Abstract
It is generally believed that during development, neurons are usually produced in excess. Cell death occurs in the developing nervous system. The survival of the developing neurons depends on many factors derived from the target sites, of which the neuronal trophic factors are by far the best known. Stem cell factor (SCF) and its receptor, c-kit, is expressed in cells of nervous system during development and adulthood. Although the role of SCF/c-kit in the nervous system is so far not clear, in vitro studies indicate that SCF/c-kit is trophic to certain neurons derived from neural crest and cerebral cortex. In this study the effects of anti-c-kit antibody on cell death in the newborn chick cerebral cortex have been investigated. Injection of anti-c-kit antibody into the cisterna magnum increased the number of cell death and resulted in thinning of the cerebral cortex as compared to that from the control group. It is concluded that SCF/c-kit is essential for cortical progenitor cell survival in the cerebral cortex. Moreover, this method may be applied to the other factors and different CNS regions, allowing identification of factors involved in cell death. It additionally re-emphasizes the importance of further investigations into the potential roles of SCF/c-kit signaling in neurodegenerative diseases.
Keywords: Stem cell factor, c-Kit, Cell death, Chick, Cerebral cortex
1. Introduction
Little is known about regionally specific signals that control the number of neuronal progenitor cells in vivo. The central nervous system (CNS) of vertebrates originates from neuroepithelial cells located within the germinal epithelium lining the dorsal portion of the telencephalic vesicles give rise to the cerebral cortex (Bayer and Altman, 1991). Several trophic factors have been identified in the CNS, many of which may become useful tools in neural repair strategies. Some of these factors regulate multiple functions in the developing brain, including cellular proliferation, migration, differentiation and survival (Bajetto et al., 2001).
Stem cell factor (SCF) also named kit ligand, steel factor (SLF). Its receptor, encoded by the proto-oncogene, c-kit, is a member of the class III family of intrinsic tyrosine kinase growth factor receptor. Both SCF and c-kit mRNAs are expressed in cells of the nervous system during development and in adulthood (Manova et al., 1992; Kim et al., 2003). c-Kit is expressed in neural stem cells and in their differentiated progeny (Erlandsson et al., 2004). During embryonic development, SCF mRNA is detectable in neural tube as early as at mouse embryonic day 9.5 (Keshet et al., 1991). In the adult nervous system, high level of SCF transcripts was found in the thalamus, cerebral cortex and cerebellum (Zhang and Fedoroff, 1997).
SCF/c-kit is important in the proliferative maintenance of retinal stem cells (James et al., 2004) and it is also a survival factor in vitro (Dolci et al., 1991; Erlandsson et al., 2004). The receptor for SCF, which possesses similarity to c-fms, the receptor for colony stimulating factor-1 (CSF-1), and to the platelet derived growth factor receptor (PDGF-R). Activation of c-fms by CSF-1 elicits neuroprotection, implying this family of receptors may protect against cellular stressors in the central nervous system (CNS), a finding supported by the ability of PDGF-R to protect against ischemic injury in both rodent and in human brains (Zhang and Fedoroff, 1999). The functional importance of the ligand–receptor complex is not well defined in the CNS. c-Kit knockout mice exhibit abnormalities in learning and memory (Motro et al., 1996; Katafuchi et al., 2000), suggesting potentially important roles for SCF/c-kit in normal brain physiology. In culture, recombinant SCF supports the survival of rat and chick neurons which express c-kit receptor (Hirata et al., 1993). Therefore, SCF may act as a neurotrophic factor for c-kit expressing neurons.
During embryonic development, the neural tube is formed by an epithelial wall, the neuroepithelium, surrounding a cavity, which is filled with cerebrospinal fluid (CSF). It is secreted by the choroids plexuses and contains growth factors (Nicholson, 1999). It has been shown that CSF is important in the survival, replication and differentiation of neuroepithelium, probably by the presence of cytokines and/or growth factors in its composition (Gato et al., 2004; Salehi and Mashayekhi, 2006; Mashayekhi and Salehi, 2006). It was shown that these molecules in the CSF can enter the brain tissues (Nicholson, 1999). Upon secretion into the ventricles peptides, growth factors and other macromolecules are conveyed by CSF bulk flow to various regions of the brain and spinal cord. This convective distribution of peptide signal and trophic factors places many neurons in contact with the products and secretion of the choroid epithelial cells (Johanson et al., 2000). Many of these peptides are secreted by the fetal choroid plexuses to provide trophic support for the developing brain (Miyan et al., 2003).
We have previously shown that infusion of anti-NGF antibody into the CSF of chick embryo leads to decrease cell production in the cerebral cortical germinal epithelium (Mashayekhi and Salehi, 2007). In this study, the effects of intracisternal administration of anti-c-kit antibody on neural cell survival using a specific neutralizing antibody were investigated.
2. Materials and methods
2.1. Animals
Fertile white Leghorn eggs were incubated at 38 °C in a humidified atmosphere to obtain chick embryos at different developmental stages. After birth the anti-c-kit antibody (Abcam, Cambridge, UK) was administered intracranially via the cisterna magnum. Vehicle solution containing 0.4% trypan blue dye was injected to assess distribution. Groups of 10 one-day chicks received IgG vehicle (control group) or anti-c-kit antibody (4 μg/chick, three repeats, n = 27). After injections, the chicks were allowed to survive for another 2 days. All the anti-c-kit antibody injected and control chicks were collected after euthanasia by intraperitoneal injection of an overdose of anesthetic (sodium pentobarbitone) and the brains were removed and processed as described. The heads were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) at pH 7.3 for 24 h. They were dehydrated in ascending concentrations of ethanol, embedded in paraffin (Merck, Darmstadt, Germany) and cut in 5 μm-thick sections with a microtome (Leitz, 1512, Germany)
All animal procedures were carried out in accordance with the Animals (Scientific Procedure) Act, 1986.
2.2. Staining of the tissue sections
2.2.1. Hematoxylin–eosin staining
The sections were cleared in citroclear for 30 min and re-hydrated with descending concentrations of ethanol (100%, 90%, 80%, 70%, and 50%) for 5 min each. They were left in tap water for 5 min and stained with hematoxylin (Sigma, Poole, UK) for 2 min and washed in tap water for 10 min. They were then dehydrated in ascending concentrations of ethanol and stained in alcoholic eosin (Sigma, Poole, UK) for 2 min. The sections were left in citroclears for 10 min and mounted with coverslip using glycerine–albumin.
2.2.2. Terminal dUTP nick end labeling
The sections were incubated for 15 min in 30 mM Tris buffer containing 2.5 mM CaCl2 (proteinase K buffer), followed by 15 min incubation in 10 mg/ml proteinase K. The sections were washed three times with distilled water and pre-incubated for 20 min in terminal transferase (TdT) buffer (30 mM Tris buffer, 140 mM sodium cacodylate and 1 mM cobalt chloride at pH 7.2), followed by incubation in 1 U/100 μl TdT and 1 U/100 μl biotinylated-d-UTP (bio-11-dUTP) at 37 °C for 60 min. The reaction was terminated by rinsing the slides in distilled water followed by 0.1 M phosphate buffered saline (PBS) for 15 min. The sections were incubated for a further 2 h with streptavidin conjugate with peroxidase (ABC complex) (Vector Laboratories, Peterborough, UK) diluted 1:200 in PBS. The reaction product was visualized with 0.05% Diaminobenzidine (DAB; Vector Laboratories, Peterborough, UK) for 5 min. Sections were dehydrated through ascending series of ethanol and xylene. The stained sections were mounted using glycerine–albumin (Sigma) and images were captured with a camera fitted to a microscope. The number of dying cells was counted using a microscope at 400× magnification.
2.2.3. Double staining
In order to assess the type of TUNEL positive cells, the sections were stained with either anti-neurofilament or anti-vimentin antibodies, which stain neurons and glia, respectively. Sections were incubated in 1% H2O2 solution in PBS for 1 h in the dark at room temperature to block endogenous peroxidase. Following a rinse with PBS, the slides were incubated in 10% normal serum in PBS for 1 h prior to flooding with primary antibody diluted in 10% normal serum in PBS/Triton X-100. The primary antibody was diluted in PBS/Triton X-100. Triton X-100 or Tween was added to the PBS to facilitate the penetration of the antibody across the cell membrane. Overnight immersion in primary antibody (anti-neurofilament antibody or anti-Vimentin antibody) solution at 4 °C was followed by washing in PBS and PBS/Triton X-100 three times (for 5 min each times) and then flooding with Biotinylated horse anti-rabbit and anti-mouse antibody (Vector laboratories, USA) in a dilution of 1:200 in 10% normal serum in PBS/Triton X-100 for 2 h. Following a rinse in PBS three times (for 5 min each time), the sections were incubated in Avidin–Biotin Complex (ABC) (Vector laboratories, USA), for 2 h at room temperature. The sections were washed in PBS and PBS/Tween three times (for 5 min each time). Finally they were stained with diaminobenzidine (DAB) (Vector Elite Kits, USA) for 30 s. All sections were washed with tap water for 5 min. Then the sections were stained with TUNEL according to the method described above and developed with DAB and mounted in glycerine–albumin.
2.3. Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). In all experiments, a minimum of 27 measurements (n = 27) were taken in order to calculate a mean ± SEM. The statistical significance was evaluated with the Student’s t-test, and P values of less than 0.05 (P ⩽ 0.05) were regarded as statistically significant.
3. Results
3.1. Analysis of the changes in the thickness of cerebral cortex
Coronal sections through the brains of control and anti-c-kit antibody injected chicks were analysed to determine the effects of anti-c-kit antibody on gross cortical morphology. The most apparent difference was in the thickness of the cerebral cortex. The thickness of the germinal epithelium and cerebral cortex in the anti-c-kit antibody injected chicks was decreased when compared to that from the control group. Statistical analysis showed that the decrease in the thickness of the cerebral cortex of the anti-c-kit antibody chicks was significant when compared to that from the control group (n = 27) (Figs. 1 and 2) (P < 0.001). The reduction in the overall size of the cerebral cortex demonstrates a significant effect of anti-c-kit antibody on the growth of the cerebral cortex.
Figure 1.
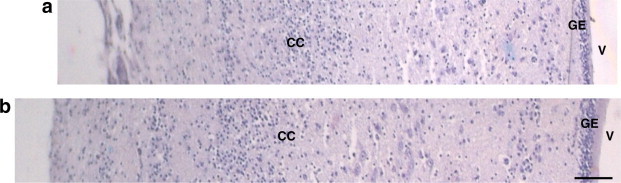
Coronal sections across the thickness of cerebral cortex from anti-c-kit antibody injected (a) and control (b). Abbreviations: V = ventricle, GE = germinal epithelium, cc = cerebral cortex. Scale bar = 100 μm.
Figure 2.
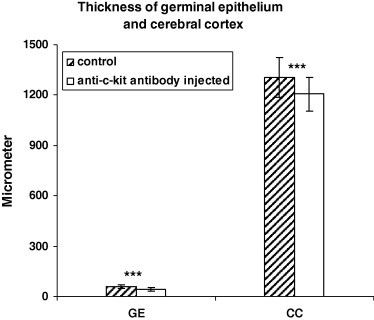
The thickness of the cerebral cortex (CC) and germinal epithelium (GE) was measured in comparable coronal sections from anti-c-kit antibody injected and controls. The brains were cut at 5 μm on a microtome. There is a significant decrease in the thickness of cerebral cortex and germinal epithelium when compared to controls. In each of the experimental groups the number of animals investigated was n = 27. Significance values are shown as stars: three stars P < 0.001.
3.2. Cell death in the cerebral cortex of anti-c-kit antibody injected and control groups
An explanation for decreased thickness of cerebral cortex observed in anti-c-kit antibody injected chicks might be due to increase in the number of cell deaths. In order to assess the number of cell deaths, the sections were stained with TUNEL. Cells with morphological features of apoptosis could be recognized easily in brain sections stained with TUNEL (Fig. 3). These cells, scattered amongst numerous healthy cells, were present throughout the cerebral cortex in all the sections examined in this experiment. Analysis of TUNEL stained sections from antibody injected chicks and the control group showed that the number of dying cells in the cerebral cortex of anti-c-kit antibody injected group was increased when compared to that from the control group. Statistical analysis showed that there is a significant increase in the number of dying cells in the cortex of antibody injected chicks when compared with controls (Fig. 4) (P < 0.001).
Figure 3.
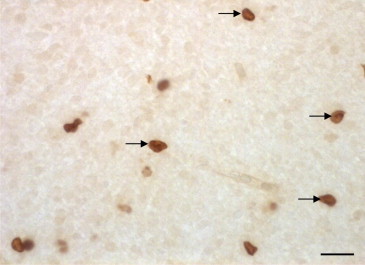
TUNEL stained sections across the thickness of the cerebral cortex from the chick brain 3 days after hatching. The brains were cut at 5 μm on a microtome. Arrows show the TUNEL positive cells. Some TUNEL positive cells have been shown by arrows. Scale bar = 30 μm.
Figure 4.
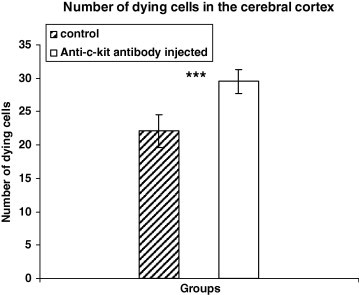
The number of dying cells in the cerebral cortical sections was counted. Sections were taken from day three after hatching. Anti-c-kit antibody was injected on day one after hatching. The brains were cut at 5 μm on a microtome. In each of the experimental groups the number of animals investigated was n = 27. Significance values are shown as stars: three stars P < 0.001.
In order to identify the type of dying cells, the sections were stained with either anti-neurofilament or anti-vimentin antibodies followed by TUNEL staining. The result from double staining has shown that the TUNEL positive cells are also anti-neurofilament positive (Fig. 5). Thus it is concluded that the TUNEL positive cells are neurons rather than glia.
Figure 5.
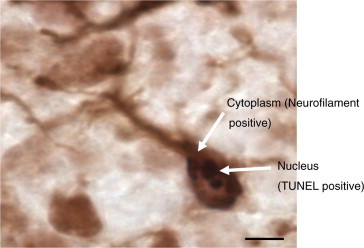
Higher magnification of double stained with both TUNEL and anti-neurofilament antibody coronal sections through the cerebral cortex of day three chick after hatching. The brains were cut at 5 μm-thick on a microtome. Scale bar = 25 μm.
4. Discussion
Neurons of both the developing and adult cerebral cortex express SCF and c-kit (Zhang and Fedoroff, 1997), although the functions of SCF/c-kit in the CNS remain largely unexplored. Transgenic mice with a deletion of the c-kit gene display marked defects in learning and memory, suggesting an important function in normal brain physiology (Katafuchi et al., 2000). SCF and c-kit also may be involved in the response to CNS damage, as both are up-regulated following brain injury and are correlated with neurogenesis (Jin et al., 2002), a mechanism for brain repair. Furthermore, the frequent overexpression of c-kit in a number of malignancies, including neuronal tumors, correlates with cellular survival (Timeus et al., 1997; Vitali et al., 2003), further indicating SCF/c-kit may enhance cytoprotection in a variety of cell types, including neurons.
We have previously shown that normal CSF circulation is important in neural cell proliferation and survival in developing chick cerebral cortex (Mashayekhi and Salehi, 2006; Salehi and Mashayekhi, 2006). We have also shown that nerve growth factor is an important factor in cerebral cortical development, stimulating neuronal precursor proliferation (Mashayekhi and Salehi, 2007). CSF contains growth factors and cytokines which are known modulator of neurogenesis and differentiation (Nicholson, 1999).
It has been demonstrated that components carried in the CSF not only circulate rapidly through the CSF pathways, but also have fast access to most regions of the brain itself, gaining entry across the pia and ependymal layers through gap junctions and by active transport or diffusion (Proescholdt et al., 2000). It has been shown that some of the cells underlying ependyma have processes passing between the ependymal cells that are in contact with the CSF (reviewed by Mashayekhi et al., 2002).
In our study using neutralizing antibody suggests that SCF/c-kit is an important factor in cerebral cortical development, preventing neural cell death (Hirata et al., 1993; Jin et al., 2002; Erlandsson et al., 2004; Dhandapani et al., 2005). Anti-c-kit antibody, which recognizes protein, specifically blocks c-kit activity and therefore increasing neural cell death.
It is proposed that the decrease of cerebral cortical thickness in anti-c-kit antibody injected chicks is partly due to the increase in neural cell death. In order to test this theory, the brain sections from anti-c-kit antibody injected and controls were stained with TUNEL. Injection of antibody may block the activity of SCF/c-kit that is necessary for neural cell survival. The results of this study could have been anticipated with some certainty given the findings of other studies that have investigated the role of SCF/c-kit in cell survival.
This study using neutralizing antibody against c-kit suggests that this SCF/c-kit is an important factor in neural cell survival. The antibody recognizes native protein, specifically blocks c-kit. In this study, we demonstrated that the neutralizing antibody specifically blocked survival effects elicited by c-kit in the cerebral cortex.
5. Conclusions
It is concluded that SCF/c-kit is important for neural cell survival in the cerebral cortex. The elucidation of the neuroprotective action of c-kit in this study adds to a growing literature suggesting a central survival role for this factor in the cerebral cortex. It additionally re-emphasizes the importance of further investigations into the potential roles of SCF/c-kit in neurodegenerative diseases.
Acknowledgements
This study was supported by the University of Guilan, Iran. I am extremely grateful to Dr. Nouri for her technical support.
References
- Bajetto A., Bonavia R., Barbero S., Florio T., Schettini G. Chemokines and their receptors in the central nervous system. Front. Neuroendocrinol. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Bayer S.A., Altman J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience. 1991;45:391–412. doi: 10.1016/0306-4522(91)90236-h. [DOI] [PubMed] [Google Scholar]
- Dhandapani K.M., Wade F.M., Wakade C., Mahesh V.B., Brann D.W. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J. Neurochem. 2005;95:9–19. doi: 10.1111/j.1471-4159.2005.03319.x. [DOI] [PubMed] [Google Scholar]
- Dolci S., Williams D.E., Ernst M.K., Resnick J.L., Brannan C.I., Lock L.F., Lyman S.D., Boswell H.S., Donovan P.J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991;29:809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Erlandsson A., Larsson J., Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp. Cell Res. 2004;10:201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Gato A., Martin P., Alonso M.I., Martin C., Pulgar M.A., Moro J.A. Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J. Exp. Zoolog. A, Comp. Exp. Biol. 2004;1:280–289. doi: 10.1002/jez.a.20035. [DOI] [PubMed] [Google Scholar]
- Hirata T., Morii E., Morimoto M., Kasugai T., Tsujimura T., Hirota S., Kanakura Y., Nomura S., Kitamura Y. Stem cell factor induces outgrowth of c-kit-positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development. 1993;119:49–56. doi: 10.1242/dev.119.1.49. [DOI] [PubMed] [Google Scholar]
- James J., Das A.V., Rahnenfuhrer J., Ahmad I. Cellular and molecular characterization of early and late retinal stem cells/progenitors: differential regulation of proliferation and context dependent role of Notch signaling. J. Neurobiol. 2004;61:359–376. doi: 10.1002/neu.20064. [DOI] [PubMed] [Google Scholar]
- Jin K., Mao X.O., Sun Y., Xie L., Greenberg D.A. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C.E., Palm D.E., Primiano M.J., McMillan P.N., Chan P., Knuckey N.W., Stopa E.G. Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell. Mol. Neurobiol. 2000;20:197–216. doi: 10.1023/A:1007097622590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T., Li A.J., Hirota S., Kitamura Y., Hori T. Impairment of spatial learning and hippocampal synaptic potentiation in c-kit mutant rats. Learn. Mem. 2000;7:383–392. doi: 10.1101/lm.33900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Lyman S.D., Williams D.E., Anderson D.M., Jenkins N.A., Copeland N.G., Parada L.F. Embryonic RNA expression patterns of the c-kit receptor and its cognate ligand suggest multiple functional roles in mouse development. EMBO J. 1991;10:2425–2435. doi: 10.1002/j.1460-2075.1991.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Im J.O., Won Y.J., Yoon S.Y., Lee E.J., Lee J.H., Hong H.N. Upregulation of c-Kit receptor and stem cell factor in cerebellar inhibitory synapses in response to kainic acid. J. Neurosci. Res. 2003;1:72–78. doi: 10.1002/jnr.10466. [DOI] [PubMed] [Google Scholar]
- Manova K., Bachvarova R.F., Huang E.J., Sanchez S., Pronovost S.M., Velazquez E., McGuire Besmer P. c-Kit receptor and ligand expression in postnatal development of the mouse cerebellum suggests a function for c-kit in inhibitory interneurons. J. Neurosci. 1992;12:4663–4676. doi: 10.1523/JNEUROSCI.12-12-04663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi F., Salehi Z. The importance of cerebrospinal fluid on neural cell proliferation in developing chick cerebral cortex. Eur. J. Neurol. 2006;6:266–272. doi: 10.1111/j.1468-1331.2006.01208.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F., Salehi Z. Infusion of anti-nerve growth factor into the cisternum magnum of chick embryo leads to decrease cell production in the cerebral cortical germinal epithelium. Eur. J. Neurol. 2007;14:181–186. doi: 10.1111/j.1468-1331.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F., Draper C.E., Bannister C.M., Pourghasem M., Owen-Lynch P.J., Miyan J.A. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain. 2002;125:1859–1874. doi: 10.1093/brain/awf182. [DOI] [PubMed] [Google Scholar]
- Miyan J.A., Nabiyouni M., Zendah M. Development of the brain: a vital role for cerebrospinal fluid. Can. J. Physiol. Pharmacol. 2003;81:317–328. doi: 10.1139/y03-027. [DOI] [PubMed] [Google Scholar]
- Motro B., Wojtowicz J.M., Bernstein A., van der Kooy D. Steel mutant mice are deficient in hippocampal learning but not long-term potentiation. Proc. Natl. Acad. Sci. USA. 1996;5:1808–1813. doi: 10.1073/pnas.93.5.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. Signals that go with the flow. Trends Neurosci. 1999;22:143–145. doi: 10.1016/s0166-2236(98)01388-5. [DOI] [PubMed] [Google Scholar]
- Proescholdt M.G., Hutto B., Brady L.S., Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience. 2000;95:577–592. doi: 10.1016/s0306-4522(99)00417-0. [DOI] [PubMed] [Google Scholar]
- Salehi Z., Mashayekhi F. The role of cerebrospinal fluid on neural cell survival in the developing chick cerebral cortex: an in vivo study. Eur. J. Neurol. 2006;3:760–764. doi: 10.1111/j.1468-1331.2006.01358.x. [DOI] [PubMed] [Google Scholar]
- Timeus F., Crescenzio N., Valle P., Pistamiglio P., Piglione M., Garelli E., Ricotti E., Rocchi P., Strippoli P., Cordero di Montezemolo L., Madon E., Ramenghi U., Basso G. Stem cell factor suppresses apoptosis in neuroblastoma cell lines. Exp. Hematol. 1997;25:1253–1260. [PubMed] [Google Scholar]
- Vitali R., Cesi V., Nicotra M.R., McDowell H.P., Donfrancesco A., Mannarino O., Natali P.G., Raschellà G., Dominici C. c-Kit is preferentially expressed in MYCN-amplified neuroblastoma and its effect on cell proliferation is inhibited in vitro by STI-571. Int. J. Cancer. 2003;106:147–152. doi: 10.1002/ijc.11187. [DOI] [PubMed] [Google Scholar]
- Zhang S.C., Fedoroff S. Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J. Neurosci. Res. 1997;1:1–15. [PubMed] [Google Scholar]
- Zhang S.C., Fedoroff S. Expression of stem cell factor and c-kit receptor in neural cells after brain injury. Acta Neuropathol. (Berl) 1999;97:393–398. doi: 10.1007/s004010051003. [DOI] [PubMed] [Google Scholar]


