| FITC | CD9 | Tspan-29 | 24–26 | Platelets, pre-B cells, activated T cells | Adhesion, migration, platelet activation |
| APC | CD10 | CALLA | 100 | B/T precursors, stromal cells | Endopeptidase |
| PE | CD13 | APN | 150 | Granulocytes, monocytes and their precursors, endothelial cells, epithelial cells, mesenchymal stem cells | Metalloproteinase |
| PE | CD14 | LPS-R | 53–55 | Monocytes, macrophages | Receptor for LPS/LPB complex |
| APC | CD15 | Lewis X | – | Granulocyte, monocyte, epithelial cells | Cell adhesion |
| PE | CD16 | FCγRIIIa | 50–65 | Neutrophils, NK, macrophages | Low affinity with FCγ receptor, mediates phagocytosis |
| APC | CD19 | Bgp95 | 95 | B cells, not on plasma cells | Signal transduction |
| FITC | CD26 | DPP IV | 110 | Mature thymocytes, T, B, NK cells | Exoprotease, costimulation |
| APC | CD28 | Tp44 | 44 | Most T cells, thymocytes, NK and plasma cells | Costimulation |
| APC | CD29 | VLA β1-chain | 130 | T, B, granulocytes, monocytes, fibroblasts, endothelial cells, NKs, platelet | Adhesion activation, embryogenesis, and development |
| FITC | CD31 | ECAM-1 | 130–140 | Monocytes, platelets, granulocytes, and endothelial cells | Cell adhesion |
| APC | CD33 | My9 | 67 | Monocytes, granulocytes, mastocytes, and myeloid progenitors | Cell adhesion |
| APC | CD34 | My10 | 105–120 | Hematopoietic stem cells and progenitors, endothelial cells | Cell adhesion |
| APC | CD36 | Platelet GPIV | 85 | Platelets, monocytes, macrophages, endothelial cells, erythroid precursors | Adhesion and phagocytosis |
| FITC | CD40 | Bp50 | 48 | Monocytes, macrophages, B cells, endothelial cells, fibroblasts, keratinocytes | Costimulation to B cells, growth, differentiation, and isotype switching |
| APC | CD44 | H-CAM | 90 | Leukocytes, erythrocytes, and epithelial cells | Rolling, homing, and aggregation |
| Per Cp | CD45 | LCA | 180–220 | Hematopoietic cells, except erythrocytes and platelets | Critical for T and B cell receptor-mediated activation |
| FITC | CD47 | IAP I | 50–55 | Hematopoietic, epithelial, endothelial, and brain mesenchymal cells | Adhesion |
| FITC | CD49d | VLA-4 | 150 | B cells, T cells, monocytes, eosinophils, basophils, NKs, dendritic cells | Adhesion, migration, homing, activation |
| APC | CD54 | ICAM-1 | 80–114 | Epithelial and endothelial cells monocytes. Low on resting lymphocytes, upregulate on activated | T cell activation |
| PE | CD56 | NCAM | 175–220 | Neural, tumors, embryonic tissue, NK | Homophilic and heterophilic adhesion |
| PE | CD58 | LFA-3 | 40–70 | Leucocytes, erythrocytes, epithelial endothelial cells, and fibroblasts | Costimulation |
| FITC | CD71 | Transferrin recepor | 95 | Reticulocytes, erythroid precursor | Controls iron intake during cell proliferation |
| APC | CD81 | TAPA-1 | 26 | B and T cells, monocytes, endothelial cells | Signal transduction |
| FITC | CD90 | Thy-1 | 25–35 | Hematopoietic stem cells, neurons, mesenchymal stem cells | Inhibition of hematopoietic stem cells and neuron differentiation |
| PE | CD99 | MIC2 | 32 | Leucocyte, NK, monocytes, endothelial and epithelial cells | Leucocyte migration, T cell activation, cell adhesion |
| PE | CD105 | Endoglin | 90 | Endothelial and mesenchymal stem cells, erythroid precursors, monocytes | Angiogenesis, modulates cellular response to TGFβ1 |
| PE | CD117 | c-kit | 145 | Hematopoietic stem cells and progenitors | Crucial for hematopoietic stem cells |
| PE | CD133 | Prominin-1 | 120 | Hematopoietic stem cell, endothelial, epithelial, and neural precursors | Unknown function, stem cell marker |
| PE | CD151 | PETA-3 | 32 | Endothelial and epithelial cells, megakaryocytes, platelets | Adhesion |
| PE | CD166 | ALCAM | 100–105 | Neurons, activated T cells, epithelial cells, mesenchymal stem cells | Adhesion, T cell activation |
| PE | CD200 | OX-2 | 33 | B cells, activated T cells, thymocytes, neurons, endothelium | Downregulatory signal for myeloid cell functions |
| FITC | CD243 | MDR-1 | 170 | Stem cells, multidrug-resistant tumors | Influences the uptake, distribution, elimination of drugs |
| APC | CD271 | NGFR | 75 | Neurons, stromal and dendritic follicular cells | Low affinity for NGF receptor |
| APC | CD324 | E-cadherin | 120 | Epithelial, keratinocytes, platelet | Adhesion, growth, differentiation |
| APC | CD338 | ABCG-2 | 72 | Hematopoietic stem cells, liver, kidney, intestine, side population of stem cells | Absorption and excretion of xenobiotics |
| FITC | HLA-ABC | Class I MHC | 46 | All nucleated cells and platelets | Antigen presentation |
| FITC | HLA-DR | Class II MHC | 30 | B cells, monocytes, myeloid progenitors, activated T and dendritic cells | Antigen presentation |
CaliBRITE beads (Becton-Dickinson, catalog no. 340486) were used as quality controls across the study as described elsewhere [30,31], according to the manufacturer's instructions. Daily control of CaliBRITE intensity showed no change in instrument sensitivity throughout the study. The relative voltage range for each detector was assessed una tantum using the eight-peak technology (Rainbow Calibration Particles, Becton-Dickinson, catalog no. 559123) at the beginning of the study.
Compensation was set in the FACS-DiVa (Becton-Dickinson) software, and compensated samples were analyzed. Samples were acquired immediately after staining using the FACSCanto II instrument, and at least 10,000 events were recorded for each monoclonal combination. Levels of CD antigen expression were displayed as median fluorescence intensity (MFI). The FACS-DiVa software (Becton-Dickinson) was used for cytometric analysis.
Differentiation potential toward the adipogenic lineage
hA-MSCs and hVAT-MSCs were cultured in the low-glucose DMEM (Sigma-Aldrich) supplemented with 10% of FBS, 2% of ultraglutamine, and 1% of nonessential amino acids at 37°C in 5% CO2 (all from Lonza). The cells were passaged twice before the addition of the differentiation medium composed of the DMEM with the addition of 10% FBS, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxhantine, 200 μM indomethacin, and 10 μg/mL insulin. Media were changed every 2 days and cells were either stained or collected for RNA extraction.
CD13 RNA interference and overexpression
hA-MSCs plated at a density of 5,000 cells/cm2 were transfected using 20 μL Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen) with 8 μg short hairpin RNAs (shRNAs) expressing plasmids (Open Biosystem) or with an 8 μg pCMV-Sport 6 Vector (Invitrogen), to silence or to overexpress CD13 mRNA, respectively. Transfected cells were induced to differentiate toward the adipogenic lineage up to 4 days.
Effect of interferon-γ on the expression of CD13 on the surface of h-MSCs
The expression of CD13 on the surface of Co- and of Ob-h-MSCs isolated from the amnion, umbilical cord, and VAT was measured after exposure of cells to 0.8 and 12.5 ng/mL interferon (IFN)-γ at 37°C for 24 h, using untreated Co- and Ob-h-MSCs as controls. At the end of incubation, the cells were harvested by trypsin, washed in PBS, counted, and adjusted to the same concentrations of 1×105 h-MSCs. Subsequently, their immunophenotype was examined by flow cytometry.
Adipocyte staining
After 14 days of differentiation, the adipocyte cultures were stained for lipid droplets, which are an index of differentiation. The cells were washed in PBS and fixed in 10% formalin for 1 h. Then, they were washed in PBS and the lipids were stained for 15 min with Oil Red O prepared by mixing vigorously three parts of a stock solution (0.5% Oil Red O in 98% isopropanol) with two parts of water, and then eliminating undissolved particles with a 0.4-μm filter. Cells were then washed with water and the number of adipocytes was evaluated with a microscope. Relative lipid levels were assessed by redissolving the Oil Red O present in stained cells in 98% isopropanol, and then determining absorbance at 550 nm.
RNA isolation
Total RNA was purified from hA-MSCs isolated from term placentas of Co- and of Ob-pregnant women using the mirVana™ miRNA isolation kit (Ambion) and its concentration was evaluated with the NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies).
Quantitative real-time PCR of mRNAs
Real-time quantitative PCR (qRT-PCR) was carried out on the Applied Biosystems 7900HT Sequence Detection system (Applied Biosystems). cDNAs were synthesized from 2 μg of total RNA using hexamer random primers and M-MuLV Reverse Transcriptase (New England BioLabs). The PCR was performed in a 20 μL final volume containing cDNA, 1×SYBR Green PCR mix, and 10 μM of each specific primer. Supplementary Table S2 lists the oligonucleotide primers used for PCR of selected genes: peroxisome proliferator-activated receptor gamma (PPARγ), CD13, protein homologous to myelin P2 (aP2), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR conditions for reverse transcription were stage 1: 50°C, 2 min; stage 2: 95°C, 10 min; stage 3: 95°C, 15 s; 60°C, 1 min/40 cycles; and stage 4: 95°C, 15 s; 60°C, 1 min. Levels of target genes were quantified using specific oligonucleotide primers and normalized for GAPDH expression.
Statistical analysis
The parameters investigated were expressed as mean and standard error of the mean (SEM) (parametric distributions) or as the median value and 25th and 75th percentiles (nonparametric distributions). The Student's t and Mann–Whitney tests were used to compare parametric and nonparametric data, respectively. P values<0.05 were considered statistically significant. Correlation analysis was performed with the SPSS package for Windows (ver. 18; SPSS, Inc.).
Results
The clinical and biochemical characteristics of the mothers and their newborns are reported in Table 2 (A and B, respectively). Weight gain was lower (P=0.05) and diastolic blood pressure was higher (P=0.05) in Ob- than in Co-pregnant women. Both the leptin concentration (P<0.0001) and the L/A ratio (P<0.0001) were higher in Ob- than in Co-pregnant women at delivery. Biometric characteristics did not differ significantly between Ob- and Co-newborns.
Table 2.
Clinical and Biochemical Characteristics of Obese and Normal Weight Control Pregnant Women at Delivery and Their Newborns
|
A | ||
|---|---|---|
| Mother's parameters | Ob-pregnant women (n=16) | Co-pregnant women (n=7) |
| Age (years) | 32.6 (0.9) | 30.7 (1.5) |
| Weight (kg)a | 110.1 (5.4) | 65.2 (3.6) |
| Height (m) | 163.3 (1.6) | 169.0 (1.7) |
| BMI prepregnancy (kg/m2)a | 40.3 (1.8) | 22.4 (1.0) |
| Weight gain in pregnancyb | 8.4 (1.3) | 14.3 (1.8) |
| Systolic blood pressure (mmHg) | 124.3 (2.7) | 117.1 (5.1) |
| Diastolic blood pressure (mmHg)c | 82.5 (2.2) | 74.2 (2.0) |
| Frequency cardiac | 79.6 (1.7) | 79.0 (3.7) |
| Gestational age | 38.4 (0.3) | 38.7 (0.2) |
| Glucose (mmol/L) | 4.3 (0.1) | 4.0 (0.3) |
| Total cholesterol (mmol/L) | 6.9 (0.4) | 7.3 (0.1) |
| Triglycerides (mmol/L) | 2.8 (0.2) | 2.3 (0.3) |
| AST (U/L) | 15d (12.2–26.5d) | 14.8 (0.7) |
| ALT (U/L) | 13d (9.2–17.7d) | 12.1 (1.1) |
| ALP (U/L) | 124.2 (11.1) | 115.0 (12.6) |
| GGT (U/L) | 11.0 (1.7) | 8.8 (1.5) |
| Leptin (L) (ng/mL)a | 38.5 (2.2) | 15.2 (3.3) |
| Adiponectin (A) (μg/mL) | 6.0 (0.7) | 7.5 (1.4) |
| L/Aa | 7.7 (0.6) | 2.6 (0.5) |
|
B | ||
|---|---|---|
| Newborn features | Ob-newborns (n=16) | Co-newborns (n=7) |
| Birth weight (kg) | 3162 (0.1) | 3401 (0.1) |
| Length (cm) | 49.6 (0.7) | 50.8 (0.7) |
| Head circumference (cm) | 34.0 (0.4) | 34.8 (0.3) |
| Apgar 1′ | 7.0d (7.0–8.0d) | 7.8 (0.2) |
| Apgar 5′ | 9.0d (8.5–9.0d) | 8.7 (0.1) |
Data are expressed as mean (SEM) (parametric distributions).
Statistically significant difference at the Student t test.
P<0.0001.
P<0.05.
P<0.05.
Median value and 25th–75th percentiles (nonparametric distributions).
Isolation of hA-MSCs
We isolated hA-MSCs from the mesenchymal layer of amniotic membranes obtained from our Ob- and Co-pregnant women at delivery. The fetal origin of all isolated hA-MSCs was confirmed by STR typing of DNA of the mother and of the hA-MSCs. Mycoplasma contamination of cultures was checked and excluded (data not shown). All isolated hA-MSCs were characterized by a high proliferation potential and collected after four population doublings. Morphologically, cultured Ob- and Co-hA-MSCs showed a similar fibroblastic-like morphology after four population doublings (Supplementary Fig. S1).
Immunophenotyping of h-MSCs
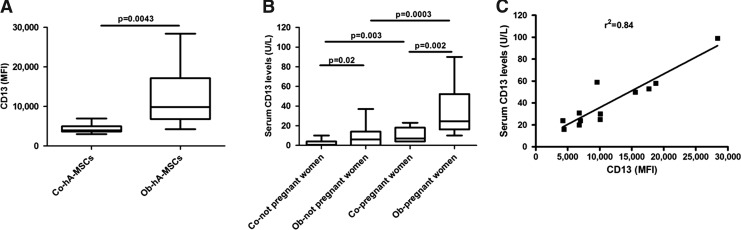
The antigenic mosaic displayed by Ob- and Co-hA-MSCs is shown in Table 3. Seventeen of the 38 antigens investigated were not expressed on the surface of hA-MSCs (hematopoietic antigens: CD14, CD15, CD16, CD19, CD28, CD33, CD34, CD45, and CD117; the endothelial marker PECAM-1/CD31; and no-lineage markers: thrombospondin receptor/CD36, Bp50/CD40, Prominin-1/CD133, MDR-1/CD243, NGFR/CD271, ABCG-2/CD338, and HLA-DR). Both Ob- and Co-hA-MSCs were positive for the following mesenchymal markers: CD9, CD10, CD13, CD26, CD29, CD44, CD47, CD49d, CD54, CD56, CD58, CD71, CD81, CD90, CD99, CD105, CD151, CD166, CD200, and HLA-ABC. A very weak positivity for the epithelial antigen E-cadherin/CD324 was also observed. Interestingly, CD13 expression was significantly higher in Ob-hA-MSCs than in Co-hA-MSCs, that is, MFI: 9,802.0 and 3,950.0, respectively (P=0.005) (Table 3 and Fig. 1A). The immunophenotype characterization confirmed the mesenchymal origin and the higher CD13 expression in hVAT-MSCs and hUC-MSCs from Ob- than from Co-women (hVAT-MSCs - MFI: 8,200.0 vs. 1,100.0 and hUC-MSCs - MFI: 4,965.0 vs. 3,155.0, respectively).
Table 3.
Immunophenotyping of Human Amniotic Mesenchymal Stem Cells Isolated from Obese and Control Pregnant Women
| |
|
Ob-hA-MSCs |
Co-hA-MSCs |
|||
|---|---|---|---|---|---|---|
| Fluorochrome | Antigen | MFI | 25th-75th Percentiles | MFI | 25th-75th Percentiles | P value |
| Not expressed antigens | ||||||
| FITC | CD31 | 364.0 | 278.3–511.3 | 306.5 | 286.8–368.0 | 0.3254 |
| CD40 | 444.0 | 336.5–568.3 | 388.0 | 357.0–457.8 | 0.4824 | |
| CD243 | 363.0 | 292.3–528.3 | 307.5 | 274.0–378.3 | 0.2061 | |
| HLA-DR | 355.0 | 283.8–530.0 | 297.0 | 272.5–374.8 | 0.2415 | |
| NC | 325.0 | 250.0–516.3 | 279.0 | 218.8–418.8 | 0.4260 | |
| PE | CD14 | 166.5 | 125.5–197.8 | 134.5 | 124.5–157.8 | 0.2815 |
| CD16 | 36.0 | 11.5–71.7 | 65.5 | 50.5–72.0 | 0.2407 | |
| CD117 | 142.5 | 109.5–189.3 | 121.0 | 116.0–176.8 | 0.6065 | |
| CD133 | 95.5 | 80.5–112.8 | 87.5 | 76.50–110.5 | 0.5423 | |
| NC | 115.5 | 91.75–181.5 | 102.5 | 93.25–135.0 | 0.5427 | |
| APC | CD15 | 122.0 | 91.0–283.5 | 122.5 | 80.0–162.5 | 0.6065 |
| CD36 | 241.5 | 194.0–388.5 | 200.5 | 145.5–267.3 | 0.2417 | |
| CD271 | 208.0 | 127.0–296.5 | 170.0 | 127.5–233.3 | 0.6065 | |
| CD338 | 200.5 | 106.5–373.0 | 107.5 | 101.8–146.8 | 0.1223 | |
| CD19 | 192.0 | 154.0–241.3 | 144.0 | 120.3–182.2 | 0.1012 | |
| CD28 | 91.0 | 5.2–228.5 | 85.5 | 0–122.0 | 0.1722 | |
| CD33 | 147.5 | 10.0–189.5 | 105.5 | 90.0–132.3 | 0.6734 | |
| CD34 | 186.5 | 105.0–241.5 | 133.0 | 17.5–206.0 | 0.4250 | |
| NC | 169.0 | 99.5–244.8 | 92.0 | 64.0–204.0 | 0.2061 | |
| Per Cp | CD45 | 215.5 | 133.8–251.3 | 167.0 | 146.0–208.0 | 0.6734 |
| NC | 305.0 | 252.0–483.3 | 288.5 | 261.3–348.8 | 0.7431 | |
| Expressed antigens | ||||||
| FITC | CD9 | 3,538.0 | 2,172.0–6,871.0 | 2,156.0 | 1,743.0–3,495.0 | 0.2417 |
| CD26 | 1,287.0 | 651.8–3,235.0 | 1,308.0 | 742.3–1,920.0 | 0.9626 | |
| CD47 | 1,339.0 | 980.3–2,312.0 | 1,287.0 | 1,106.0–1,344.0 | 0.4824 | |
| CD49d | 1,185.0 | 946.8–1,393.0 | 941.0 | 708.3–1,140.0 | 0.2061 | |
| CD71 | 1,271.0 | 796.5–2,147.0 | 1,093.0 | 897.0–1,538.0 | 0.7431 | |
| CD90 | 37,140.0 | 22,740.0–52,690.0 | 36,210.0 | 21,640.0–50,260.0 | 0.8149 | |
| CD324 | 517.0 | 463.0–551.0 | 436.0 | 375.0–545.0 | 0.3027 | |
| HLA-ABC | 9,363.0 | 4,033.0–14,180.0 | 5,424.0 | 3,987.0–6,539.0 | 0.1223 | |
| NC | 325.0 | 250.0–516.3 | 279.0 | 218.8–418.8 | 0.4260 | |
| PE | CD13 | 9,802.0 | 6,786.0–17,130.0 | 3,950.0 | 3,634.0–4,961.0 | 0.0043a |
| CD56 | 496.0 | 293.8–711.0 | 528.0 | 151.0–1,048.0 | 0.9626 | |
| CD58 | 2,432.0 | 1,723.0–2,792.0 | 2,009.0 | 1,798.0–2,459.0 | 0.5427 | |
| CD99 | 405.0 | 296.5–586.3 | 467.5 | 360.5–651.0 | 0.3736 | |
| CD105 | 652.0 | 507.0–1,329.0 | 790.0 | 746.0–847.8 | 0.6734 | |
| CD151 | 16,010.0 | 10,970.0–21,430.0 | 19,410.0 | 11,090.0–23,690.0 | 0.4260 | |
| CD166 | 5,215.0 | 3,551.0–7,382.0 | 4,634.0 | 3,962.0–5,608.0 | 0.6734 | |
| CD200 | 722.5 | 205.8–1,699.0 | 1,137.0 | 631.5–1,444.0 | 0.3736 | |
| NC | 115.5 | 91.7–181.5 | 102.5 | 93.2–135.0 | 0.5427 | |
| APC | CD10 | 1,247.0 | 999.3–2,319.0 | 1,890.0 | 1,122.0–3,031.0 | 0.3736 |
| CD29 | 45,150.0 | 25,130.0–54,610.0 | 24,240.0 | 17,660.0–40,000.0 | 0.0832 | |
| CD44 | 11,440.0 | 8,186.0–16,290.0 | 7,259.0 | 6,613.0–9,753.0 | 0.0678 | |
| CD54 | 9,910.0 | 5,404.0–14,260.0 | 10,660.0 | 9,486.0–24,670.0 | 0.3736 | |
| CD81 | 31,240.0 | 19,110.0–55,050.0 | 38,890.0 | 24,500.0–44,640.0 | 0.8149 | |
| NC | 169.0 | 99.5–244.8 | 92.0 | 64.0–204.0 | 0.2061 | |
Significant P value at the Mann–Whitney test.
hA-MSC, human amniotic mesenchymal stem cells; MFI, median fluorescence intensity.
FIG. 1.
Expression of CD13 antigen in control (Co-) and obese (Ob-) pregnant women. (A) Ob-Human amniotic mesenchymal stem cells (hA-MSCs) expressed significantly higher amounts (at Mann–Whitney test) of CD13 surface antigen compared with Co-hA-MSCs (P=0.005); (B) serum levels of CD13 were significantly higher both in Ob- than in Co-not pregnant women (P=0.05) and in Ob- than in Co-women at delivery (P=0.005); (C) serum CD13 levels were correlated with CD13 surface expression levels in Ob-pregnant women (r2=0,84; P<0.0001). The box plots provide a vertical view of the data expressed as median, 25th percentile, 75th percentile, and extreme values.
APN/CD13 serum levels
We first measured baseline serum levels of CD13 in a small group of not pregnant obese and normal weight women and found significantly higher values in the obese subset (medians: 6.00 and 1.00 U/L, P=0.05, respectively) (Fig. 1B). The serum levels of CD13 were also significantly higher in Ob- than in Co-pregnant women at delivery (medians: 24.00 and 7 U/L, P=0.005, respectively), (Fig. 1B). CD13 levels were significantly higher in Ob- and Co-pregnant women than in not pregnant Ob- and Co-women: four (P=0.0005) and seven times (P=0.005), respectively. Furthermore, in Ob-pregnant women, serum CD13 levels were significantly correlated to the levels of CD13 on the surface of hA-MSCs (r2=0.84; P<0.0001) (Fig. 1C).
CD13 h-MSC expression and adipogenic differentiation
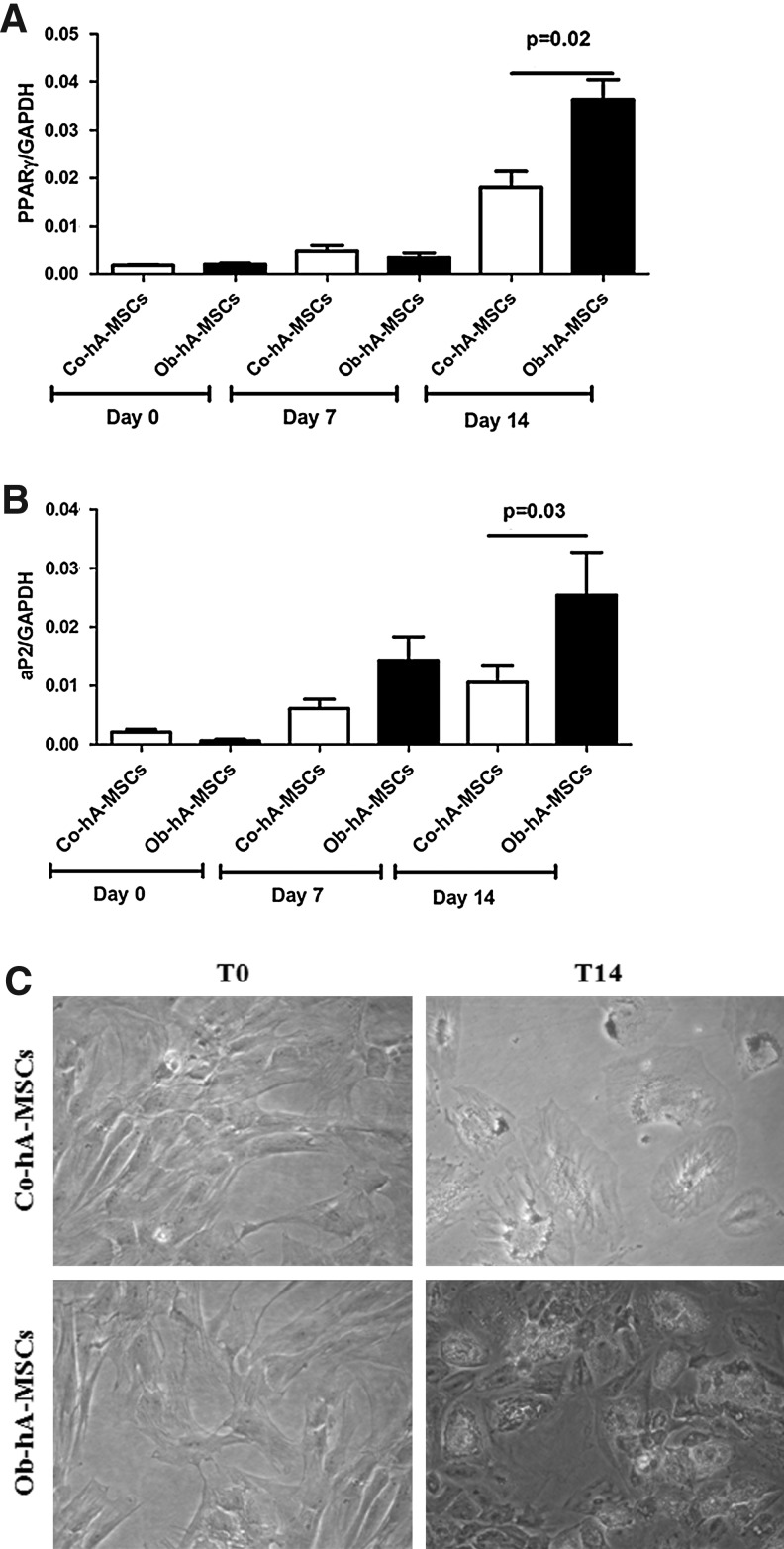
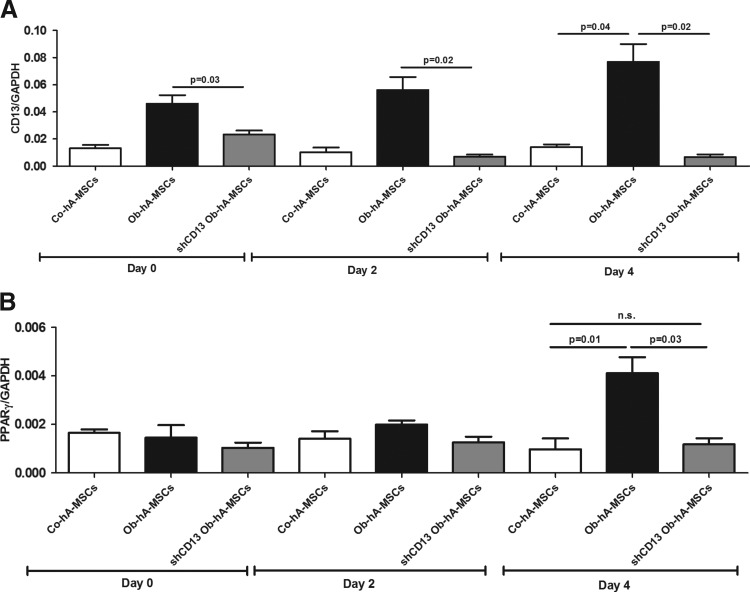
To investigate whether CD13 is involved in adipogenesis, we cultured Ob- and Co-hA-MSCs for 14 days in the adipogenic induction medium. At the end of incubation, the adipogenic potential, as measured by PPARγ and aP2 mRNA levels, was higher in Ob- than in Co-hA-MSCs. In fact, as shown in Fig. 2A and B, the mean RQs at day 14 were 0.04 and 0.02, respectively, for PPARγ (P=0.05), and 0.02 and 0.01, respectively, for aP2 (P=0.05). The same results were obtained with Oil Red staining; in fact, staining was more intense in Ob- than in Co-hA-MSCs at day 14 of differentiation [Abs (550 nm)=0.6 and 0.4, P=0.05, respectively] (Fig. 2C). During adipogenesis, CD13 mRNA levels remained higher in Ob- than in Co-hA-MSCs. CD13 silencing by shRNA in Ob-hA-MSCs resulted in a switch-off of CD13 mRNA expression, as evaluated by RT-PCR (Fig. 3A), and at the same time, the adipogenic potential of these cells did not differ from that observed in Co-hA-MSCs, as shown by similar PPARγ mRNA levels measured in silenced Ob-hA-MSCs and in Co-hA-MSCs (P=0.71) (Fig. 3B). In agreement to CD13 involvement in adipogenesis, we overexpressed CD13 in Co-hA-MSCs (mRNA CD13 mean RQ=7.23) and observed at day 4 of differentiation that PPARγ mRNA levels were higher in treated (mean RQ=0.015) than in untreated (mean RQ=0.001) Co-hA-MSCs. The adipogenic potential at day 14 was also higher in Ob- than in Co-hVAT-MSCs isolated from not pregnant women [aP2: RQs were 0.050 and 0.036; Oil Red O Abs (550 nm): 0.559 and 0.437, respectively].
FIG. 2.
Adipogenic potential in Ob-hA-MSCs and in Co-hA-MSCs. The statistically significant higher mRNA expression levels of PPARγ (P=0.05) (A) and of aP2 (P=0.05) (B) measured 14 days after the adipogenic induction, indicated increased adipogenesis in Ob- versus Co-hA-MSCs. (C) The higher adipogenesis in Ob- than in Co-hA-MSCs was also confirmed by Oil Red staining [Abs (550 nm)=0.6 and 0.4, P=0.05, respectively].
FIG. 3.
Role of CD13 in adipogenesis. (A) mRNA expression levels of CD13 were significantly higher in Ob- than in Co-hA-MSCs at day 0 (P=0.05), day 2 (P=0.05), and day 4 (P=0.05) when cultured with the adipogenic medium. CD13 mRNA expression was switched-off in Ob-hA-MSCs after CD13 silencing with shRNA. (B) At day 4 of adipogenic induction, PPARγ mRNA expression levels that were significantly higher in Ob-hA-MSCs than in Co-hA-MSCs (P=0.02), decreased to the levels detected in Co-hA-MSCs after CD13 silencing (P=0.71), which indicates that CD13 enhances adipogenesis in hA-MSCs. n.s.: not statistically significant difference.
Upregulation of CD13 h-MSC expression by IFN-γ
We next evaluated if CD13 expression could be upregulated in h-MSCs by IFN-γ as occurs in murine cellular models [32]. To this aim, we treated the Co- and Ob-hA-MSCs with 0.8 ng/mL or 12.5 ng/mL IFN-γ for 24 h. We found that CD13 expression was significantly higher on membranes of Co-hA-MSCs treated with 12.5 ng/mL IFN-γ (P=0.05) than in untreated cells, whereas there was a slight, not significant, increase in treated Ob-hA-MSCs (Supplementary Fig. S2) versus the untreated counterpart cells. In addition, IFN-γ treatment (12.5 ng/mL at 37°C for 24 h) induced the increase of CD13 membrane expression in hVAT-MSCs (Ob- and Co-MSCs: 39% and 8%, respectively), and in Co-hUC-MSCs (4%) versus the untreated counterpart cells, but not in Ob-hUC-MSCs. Our results suggest that high levels of INF-γ drive the upregulation of CD13 expression in Co-h-MSCs, irrespective of their source and of pregnancy, whereas its effect on Ob-h-MSC CD13 expression during obesity is ambiguous.
Discussion
Human amniotic membrane is a readily available source of abundant fetal MSCs that are free from ethical concerns [33]. hA-MSCs isolated from normal weight healthy women at delivery have been characterized [24,34,35], but, to our knowledge, the features of hA-MSCs from obese women are largely unknown. In this study, we used flow cytometry to characterize hA-MSCs isolated at delivery from two groups of women: prepregnancy normal weight and prepregnancy severely obese women. The immunophenotypic characterization confirmed the mesenchymal origin of the isolated cells [36]. In particular, the distribution of CD56 was in agreement with the placental origin of the isolated hA-MSCs. In fact, this marker is absent from bone marrow [34] and from adipose tissue-derived mesenchymal stem cells [37]. Similarly, the endothelial marker PECAM-1/CD31, and the hematopoietic antigens CD14, CD15, CD16, CD19, CD28, CD33, CD34, CD45, and CD117 were absent from isolated Ob- and Co-hA-MSCs. Staining for the E-cadherin/CD324 epithelial antigen was very weak in our Ob- and Co-hA-MSC preparations; the coexpression of epithelial, although at a low intensity, and mesenchymal markers on our hA-MSCs was in agreement with previous findings [38,39]. Overall, our results are similar to those reported by Parolini et al. [24] and/or Roubelakis [35] regarding the expressed (CD49d, CD90, HLA-ABC, CD13, CD56, CD105, CD166, CD10, CD29, CD44, and CD54) and not expressed (PECAM-1/CD31, HLA-DR, CD14, Prominin-1/CD133, NGFR/CD271, CD34, and CD45) membrane-bound antigens in hA-MSCs. We found that the Ob-hA-MSC immunophenotype is characterized by a significantly higher expression of the APN/CD13 antigen with respect to the Co-hA-MSC phenotype. Besides amnion, CD13 was overexpressed in h-MSCs isolated from the umbilical cord in obese women and in those isolated from VAT in not pregnant obese women.
Type II metalloprotease APN/CD13 (EC. 3.4.11.2) is a heavily glycosylated membrane-bound protein (∼960aa, ∼150 kDa) that is encoded by the human ANPEP gene located on chromosome 15 (q25-q26) [40]. This protein exists also in a soluble form. APN/CD13 is a ubiquitous enzyme present in a wide variety of human organs, tissues, and cell types, including the placenta, human umbilical vein endothelial cells, monocytes, lymphocytes T, hypothalamus, and epithelial intestinal cells [41]. It has various mechanisms of action: enzymatic cleavage of peptides, endocytosis, and signal transduction [42]. APN/CD13 is involved in inflammation, cellular differentiation and proliferation, apoptosis, cell adhesion, and motility [42]. Dysregulated expression of membrane and/or soluble forms of APN/CD13 has been observed in many diseases [43], but until now, it has never been associated with obesity. Here we provide the first demonstration that the CD13 antigen is increased on hA-MSCs during obesity and could play a role in adipogenesis. In fact, we first detected a higher adipogenic potential in Ob- than in Co-hA-MSCs after 14 days of adipogenic differentiation, and then observed that the adipogenic potential of Ob-hA-MSCs was comparable to that of Co-hA-MSCs after CD13 silencing. Conversely, the adipogenic potential increased in Co-hA-MSCs after CD13 overexpression. Furthermore, we provide evidence that INFγ upregulated CD13 expression in Co-hA-MSCs.
Intriguingly, in Ob-pregnant women, APN/CD13 serum levels at delivery were higher than in Co-pregnant women and correlated with CD13 surface Ob-hA-MSC expression (r2=0.84, P<0.0001), which support the hypothesis that the placenta is the major source of the high CD13 levels measured in maternal serum [44]. We also found that the leptin concentration and the L/A ratio were increased in Ob-maternal serum at delivery. This finding confirms the concept that these two parameters are obesity risk markers [45,46].
In conclusion, this characterization of Ob-hA-MSCs shows that antigen CD13, by influencing the adipogenic potential of these cells, could be an in utero risk factor for obesity. Our data strengthen the hypothesis that high serum CD13 and mesenchymal stem cell CD13 are markers of obesity.
Supplementary Material
Acknowledgments
The present work was supported by grants from CEINGE Regione Campania (DGRC 1901/2009) and by MIUR-PRIN 2008. We thank Jean Ann Gilder (Scientific Communication srl, Naples, Italy) for revising and editing the manuscript.
Author Disclosure Statement
The authors declare no financial conflict of interests.
References
- 1.Guelinckx I. Devlieger R. Beckers K. Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9:140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 2.Heslehurst N. Ells LJ. Simpson H. Batterham A. Wilkinson J. Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY. Dietz PM. England L. Morrow B. Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 4.Pipe NG. Smith T. Halliday D. Edmonds CJ. Williams C. Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol. 1979;86:929–940. doi: 10.1111/j.1471-0528.1979.tb11240.x. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenberg HM. Huston-Presley L. Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 6.Capobianco V. Nardelli C. Ferrigno M. Iaffaldano L. Pilone V. Forestieri P. Zambrano N. Sacchetti L. miRNA and protein expression profiles of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J Proteome Res. 2012;11:3358–3369. doi: 10.1021/pr300152z. [DOI] [PubMed] [Google Scholar]
- 7.Drolet R. Richard C. Sniderman AD. Mailloux J. Fortier M. Huot C. Rhéaume C. Tchernof A. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond) 2008;32:283–291. doi: 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- 8.Bartha JL. Marín-Segura P. González-González NL. Wagner F. Aguilar-Diosdado M. Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring) 2007;15:2233–2239. doi: 10.1038/oby.2007.265. [DOI] [PubMed] [Google Scholar]
- 9.Harvey NC. Poole JR. Javaid MK. Dennison EM. Robinson S. Inskip HM. Godfrey KM. Cooper C. Sayer AA SWS Study Group. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu SY. Callaghan WM. Kim SY. Schmid CH. Lau J. England LJ. Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien TE. Ray JG. Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Metwally M. Ong KJ. Ledger WL. Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception?. A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 13.Sattar N. Clark P. Holmes A. Lean ME. Walker I. Greer IA. Antenatal waist circumference and hypertension risk. Obstet Gynecol. 2001;97:268–271. doi: 10.1016/s0029-7844(00)01136-4. [DOI] [PubMed] [Google Scholar]
- 14.Li C. Kaur H. Choi WS. Huang TT. Lee RE. Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13:362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 15.Fowden AL. Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 16.Fowden AL. Forhead AJ. Coan PM. Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 17.Okawa H. Okuda O. Arai H. Sakuragawa N. Sato K. Amniotic epithelial cells transform into neuron-like cells in the ischemic brain. Neuroreport. 2001;12:4003–4007. doi: 10.1097/00001756-200112210-00030. [DOI] [PubMed] [Google Scholar]
- 18.Zeigler BM. Sugiyama D. Chen M. Guo Y. Downs KM. Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 19.Fukuchi Y. Nakajima H. Sugiyama D. Hirose I. Kitamura T. Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 20.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 21.In 't Anker PS. Scherjon SA. Kleijburg-van der Keur C. de Groot-Swings GM. Claas FH. Fibbe WE. Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 22.Katz AJ. Tholpady A. Tholpady SS. Shang H. Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH. Lee MJ. Seok OS. Paek YC. Cho GJ. Seol HJ. Lee JK. Oh MJ. Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine. 2010;49:95–101. doi: 10.1016/j.cyto.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Parolini O. Alviano F. Bagnara GP. Bilic G. Bühring HJ. Evangelista M. Hennerbichler S. Liu B. Magatti M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 25.Grant A. Palzer S. Hartnett C. Bailey T. Tsang M. Kalyuzhny AE. A cell-detachment solution can reduce background staining in the ELISPOT assay. Methods Mol Biol. 2005;302:87–94. doi: 10.1385/1-59259-903-6:087. [DOI] [PubMed] [Google Scholar]
- 26.Mariotti E. Mirabelli P. Di Noto R. Fortunato G. Salvatore F. Rapid detection of mycoplasma in continuous cell lines using a selective biochemical test. Leuk Res. 2008;32:323–326. doi: 10.1016/j.leukres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Bieback K. Kern S. Klüter H. Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 28.Schäffler A. Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 29.Perfetto SP. Ambrozak D. Nguyen R. Chattopadhyay P. Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protocols. 2006;1:1522–1530. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- 30.Lamoreaux L. Roederer M. Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protocols. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 31.Maeker HT. Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A. 2006;69A:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovac J. Cupić B. Zivković E. Horvat L. Majhen D. Expression, regulation and functional activities of aminopeptidase N (EC 3.4.11.2; APN; CD13) on murine macrophage J774 cell line. Immunobiology. 2011;216:132–144. doi: 10.1016/j.imbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Miki T. Lehmann T. Cai H. Stolz DB. Stromk SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 34.Mariotti E. Mirabelli P. Abate G. Schiattarella M. Martinelli P. Fortunato G. Di Noto R. Del Vecchio L. Comparative characteristics of mesenchymal stem cells from human bone marrow and placenta: CD10, CD49d, and CD56 make a difference. Stem Cells Dev. 2008;17:1039–1041. doi: 10.1089/scd.2008.0212. [DOI] [PubMed] [Google Scholar]
- 35.Roubelakis MG. Trohatou O. Anagnou NP. Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells Int. 20122012:107836. doi: 10.1155/2012/107836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delorme B. Ringe J. Gallay N. Le Vern Y. Kerboeuf D. Jorgensen C. Rosset P. Sensebé L. Layrolle P. Häupl T. Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 37.Gronthos S. Franklin DM. Leddy HA. Robey PG. Storms RW. Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 38.Sakuragawa N. Kakinuma K. Kikuchi A. Okano H. Uchida S. Kamo I. Kobayashi M. Yokoyama Y. Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res. 2004;78:208–214. doi: 10.1002/jnr.20257. [DOI] [PubMed] [Google Scholar]
- 39.Soncini M. Vertua E. Gibelli L. Zorzi F. Denegri M. Albertini A. Wengler GS. Parolini O. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 40.Watt VM. Willard HF. The human aminopeptidase N gene: isolation, chromosome localization, and DNA polymorphism analysis. Hum Genet. 1990;85:651–654. doi: 10.1007/BF00193592. [DOI] [PubMed] [Google Scholar]
- 41.Lai A. Ghaffari A. Ghahary A. Inhibitory effect of anti-aminopeptidase N/CD13 antibodies on fibroblast migration. Mol Cell Biochem. 2010;343:191–199. doi: 10.1007/s11010-010-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luan Y. Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14:639–647. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- 44.Kawai M. Araragi K. Shimizu Y. Hara Y. Identification of placental leucine aminopeptidase and triton-slowed aminopeptidase N in serum of pregnant women. Clin Chim Acta. 2009;400:37–41. doi: 10.1016/j.cca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Labruna G. Pasanisi F. Nardelli C. Tarantino G. Vitale DF. Bracale R. Finelli C. Genua MP. Contaldo F. Sacchetti L. UCP1-3826 AG+GG genotypes, adiponectin, and leptin/adiponectin ratio in severe obesity. J Endocrinol Invest. 2009;32:525–529. doi: 10.1007/BF03346500. [DOI] [PubMed] [Google Scholar]
- 46.Labruna G. Pasanisi F. Nardelli C. Caso R. Vitale DF. Contaldo F. Sacchetti L. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring) 2011;19:1492–1496. doi: 10.1038/oby.2010.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.